Milkshake Acutely Stimulates Dopamine Release in Ventral and Dorsal Striatum in Healthy-Weight Individuals and Patients with Severe Obesity Undergoing Bariatric Surgery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Questionnaire Measures
2.2.2. Imaging Protocol
2.2.3. Image Acquisition and Reconstruction
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
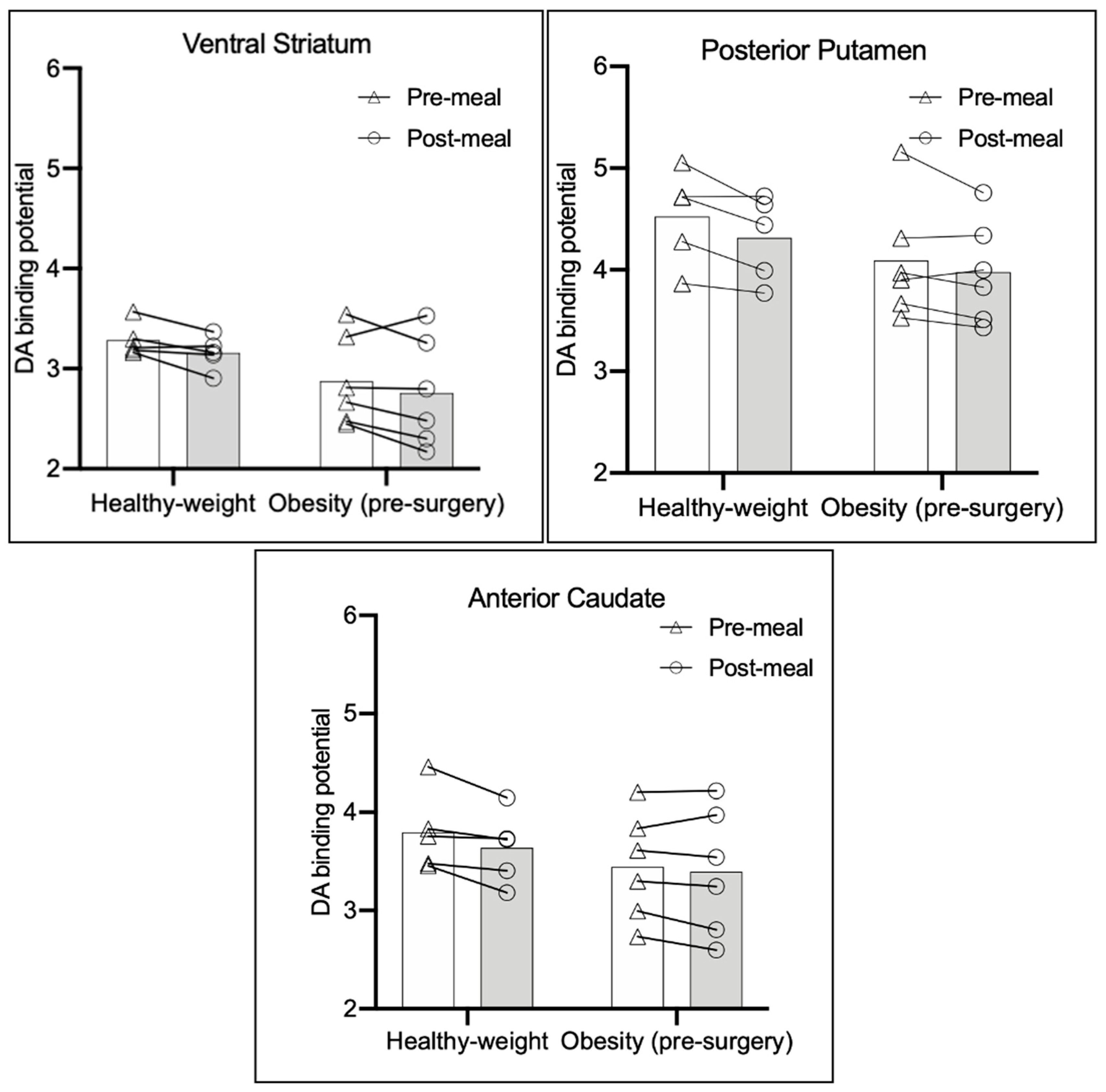
3.2. Meal-Stimulated Change in DA BPND in the Healthy-Weight Group and Severe Obesity Group Pre-Surgery
3.3. Meal-Stimulated Change in DA BPND in the Severe Obesity Group from Pre- to Post-Surgery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BAS | Behavioral Activation Scales |
| BMI | Body mass index |
| BPND | Nondisplaceable binding potential |
| CBC | Complete blood count |
| DA | Dopamine |
| DARel | Dopamine release |
| ECG | Electrocardiogram |
| FCI | Food-Craving Inventory |
| fMRI | Functional magnetic resonance imaging |
| g | Gram |
| HRRT | High-Resolution Research Tomography |
| IRB | Institutional Review Board |
| JHMI | Johns Hopkins Medical Institutions |
| JHU | Johns Hopkins University |
| kCal | kilocalorie |
| MBqs | Megabequerels (MBqs |
| mCis | Millicuries (mCis) |
| mL | Milliliters |
| MRI | Magnetic resonance imaging |
| MRTM2 | Multilinear reference tissue method with 2 parameters |
| N | Number |
| NAcc | Nucleus accumbens |
| PET | Positron emission tomography |
| RYGB | Roux-en-Y gastric bypass |
| SD | Standard deviation |
| VSG | Vertical sleeve gastrectomy |
References
- Tellez, L.A.; Han, W.; Zhang, X.; Ferreira, T.; Perez, I.O.; Shammah-Lagnado, S.J.; Pol, A.N.V.D.; De Araujo, I.E. Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci. 2016, 19, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 2004, 43, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.H. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann. N. Y. Acad. Sci. 1989, 575, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, A.; Smith, G.P.; Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R31–R37. [Google Scholar] [CrossRef]
- Geiger, B.M.; Haburcak, M.; Avena, N.M.; Moyer, M.C.; Hoebel, B.G.; Pothos, E.N. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Van de Giessen, E.; la Fleur, S.E.; Eggels, L.; de Bruin, K.; van den Brink, W.; Booij, J. High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int. J. Obes. 2013, 37, 754–757. [Google Scholar] [CrossRef]
- Babbs, R.K.; Sun, X.; Felsted, J.; Chouinard-Decorte, F.; Veldhuizen, M.G.; Small, D.M. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav. 2013, 121, 103–111. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Blum, K.; Bohon, C. Weight gain is associated with reduced striatal response to palatable food. J. Neurosci. 2010, 30, 13105–13109. [Google Scholar] [CrossRef]
- Stice, E.; Burger, K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 2019, 68, 38–53. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.K.; Horstmann, A. Molecular Imaging of Central Dopamine in Obesity: A Qualitative Review across Substrates and Radiotracers. Brain Sci. 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.-C.; Ichise, M.; et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Van de Giessen, E.; Celik, F.; Schweitzer, D.H.; van den Brink, W.; Booij, J. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 2014, 28, 866–873. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Thanos, P.K.; Logan, J.; Alexoff, D.; Ding, Y.-S.; Wong, C.; Ma, Y.; et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage 2008, 42, 1537–1543. [Google Scholar] [CrossRef]
- De Weijer, B.A.; van de Giessen, E.; van Amelsvoort, T.A.; Boot, E.; Braak, B.; Janssen, I.M.; van de Laar, A.; Fliers, E.; Serlie, M.J.; Booij, J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011, 1, 37. [Google Scholar] [CrossRef]
- Steele, K.E.; Prokopowicz, G.P.; Schweitzer, M.A.; Magunsuon, T.H.; Lidor, A.O.; Kuwabawa, H.; Kumar, A.; Brasic, J.; Wong, D.F. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 2010, 20, 369–374. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Tuominen, L.; Tuulari, J.J.; Hirvonen, J.; Parkkola, R.; Helin, S.; Salminen, P.; Nuutila, P.; Nummenmaa, L. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J. Neurosci. 2015, 35, 3959–3965. [Google Scholar] [CrossRef]
- Eisenstein, S.A.; Antenor-Dorsey, J.A.V.; Gredysa, D.M.; Koller, J.M.; Bihun, E.C.; Ranck, S.A.; Arbeláez, A.M.; Klein, S.; Perlmutter, J.S.; Moerlein, S.M.; et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse 2013, 67, 748–756. [Google Scholar] [CrossRef]
- Guo, J.; Simmons, W.K.; Herscovitch, P.; Martin, A.; Hall, K.D. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol. Psychiatry 2014, 19, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Stefater, M.A.; Wilson-Pérez, H.E.; Chambers, A.P.; Sandoval, D.A.; Seeley, R.J. All bariatric surgeries are not created equal: Insights from mechanistic comparisons. Endocr. Rev. 2012, 33, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.P.; Cowan, R.L.; Volkow, N.D.; Feurer, I.D.; Li, R.; Williams, D.B.; Kessler, R.M.; Abumrad, N.N. Decreased dopamine type 2 receptor availability after bariatric surgery: Preliminary findings. Brain Res. 2010, 1350, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Jones-Gotman, M.; Dagher, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Geliebter, A.; Volkow, N.D.; Telang, F.W.; Logan, J.; Jayne, M.C.; Galanti, K.; Selig, P.A.; Han, H.; Zhu, W.; et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity 2011, 19, 1601–1608. [Google Scholar] [CrossRef]
- Haltia, L.T.; Rinne, J.O.; Merisaari, H.; Maguire, R.; Savontaus, E.; Helin, S.; Någren, K.; Kaasinen, V. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 2007, 61, 748–756. [Google Scholar] [CrossRef]
- Manza, P.; Shokri-Kojori, E.; Wiers, C.E.; Kroll, D.; Feldman, D.; McPherson, K.; Biesecker, E.; Dennis, E.; Johnson, A.; Kelleher, A.; et al. Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol. Psychiatry 2022, 27, 939–946. [Google Scholar] [CrossRef]
- Smith, K.R.; Papantoni, A.; Veldhuizen, M.G.; Kamath, V.; Harris, C.; Moran, T.H.; Carnell, S.; Steele, K.E. Taste-related reward is associated with weight loss following bariatric surgery. J. Clin. Investig. 2020, 130, 4370–4381. [Google Scholar] [CrossRef]
- Dugan, N.; Thompson, K.J.; Barbat, S.; Prasad, T.; McKillop, I.H.; Maloney, S.R.; Roberts, A.; Gersin, K.S.; Kuwada, T.S.; Nimeri, A. Male gender is an independent risk factor for patients undergoing laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass: An MBSAQIP® database analysis. Surg. Endosc. 2020, 34, 3574–3583. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Williams, L.; Jones, D.T.; Benarroch, E.E. Caudate nucleus as a component of networks controlling behavior. Neurology 2017, 89, 2192–2197. [Google Scholar] [CrossRef]
- White, M.A.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and validation of the food-craving inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Rahmim, A.; Cheng, J.-C.; Blinder, S.; Camborde, M.-L.; Sossi, V. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Phys. Med. Biol. 2005, 50, 4887–4912. [Google Scholar] [CrossRef] [PubMed]
- Ichise, M.; Liow, J.-S.; Lu, J.-Q.; Takano, A.; Model, K.; Toyama, H.; Suhara, T.; Suzuki, K.; Innis, R.B.; Carson, R.E. Linearized reference tissue parametric imaging methods: Application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003, 23, 1096–1112. [Google Scholar] [CrossRef]
- Seo, S.; Kim, S.J.; Kim, Y.K.; Lee, J.-Y.; Jeong, J.M.; Lee, D.S.; Lee, J.S. Comparative assessment of parametric neuroreceptor mapping approaches based on the simplified reference tissue model using [11C]ABP688 PET. J. Cereb. Blood Flow Metab. 2015, 35, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Oswald, L.M.; Wong, D.F.; McCaul, M.; Zhou, Y.; Kuwabara, H.; Choi, L.; Brasic, J.; Wand, G.S. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 2005, 30, 821–832. [Google Scholar] [CrossRef]
- Baumann, B.; Danos, P.; Krell, D.; Diekmann, S.; Leschinger, A.; Stauch, R.; Wurthmann, C.; Bernstein, H.-G.; Bogerts, B. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: Preliminary data from a postmortem study. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 71–78. [Google Scholar] [CrossRef]
- Martinez, D.; Slifstein, M.; Broft, A.; Mawlawi, O.; Hwang, D.-R.; Huang, Y.; Cooper, T.; Kegeles, L.; Zarahn, E.; Abi-Dargham, A.; et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003, 23, 285–300. [Google Scholar] [CrossRef]
- Mawlawi, O.; Martinez, D.; Slifstein, M.; Broft, A.; Chatterjee, R.; Hwang, D.-R.; Huang, Y.; Simpson, N.; Ngo, K.; Van Heertum, R.; et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001, 21, 1034–1057. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Tziortzi, A.C.; Haber, S.N.; Searle, G.E.; Tsoumpas, C.; Long, C.J.; Shotbolt, P.; Douaud, G.; Jbabdi, S.; Behrens, T.E.J.; Rabiner, E.A.; et al. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb. Cortex 2014, 24, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.; Beck, K.; Jauhar, S.; Howes, O.D. Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophr. Bull. 2018, 44, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Stenger, V.A.; Fiez, J.A. Motivation-dependent responses in the human caudate nucleus. Cereb. Cortex 2004, 14, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Joshi, A.; Schott, M.; la Fleur, S.E.; Barrot, M. Role of the striatal dopamine, GABA and opioid systems in mediating feeding and fat intake. Neurosci. Biobehav. Rev. 2022, 139, 104726. [Google Scholar] [CrossRef]
- Wong, D.F.; Brašić, J.R.; Singer, H.S.; Schretlen, D.J.; Kuwabara, H.; Zhou, Y.; Nandi, A.; Maris, M.A.; Alexander, M.; Ye, W.; et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 2008, 33, 1239–1251. [Google Scholar] [CrossRef]
- Earley, C.J.; Kuwabara, H.; Wong, D.F.; Gamaldo, C.; Salas, R.M.E.; Brašić, J.R.; Ravert, H.T.; Dannals, R.F.; Allen, R.P. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep 2013, 36, 51–57. [Google Scholar] [CrossRef]
- Oswald, L.M.; Wand, G.S.; Kuwabara, H.; Wong, D.F.; Zhu, S.; Brasic, J.R. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology 2014, 231, 2417–2433. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Wong, D.F.; Oswald, L.M.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W.; et al. Sex differences in striatal dopamine release in healthy adults. Biol. Psychiatry 2006, 59, 966–974. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Oswald, L.M.; Wong, D.F.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W.; et al. Striatal dopamine release and family history of alcoholism. Alcohol Clin. Exp. Res. 2006, 30, 1143–1151. [Google Scholar] [CrossRef]
- Thanarajah, S.E.; Backes, H.; DiFeliceantonio, A.G.; Albus, K.; Cremer, A.L.; Hanssen, R.; Lippert, R.N.; Cornely, O.A.; Small, D.M.; Brüning, J.C.; et al. Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metab. 2019, 29, 695–706.e4. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief; CDC National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8.
- Keski-Rahkonen, A. Epidemiology of binge eating disorder: Prevalence, course, comorbidity, and risk factors. Curr. Opin. Psychiatry 2021, 34, 525–531. [Google Scholar] [CrossRef] [PubMed]
- DiFeliceantonio, A.G.; Coppin, G.; Rigoux, L.; Edwin Thanarajah, S.; Dagher, A.; Tittgemeyer, M.; Small, D.M. Supra-Additive Effects of Combining Fat and Carbohydrate on Food Reward. Cell Metab. 2018, 28, 33–44.e3. [Google Scholar] [CrossRef]
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef]
- Brown, H.D.; McCutcheon, J.E.; Cone, J.J.; Ragozzino, M.E.; Roitman, M.F. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur. J. Neurosci. 2011, 34, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Kuwabara, H.; Schretlen, D.J.; Bonson, K.R.; Zhou, Y.; Nandi, A.; Brašić, J.R.; Kimes, A.S.; Maris, M.A.; Kumar, A.; et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006, 31, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Aghababian, A.; Papantoni, A.; Veldhuizen, M.G.; Kamath, V.; Harris, C.; Moran, T.H.; Carnell, S.; Steele, K.E. One Year Follow-Up of Taste-Related Reward Associations with Weight Loss Suggests a Critical Time to Mitigate Weight Regain Following Bariatric Surgery. Nutrients 2021, 13, 3943. [Google Scholar] [CrossRef]
- Alamuddin, N.; Vetter, M.L.; Ahima, R.S.; Hesson, L.; Ritter, S.; Minnick, A.; Faulconbridge, L.F.; Allison, K.C.; Sarwer, D.B.; Chittams, J.; et al. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: An 18-Month Prospective Study. Obes. Surg. 2017, 27, 1563–1572. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Veltman, D.J.; Kulve, J.S.T.; Groot, P.F.C.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.E.; King, W.C.; Chen, J.-Y.; Devlin, M.J.; Flum, D.; Garcia, L.; Inabet, W.; Pender, J.R.; Kalarchian, M.A.; Khandelwal, S.; et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity 2014, 22, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Newcorn, J.H.; Kollins, S.H.; Wigal, T.L.; Telang, F.; Fowler, J.S.; Goldstein, R.Z.; Klein, N.; Logan, J.; et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry 2011, 16, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Hietala, J.; Ruotsalainen, U.; Säkö, E.; Laihinen, A.; Någren, K.; Lehikoinen, P.; Oikonen, V.; Syvälahti, E. Decrease in human striatal dopamine D2 receptor density with age: A PET study with [11C]raclopride. J. Cereb. Blood Flow Metab. 1993, 13, 310–314. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking Food Reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef] [PubMed]
| Healthy-Weight (n = 5) | Severe Obesity (n = 6) | ||
|---|---|---|---|
| Pre-Surgery | Post-Surgery | ||
| Age | 24.0 ± 4.18 † | 43.5 ± 8.02 † | |
| Race (n Black; n White; n Asian) | 1; 1; 3 | 4; 2; 0 | |
| BMI | 21.8 ± 0.16 † | 41.8 ± 5.48 †,* | 34.78 ± 4.22 †,* |
| Food-Craving Inventory (FCI) | (n = 5) | (n = 5) | |
| - | 1.78 ± 0.67 | 1.23 ± 0.18 |
| - | 1.85 ± 0.54 | 1.44 ± 0.14 |
| - | 1.88 ± 0.60 * | 1.32 ± 0.33 * |
| - | 2.15 ± 0.76 | 2.10 ± 0.38 |
| Behavioral Activation Scales (BAS) | (n = 5) | (n = 5) | |
| - | 3.52 ± 0.33 | 3.48 ± 0.33 |
| - | 2.75 ± 0.53 * | 3.10 ± 0.38 * |
| - | 3.15 ± 0.22 | 3.10 ± 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnell, S.; Steele, K.E.; Thapaliya, G.; Kuwubara, H.; Aghababian, A.; Papantoni, A.; Nandi, A.; Brašić, J.R.; Moran, T.H.; Wong, D.F. Milkshake Acutely Stimulates Dopamine Release in Ventral and Dorsal Striatum in Healthy-Weight Individuals and Patients with Severe Obesity Undergoing Bariatric Surgery: A Pilot Study. Nutrients 2023, 15, 2671. https://doi.org/10.3390/nu15122671
Carnell S, Steele KE, Thapaliya G, Kuwubara H, Aghababian A, Papantoni A, Nandi A, Brašić JR, Moran TH, Wong DF. Milkshake Acutely Stimulates Dopamine Release in Ventral and Dorsal Striatum in Healthy-Weight Individuals and Patients with Severe Obesity Undergoing Bariatric Surgery: A Pilot Study. Nutrients. 2023; 15(12):2671. https://doi.org/10.3390/nu15122671
Chicago/Turabian StyleCarnell, Susan, Kimberley E. Steele, Gita Thapaliya, Hiroto Kuwubara, Anahys Aghababian, Afroditi Papantoni, Ayon Nandi, James R. Brašić, Timothy H. Moran, and Dean F. Wong. 2023. "Milkshake Acutely Stimulates Dopamine Release in Ventral and Dorsal Striatum in Healthy-Weight Individuals and Patients with Severe Obesity Undergoing Bariatric Surgery: A Pilot Study" Nutrients 15, no. 12: 2671. https://doi.org/10.3390/nu15122671
APA StyleCarnell, S., Steele, K. E., Thapaliya, G., Kuwubara, H., Aghababian, A., Papantoni, A., Nandi, A., Brašić, J. R., Moran, T. H., & Wong, D. F. (2023). Milkshake Acutely Stimulates Dopamine Release in Ventral and Dorsal Striatum in Healthy-Weight Individuals and Patients with Severe Obesity Undergoing Bariatric Surgery: A Pilot Study. Nutrients, 15(12), 2671. https://doi.org/10.3390/nu15122671






