Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion
Abstract
1. Introduction
2. Materials and Methods
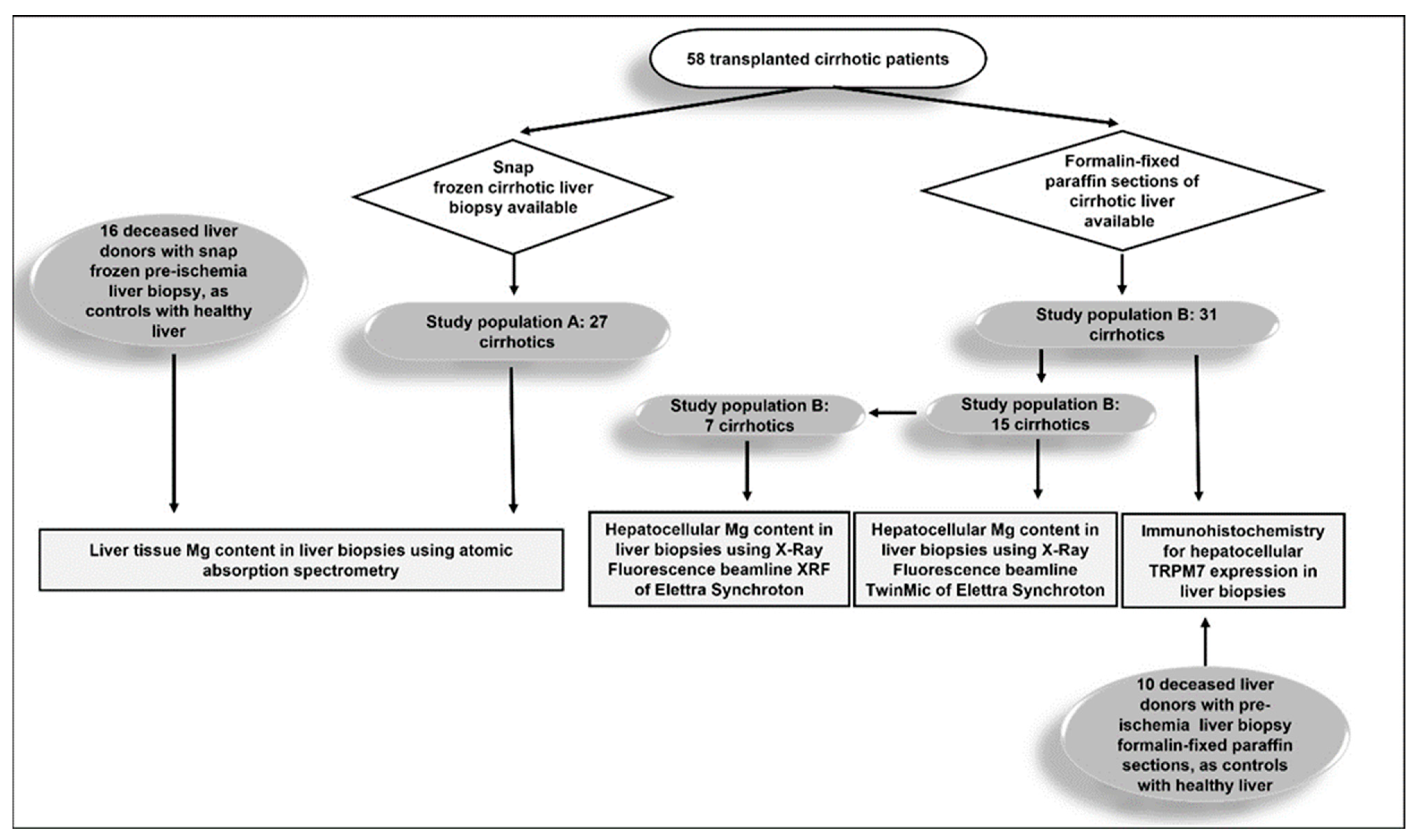
2.1. Study Design
2.2. Study Population and Data Collection
2.3. Magnesium Quantification in Liver Tissue Using Atomic Absorption Spectrometry
2.4. Determination of Magnesium in Hepatocytes by Synchrotron Based X-ray Fluorescence Microscopy
2.5. Immunohistochemistry Analysis for TRPM7 in Hepatocytes
2.6. Statistical Analysis
3. Results
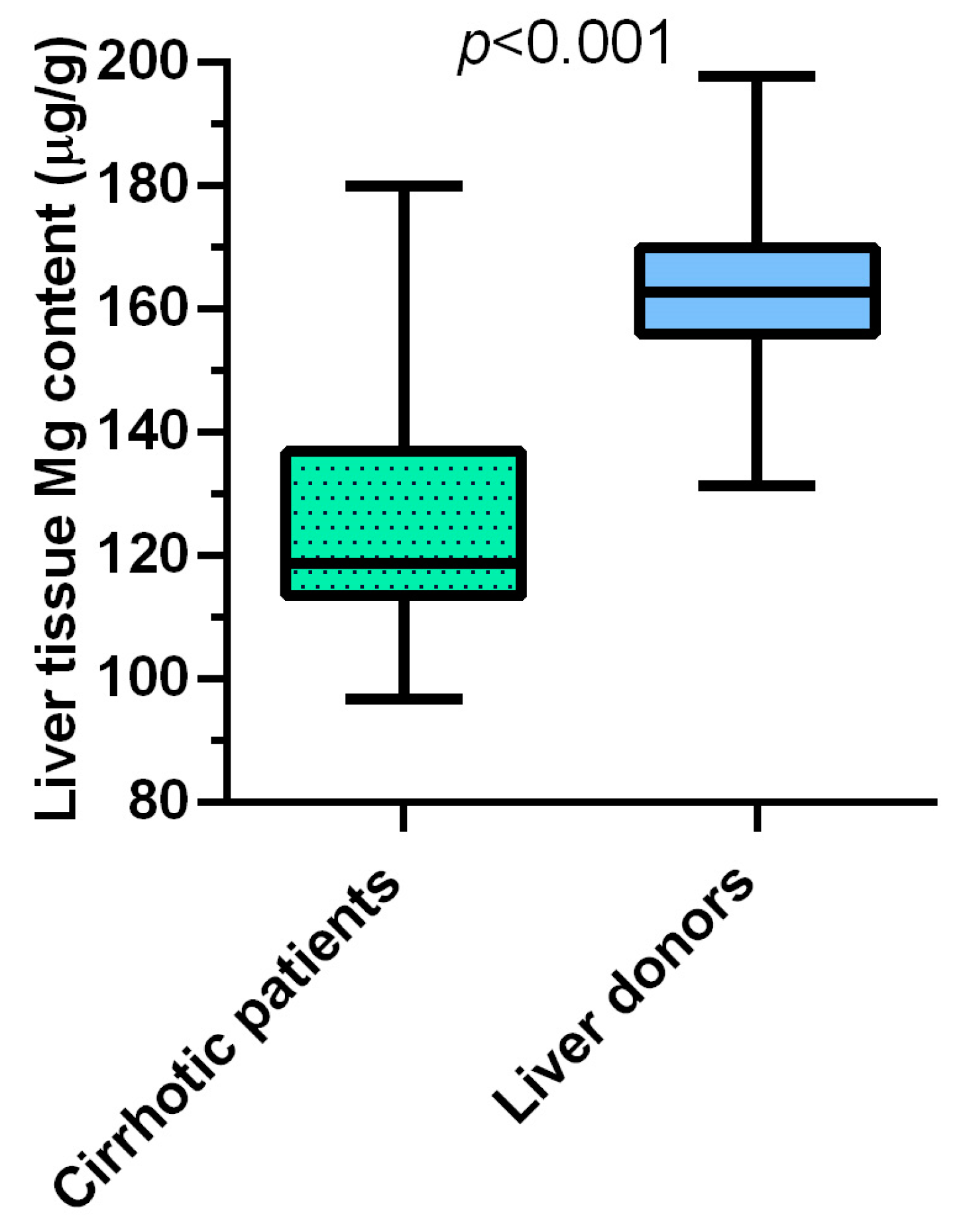
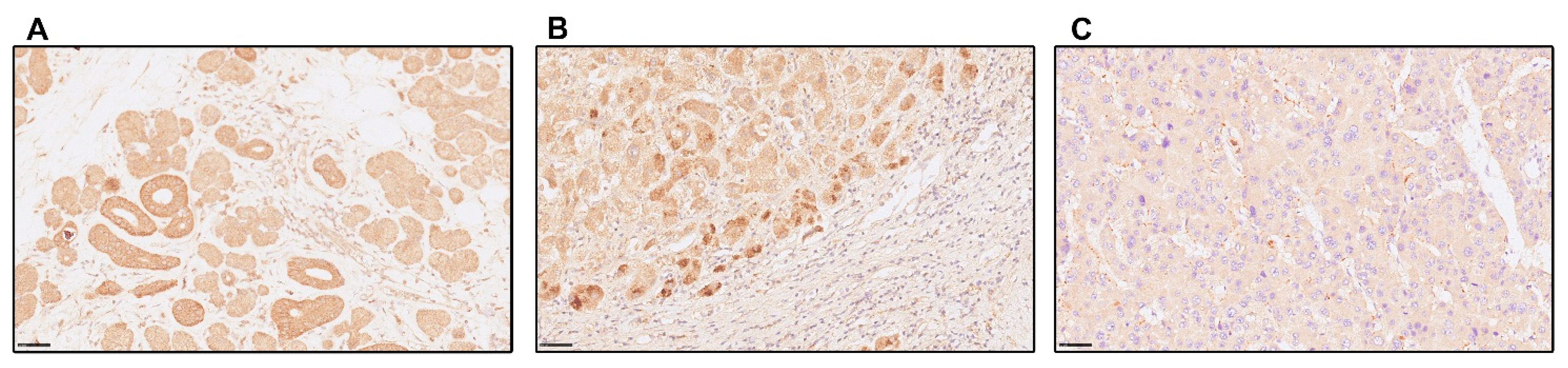
3.1. In Liver Cirrhosis, Hepatic Magnesium Content Is Lower, and TRPM7 Expression in Hepatocytes Is Higher Than in Healthy Liver
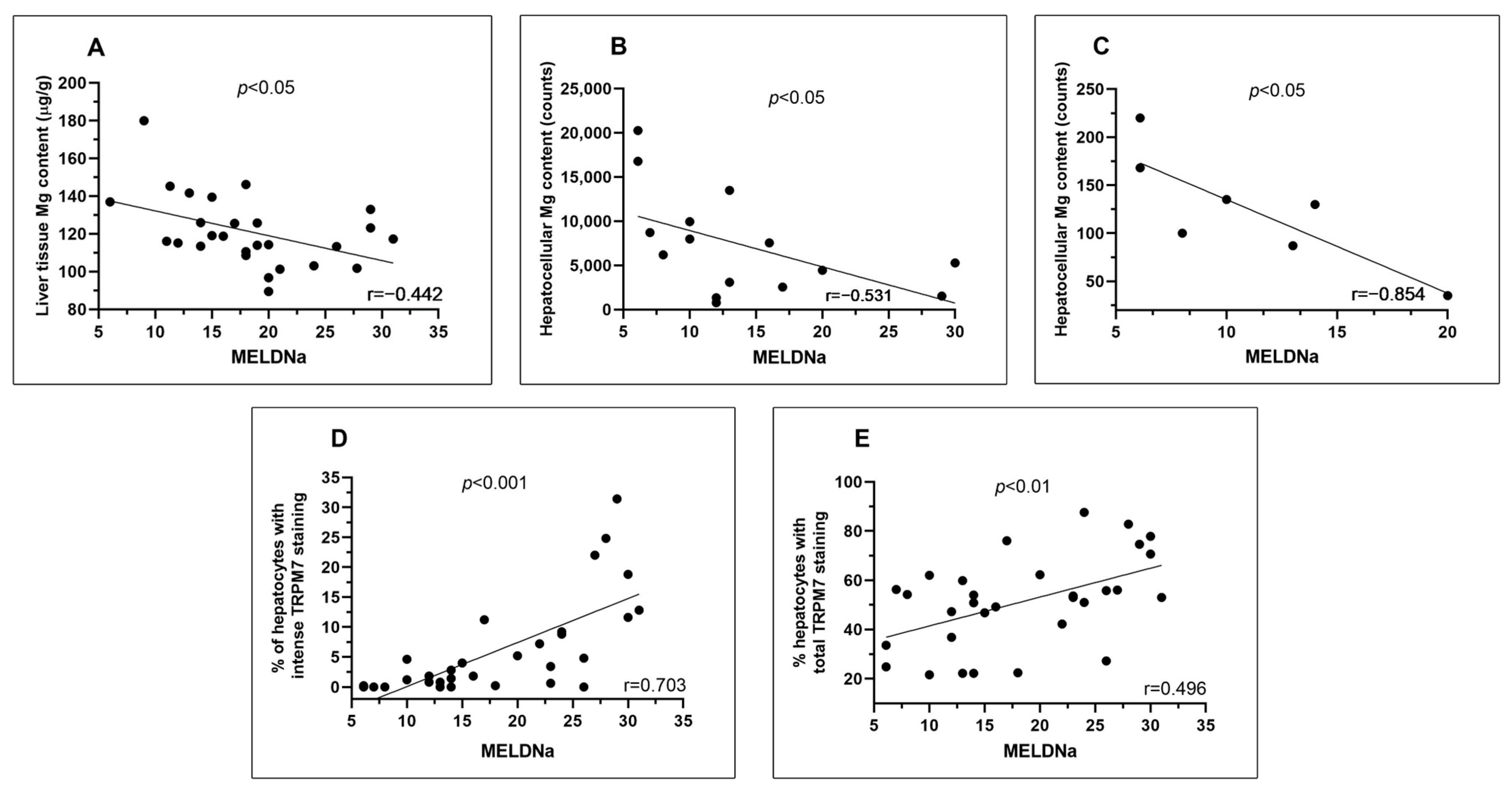
3.2. In Liver Cirrhosis, MELDNa at the Time of Liver Transplantation Is Inversely Correlated with the Content of Magnesium in Liver Tissue and Hepatocytes and Directly with the Hepatocellular Expression of TRPM7
3.3. In Liver Cirrhosis, Serum AST Activity at the Time of Liver Transplantation Is Inversely Correlated with Magnesium Content in Liver Tissue and Hepatocytes and Directly with Hepatocellular Expression of TRPM7
3.4. In Liver Cirrhosis, the Worsening of MELDNa during the Waitlist Time Is Inversely Correlated with the Content of Magnesium in Biopsies of Liver Tissue and Directly with the Hepatocellular Expression of TRPM7 at the Time of Liver Transplantation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Renal. Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef]
- Rubin, H. Magnesium: The missing element in molecular views of cell proliferation control. BioEssays 2005, 27, 311–320. [Google Scholar] [CrossRef]
- Kubota, T.; Shindo, Y.; Tokuno, K.; Komatsu, H.; Ogawa, H.; Kudo, S.; Kitamura, Y.; Suzuki, K.; Oka, K. Mitochondria are intracellular magnesium stores: Investigation by simultaneous fluorescent imagings in PC12 cells. Biochim. Biophys. Acta 2005, 1744, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Frassineti, C.; Sabatini, A.; Vacca, A.; Barbiroli, B. Quantitative mathematical expressions for accurate in vivo assessment of cytosolic [ADP] and DeltaG of ATP hydrolysis in the human brain and skeletal muscle. Biochim. Biophys. Acta 2005, 1708, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Castiglioni, S.; Olivi, E.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone, C.; Merolle, L.; Malucelli, E.; Ventura, C.; et al. Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling. Int. J. Mol. Sci. 2018, 19, 1410. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes-a 2019 update. Nucleic Acids Res. 2020, 48, D445–53. [Google Scholar] [CrossRef]
- Whang, R.; Ryder, K.W. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA 1990, 263, 3063–3064. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; González, E.A.; Slatopolsky, E. Clinical Consequences and Management of Hypomagnesemia. J. Am. Soc. Nephrol. 2009, 20, 2291–2295. [Google Scholar] [CrossRef]
- Van Laecke, S. Hypomagnesemia and hypermagnesemia. Acta Clin. Belg. 2019, 74, 41–47. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R. ‘Magnesium’-the master cation-as a drug—Possibilities and evidences. BioMetals 2021, 34, 955–986. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and liver disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.H.; Fulda, K.G. Association of Magnesium Intake with Liver Fibrosis among Adults in the United States. Nutrients 2021, 13, 142. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, X.; Fan, L.; Kabagambe, E.K.; Song, Y.; Tao, M.; Zhong, X.; Hou, L.; Shrubsole, M.J.; Liu, J.; et al. Magnesium intake and mortality due to liver diseases: Results from the Third National Health and Nutrition Examination Survey Cohort. Sci. Rep. 2017, 7, 17913. [Google Scholar] [CrossRef]
- Lu, L.; Chen, C.; Li, Y.; Guo, W.; Zhang, S.; Brockman, J.; Shikany, J.M.; Kahe, K. Magnesium intake is inversely associated with risk of non-alcoholic fatty liver disease among American adults. Eur. J. Nutr. 2022, 61, 1245–1254. [Google Scholar] [CrossRef]
- Rayssiguier, Y.; Chevalier, F.; Bonnet, M.; Kopp, J.; Durlach, J. Influence of Magnesium Deficiency on Liver Collagen after Carbon Tetrachloride or Ethanol Administration to Rats. J. Nutr. 1985, 115, 1656–1662. [Google Scholar] [CrossRef]
- Fengler, V.H.; Macheiner, T.; Goessler, W.; Ratzer, M.; Haybaeck, J.; Sargsyan, K. Hepatic Response of Magnesium-Restricted Wild Type Mice. Metabolites 2021, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Scarpa, A. Mg2+ Control of Respiration in Isolated Rat Liver Mitochondria. Biochemistry 1996, 35, 12849–12856. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Dolva, L.O.; Soyland, E.; Manger, A.T.; Falch, D.; Kjekshus, J. Oral Magnesium Supplementation Improves Metabolic Variables and Muscle Strength in Alcoholics. Alcohol. Clin. Exp. Res. 1992, 16, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Poikolainen, K.; Alho, H. Magnesium treatment in alcoholics: A randomized clinical trial. Subst. Abuse Treat Prev. Policy 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, H.; Cervantes-Huerta, M.; Rodriguez-Moran, M.; Guerrero-Romero, F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnes Res. 2010, 23, 90–96. [Google Scholar]
- Wang, Y.; Wang, Z.; Gao, M.; Zhong, H.; Chen, C.; Yao, Y.; Zhang, Z.; Zhang, X.; Li, F.; Zhang, J.; et al. Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: A phase II trial. Liver Int. 2019, 39, 2102–2111. [Google Scholar] [CrossRef]
- Markiewicz-Górka, I.; Zawadzki, M.; Januszewska, L.; Hombek-Urban, K.; Pawlas, K. Influence of selenium and/or magnesium on alleviation alcohol induced oxidative stress in rats, normalization function of liver and changes in serum lipid parameters. Hum. Exp. Toxicol. 2011, 30, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shi, H.; Sun, Z.; Wu, J.; Xia, Y.; Wang, Y.; Wu, Y.; Li, X.; Chen, W.; Wang, A.; et al. Protective Effects of Magnesium Glycyrrhizinate on Methotrexate-Induced Hepatotoxicity and Intestinal Toxicity May Be by Reducing COX-2. Front. Pharmacol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Shafeeq, S.; Mahboob, T. Magnesium supplementation ameliorates toxic effects of 2,4-dichlorophenoxyacetic acid in rat model. Hum. Exp. Toxicol. 2020, 39, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, P.H.; Zhou, X.L.; Zhang, D.L.; Gu, Q.; Zhang, S.J.; Zhang, J.; Zhang, J.S.; Qian, Z.Y. Effect of magnesium gluconate administration on lipid metabolism, antioxidative status, and related gene expression in rats fed a high-fat diet. Magnes Res. 2018, 31, 117–130. [Google Scholar]
- Paik, Y.-H.; Yoon, Y.J.; Lee, H.C.; Kil Jung, M.; Kang, S.H.; Chung, S.I.; Kim, J.K.; Cho, J.Y.; Lee, K.S.; Han, K.-H. Antifibrotic effects of magnesium lithospermate B on hepatic stellate cells and thioacetamide-induced cirrhotic rats. Exp. Mol. Med. 2011, 43, 341–349. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, W.H.; Sabry, D.; Abd Al Haleem, E.N. Comparative study of antifibrotic activity of some magnesium-containing supplements on experimental liver toxicity. Molecular study. Drug Chem. Toxicol. 2017, 40, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Chen, X.; Zhang, C.; Jin, H.; Wang, F.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Magnesium isoglycyrrhizinate promotes the activated hepatic stellate cells apoptosis via endoplasmic reticulum stress and ameliorates fibrogenesis in vitro and in vivo. BioFactors 2017, 43, 836–846. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wang, F.; Cai, Z.; Liu, S.; Xi, J.; He, R.; Zhao, Y.; Zhuang, R. Protective effect of apigenin magnesium complex on H2O2-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm. Biol. 2020, 58, 553–560. [Google Scholar] [CrossRef]
- Rocchi, E.; Borella, P.; Borghi, A.; Paolillo, F.; Pradelli, M.; Farina, F.; Casalgrandi, G. Zinc and magnesium in liver cirrhosis. Eur. J. Clin. Investig. 1994, 24, 149–155. [Google Scholar] [CrossRef]
- Koivisto, M.; Valta, P.; Höckerstedt, K.; Lindgren, L. Magnesium depletion in chronic terminal liver cirrhosis. Clin. Transplant. 2002, 16, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Kar, K.; Dasgupta, A.; Vijaya Bhaskar, M.; Sudhakar, K. Alteration of Micronutrient Status in Compensated and Decompensated Liver Cirrhosis. Indian J. Clin. Biochem. 2014, 29, 232–237. [Google Scholar] [CrossRef]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of Trace Elements in Liver Cirrhosis Patients and Their Role in Prognosis of Disease. Biol. Trace Elem. Res. 2015, 165, 35–40. [Google Scholar] [CrossRef]
- Cohen-Hagai, K.; Feldman, D.; Turani-Feldman, T.; Hadary, R.; Lotan, S.; Kitay-Cohen, Y. Magnesium Deficiency and Minimal Hepatic Encephalopathy among Patients with Compensated Liver Cirrhosis. Isr. Med. Assoc. J. 2018, 20, 533–538. [Google Scholar]
- Peng, X.; Xiang, R.; Li, X.; Tian, H.; Li, C.; Peng, Z.; Xiang, M. Magnesium deficiency in liver cirrhosis: A retrospective study. Scand. J. Gastroenterol. 2021, 56, 463–468. [Google Scholar] [CrossRef]
- Chacko, R.T.; Chacko, A. Serum & muscle magnesium in Indians with cirrhosis of liver. Indian J. Med. Res. 1997, 106, 469–474. [Google Scholar] [PubMed]
- Rahelić, D.; Kujundzić, M.; Romić, Z.; Brkić, K.; Petrovecki, M. Serum concentration of zinc, copper, manganese and magnesium in patients with liver cirrhosis. Coll. Antropol. 2006, 30, 523–528. [Google Scholar] [PubMed]
- Chaudhry, A.; Toori, K.U.; Shaikh, J.I. To determine correlation between biochemical parameters of nutritional status with disease severity in HCV related liver cirrhosis. Pak. J. Med. Sci. 2018, 34, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Llibre-Nieto, G.; Lira, A.; Vergara, M.; Solé, C.; Casas, M.; Puig-Diví, V.; Solé, G.; Humanes, A.; Grau, L.; Barradas, J.M.; et al. Micronutrient Deficiencies in Patients with Decompensated Liver Cirrhosis. Nutrients 2021, 13, 1249. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, N.K.; Andersen, H.; Vilstrup, H.; Clausen, T.; Jakobsen, J.; Dorup, I. Muscle strength, Na,K-pumps, magnesium and potassium in patients with alcoholic liver cirrhosis-relation to spironolactone. J. Intern. Med. 2002, 252, 56–63. [Google Scholar] [CrossRef]
- Göksu, N.; Özsoylu, Ş. Hepatic and Serum Levels of Zinc, Copper, and Magnesium in Childhood Cirrhosis. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 459–462. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef]
- Cai, N.; Lou, L.; Al-Saadi, N.; Tetteh, S.; Runnels, L.W. The kinase activity of the channel-kinase protein TRPM7 regulates stability and localization of the TRPM7 channel in polarized epithelial cells. J. Biol. Chem. 2018, 293, 11491–11504. [Google Scholar] [CrossRef]
- Shi, R.; Fu, Y.; Zhao, D.; Boczek, T.; Wang, W.; Guo, F. Cell death modulation by transient receptor potential melastatin channels TRPM2 and TRPM7 and their underlying molecular mechanisms. Biochem. Pharmacol. 2021, 190, 114664. [Google Scholar] [CrossRef]
- Fang, L.; Zhan, S.; Huang, C.; Cheng, X.; Lv, X.; Si, H.; Li, J. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol. Appl. Pharmacol. 2013, 272, 713–725. [Google Scholar] [CrossRef]
- Cai, S.; Wu, L.; Yuan, S.; Liu, G.; Wang, Y.; Fang, L.; Xu, D. Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur. J. Pharmacol. 2021, 898, 173982. [Google Scholar] [CrossRef]
- Ogunrinde, A.; Pereira, R.D.; Beaton, N.; Lam, D.H.; Whetstone, C.; Hill, C.E. Hepatocellular differentiation status is characterized by distinct subnuclear localization and form of the chanzyme TRPM7. Differentiation 2017, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Kozai, D.; Sakaguchi, R.; Numata, T.; Mori, Y. Different Contribution of Redox-Sensitive Transient Receptor Potential Channels to Acetaminophen-Induced Death of Human Hepatoma Cell Line. Front. Pharmacol. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Biggins, S.W.; Kremers, W.K.; Wiesner, R.H.; Kamath, P.S.; Benson, J.T.; Edwards, E.; Therneau, T.M. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N. Engl. J. Med. 2008, 359, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Kaulich, B.; Alberti, R.; Klatka, T.; Longoni, A.; de Marco, A.; Marcello, A.; Kiskinova, M. Simultaneous soft X-ray transmission and emission microscopy. Nucl. Instrum. Methods Phys. Res. A 2009, 608, 195–198. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Kourousias, G.; Merolle, L.; Altissimo, M.; Bianco, A. Current status of the TwinMic beamline at Elettra: A soft X-ray transmission and emission microscopy station. J. Synchrotron. Radiat. 2016, 23, 1526–1537. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Kourousias, G.; Stolfa, A.; Kaulich, B. Recent developments at the TwinMic beamline at ELETTRA: An 8 SDD detector setup for low energy X-ray Fluorescence. J. Phys. Conf. Ser. 2013, 425, 182001. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Morrison, G.R.; Kaulich, B.; Bacescu, D.; Kovac, J. Scanning transmission x-ray microscopy with a configurable detector. Appl. Phys. Lett. 2006, 89, 251117. [Google Scholar] [CrossRef]
- Karydas, A.G.; Czyzycki, M.; Leani, J.J.; Migliori, A.; Osan, J.; Bogovac, M.; Wrobel, P.; Vakula, N.; Padilla-Alvarez, R.; Menk, R.H.; et al. An IAEA multi-technique X-ray spectrometry endstation at Elettra Sincrotrone Trieste: Benchmarking results and interdisciplinary applications. J. Synchrotron. Radiat. 2018, 25, 189–203. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Petersson, S.; Sehlstedt, K. Variable Selection Techniques for the Cox Proportional Hazards Model: A Comparative Study. Master’s Thesis, University of Gothenburg, Gothenburg, Sweden, 2018. [Google Scholar]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Alexander, R.T.; Dimke, H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am. J. Physiol.-Ren. Physiol. 2017, 312, F998–F1015. [Google Scholar] [CrossRef]
- Parisse, S.; Ferri, F.; Persichetti, M.; Mischitelli, M.; Abbatecola, A.; Di Martino, M.; Lai, Q.; Carnevale, S.; Lucatelli, P.; Bezzi, M.; et al. Low serum magnesium concentration is associated with the presence of viable hepatocellular carcinoma tissue in cirrhotic patients. Sci. Rep. 2021, 11, 15184. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Janjetovic, K.; Velimirovic, M.; Milenkovic, M.; Stojkovic, T.; Puskas, N.; Zaletel, I.; De Luka, S.R.; Jankovic, S.; Stefanovic, S.; et al. Effects of IL-33/ST2 pathway in acute inflammation on tissue damage, antioxidative parameters, magnesium concentration and cytokines profile. Exp. Mol. Pathol. 2016, 101, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Locatelli, L.; Maier, J.A. Burning magnesium, a sparkle in acute inflammation: Gleams from experimental models. Magnes Res. 2017, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- González-Recio, I.; Simón, J.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Mercado-Gómez, M.; Rodríguez-Agudo, R.; Lachiondo-Ortega, S.; Gil-Pitarch, C.; Fernández-Rodríguez, C.; Castellana, D.; et al. Restoring cellular magnesium balance through Cyclin M4 protects against acetaminophen-induced liver damage. Nat. Commun. 2022, 13, 6816. [Google Scholar] [CrossRef] [PubMed]


| Liver Donors (Controls of Study Population A) | Cirrhotic Patients of Study Population A | Liver Donors (Controls of Study Population B) | Cirrhotic Patients of Study Population B | p Value Liver Donors (Controls of Study Population A) vs. Cirrhotic Patients Study Population A | p Value Liver Donors (Controls of Study Population B) vs. Cirrhotic Patients Study Population B | p Value Cirrhotic Patients Study Population A vs. Cirrhotic Patients Study Population B | |
|---|---|---|---|---|---|---|---|
| 16 | 27 | 10 | 31 | ||||
| Age, median (IQR) | 53 (35–61.5) | 52.0 (48.0–60.0) | 62,5 (36.0–75.8) | 57.3 (52.3–63.2) | 0.890 | 0.984 | 0.050 |
| Sex, male, n (%) | 11 (68.8) | 22 (81.5) | 6 (60.0) | 27 (87.1) | 0.460 | 0.082 | 0.720 |
| BMI (Kg/m2), median (IQR) | 25.6 (24.8–27.3) | 24.3 (22.6–27.4) | 25.5 (24.2–27.6) | 25.9 (24.4–29.0) | 0.880 | 0.869 | 0.210 |
| Cirrhosis aetiology, n (%): | NA | NA | - | - | |||
| MAFLD | 16 (59.3) | 17 (54.8) | 0.735 | ||||
| Alcohol | 10 (37) | 16 (51.6) | 0.266 | ||||
| Viral | 19 (70.4) | 12 (38.7) | 0.016 | ||||
| HCC, yes (%) | 0 (0) | 13 (48.1) | 0 (0) | 17 (54.8) | <0.001 | <0.001 | 0.820 |
| Diuretic treatment, n (%): | NAV | NAV | - | - | 0.938 | ||
| None | 8 (29.6) | 11 (35.5) | |||||
| K sparing diuretics only | 3 (11.1) | 4 (12.9) | |||||
| Loop diuretics plus K sparing diuretics | 16 (59.3) | 16 (51.6) | |||||
| Serum AST (IU/L), median (IQR) | 35.5 (26.3–47.3) | 63.0 (40.0–98.0) | 52.5 (35.0–237.5) | 59.0 (33.0–100.0) | 0.873 | 0.665 | 0.925 |
| Serum ALT (IU/L), median, (IQR) | 23 (18–30.5) | 47.0 (30.0–60.0) | 67.0 (20.0–102.3) | 43.0 (25.0–70.0) | 0.792 | 0.665 | 0.911 |
| MELDNa, at the time of liver transplant, median (IQR) | NA | 18.0 (14.0–21.0) | NA | 17.0 (12.0–26.0) | NA | NA | 0.938 |
| MELDNa, at the time of listing, median (IQR) | NA | 15.7 (11.5–18.1) | NA | 16.5 (12.0–23.4) | NA | NA | 0.803 |
| Days on the waiting list before liver transplant, median (IQR) | NA | 128.0 (49.0–390.0) | NA | 125.0 (50.0–186.0) | NA | NA | 0.294 |
| Δ-MELDNa, median (IQR) | NA | 0.18 (0.00–1.28) | NA | 0.92 (−0.31–3.43) | NA | NA | 0.692 |
| Indipendent Variable | B | 95% C.I. for B | p Value | ||
|---|---|---|---|---|---|
| Study population A | Model 1 (Mg liver content measured by atomic absorption) * | Liver Mg content (ug/g) | −0.102 | −0.207–0.005 | 0.048 |
| HCC, yes | −6.757 | −10.785–−2.571 | 0.008 | ||
| Study population B | Model 2 (percentage of hepatocytes with intense TRPM7 expression) ** | Percentage of hepatocytes with intense TRPM7 expression | 0.297 | 0.175–0.578 | 0.005 |
| HCC, yes | −10.921 | −13.683–−8.046 | 0.001 | ||
| Model 3 (percentage of hepatocytes with total, i.e., weak and intense, TRPM7 expression) *** | Percentage of hepatocytes with total TRPM7 expression | 0.101 | 0.023–0.179 | 0.021 | |
| HCC, yes | −12.413 | −15.274–−9.923 | 0.001 | ||
| Independent Variable | B | 95% C.I. for B | p Value | ||
|---|---|---|---|---|---|
| Study population A | Model 1 (Mg liver content measured by atomic absorption) * | Liver Mg content (ug/g) | −0.824 | −1.560–−0.288 | 0.007 |
| Age, years | −0.946 | −2.209–0.763 | 0.155 | ||
| Study population B | Model 2 (percentage of hepatocytes with intense TRPM7 expression) ** | Percentage of hepatocytes with intense TRPM7 expression | 3.256 | 0.758–6.208 | 0.040 |
| Age, years | −2.787 | −5.162–−0.501 | 0.029 | ||
| Model 3 (percentage of hepatocytes with total, i.e., weak and intense, TRPM7 expression) *** | Percentage of hepatocytes with total TRPM7 expression | 0.884 | −0.078–1.958 | 0.114 | |
| HCC, yes | −33.783 | −65.886–−8.639 | 0.041 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisse, S.; Gianoncelli, A.; Isani, G.; Gambaro, F.L.; Andreani, G.; Malucelli, E.; Aquilanti, G.; Carlomagno, I.; Carletti, R.; Mischitelli, M.; et al. Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion. Nutrients 2023, 15, 2626. https://doi.org/10.3390/nu15112626
Parisse S, Gianoncelli A, Isani G, Gambaro FL, Andreani G, Malucelli E, Aquilanti G, Carlomagno I, Carletti R, Mischitelli M, et al. Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion. Nutrients. 2023; 15(11):2626. https://doi.org/10.3390/nu15112626
Chicago/Turabian StyleParisse, Simona, Alessandra Gianoncelli, Gloria Isani, Francesco Luigi Gambaro, Giulia Andreani, Emil Malucelli, Giuliana Aquilanti, Ilaria Carlomagno, Raffaella Carletti, Monica Mischitelli, and et al. 2023. "Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion" Nutrients 15, no. 11: 2626. https://doi.org/10.3390/nu15112626
APA StyleParisse, S., Gianoncelli, A., Isani, G., Gambaro, F. L., Andreani, G., Malucelli, E., Aquilanti, G., Carlomagno, I., Carletti, R., Mischitelli, M., Ferri, F., Paterna, V., Lai, Q., Mennini, G., Melandro, F., Di Gioia, C., Rossi, M., Iotti, S., Fratini, M., & Ginanni Corradini, S. (2023). Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion. Nutrients, 15(11), 2626. https://doi.org/10.3390/nu15112626







