Abstract
Royal jelly (RJ) is a naturally occurring substance synthesized by honeybees and has various health benefits. Herein, we focused on the medium-chain fatty acids (MCFAs) unique to RJ and evaluated their therapeutic efficacy in treating non-alcoholic fatty liver disease (NAFLD). We examined db/m mice that were exclusively fed a normal diet, db/db mice exclusively fed a normal diet, and db/db mice fed varying RJ quantities (0.2, 1, and 5%). RJ improved NAFLD activity scores and decreased gene expression related to fatty acid metabolism, fibrosis, and inflammation in the liver. RJ regulated innate immunity-related inflammatory responses in the small intestine and decreased the expression of genes associated with inflammation and nutrient absorption transporters. RJ increased the number of operational taxonomic units, the abundance of Bacteroides, and seven taxa, including bacteria that produce short-chain fatty acids. RJ increased the concentrations of RJ-related MCFAs (10-hidroxy-2-decenoic acid, 10-hydroxydecanoic acid, 2-decenedioic acid, and sebacic acid) in the serum and liver. These RJ-related MCFAs decreased saturated fatty acid deposition in HepG2 cells and decreased the gene expression associated with fibrosis and fatty acid metabolism. RJ and RJ-related MCFAs improved dysbiosis and regulated the expression of inflammation-, fibrosis-, and nutrient absorption transporter-related genes, thereby preventing NAFLD.
1. Introduction
The occurrence of non-alcoholic fatty liver disease (NAFLD) has been progressively increasing, particularly in developed nations, parallel to the rising prevalence of obesity and diabetes [1,2]. Overeating and a sedentary lifestyle lead to the onset of NAFLD; thus, metabolic diseases, such as obesity, diabetes, and dyslipidemia, often coexist with NAFLD in many patients [3,4]. Patients with NAFLD have a higher mortality rate compared to those without NAFLD [5]; thus, the prevention of NAFLD is an issue of utmost importance.
Dysbiosis, referring to the imbalance or disruption of the gut microbiota, is closely related to the incidence of NAFLD [6]. This condition is characterized by a reduction or loss of some beneficial commensal microorganisms and an increase in potentially pathogenic commensal microorganisms and can arise from various causes, such as dietary choices, food additives, antibiotics, and lifestyle [7]. Furthermore, dysbiosis is associated with the dysfunction of the intestinal barrier and hepatic inflammation due to infiltration of the liver by microbial or food antigens via the portal vein [6].
Royal jelly (RJ) is a naturally occurring substance synthesized by honeybees and fed to both the queen bee and young worker larvae [8,9]. RJ contains three major nutrients, namely proteins (including abundant essential amino acids), carbohydrates, and fats, and is a source of various vitamins and minerals. RJ plays a vital role in promoting the optimal growth of queen honeybees and is closely linked to their reproductive capabilities and lifespan. Research has provided insights into the fact that RJ is a functional food that possesses diverse properties beneficial for human health. For instance, RJ has been reported to have various effects on metabolic diseases such as glucose intolerance, lipid disorders, and hypertension [10,11,12,13]. Notably, RJ and major RJ proteins have been reported to improve NAFLD [14,15,16]. Four main types of medium-chain fatty acids (MCFAs) have been identified in RJ: 10-hydroxy-2-decenoic acid (10 H2DA), 10-hydroxydecanoic acid (10 HDAA), 2-decenedioic acid (2-DA), and sebacic acid (SA). 10 HDAA and SA are metabolites of 10 H2DA and 2-DA, respectively [17]. However, the anti-NAFLD effects of these MCFAs are still unclear.
This study focused on the MCFAs present in RJ and dysbiosis. We attempted to elucidate the mechanism by which RJ improves NAFLD in db/db mice with severe NAFLD by measuring the tissue concentrations of RJ-related MCFAs and evaluating intestinal tissues, small intestinal inflammation, gene expression in the intestine, and gut microbiota dysbiosis.
2. Materials and Methods
2.1. Mice
We purchased 7-week-old male diabetic homozygous db/db mice and non-diabetic heterozygous db/m mice from Shimizu Laboratory Supplies (Kyoto, Japan). We used db/db mice because they exhibit overeating, obesity, hyperglycemia, and NAFLD owing to leptin receptor abnormalities. Female mice have altered innate immunity as a result of their sex hormone cycle. Male mice, on the other hand, have less altered innate immunity due to the sex hormone cycle, so we employed male mice [18]. Therefore, we used male mice in our study. The RJ powder used in this study was derived from RJ treated with alkaline proteases to eliminate allergens and standardized to contain 3.5% 10 H2DA and 0.6% 10 HDAA (lot: YRP-M-210728-1; Yamada Bee Company, Inc., Okayama, Japan) [19]. Mice (8-week-old) were fed either a normal diet (ND; 344.9 kcal/100 g, fat kcal 4.6%; Oriental Yeast Japan, Tokyo, Japan) or ND supplemented with RJ (0.2, 1, or 5% w/w) for 8 weeks. The mice were categorized into five groups (n = 6 each): (1) db/m mice fed a diet without RJ (db/m), (2) db/db mice fed a diet without RJ (db/db), (3) db/db mice fed an ND supplemented with 0.2% RJ (db/db + 0.2% RJ), (4) db/db mice fed an ND supplemented with 1% RJ (db/db + 1% RJ), and (5) db/db mice fed an ND supplemented with 5% RJ (db/db + 5% RJ) (Figure 1A). We supplied equal amounts of feed to both db/m and db/db mice and performed paired feeding. After an overnight fasting period, euthanasia was performed on all mice at 16 weeks of age using a combination of anesthetics, including 5.0 mg/kg of butorphanol, 0.3 mg/kg of medetomidine, and 4.0 mg/kg of midazolam. All animal experiments were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine, Kyoto, Japan (approval number: M2020-48).
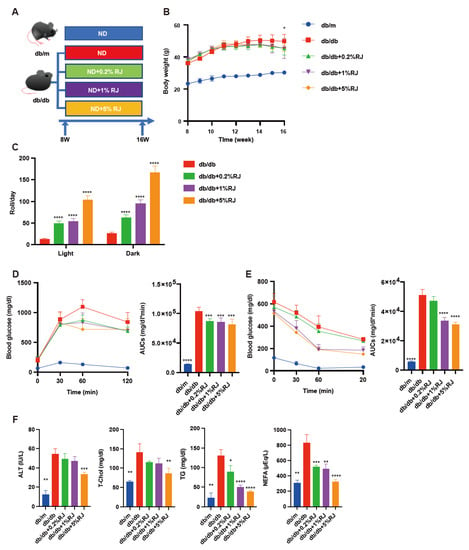
Figure 1.
RJ-fed db/db mice showed higher locomotor activity, lower blood glucose levels, and lower liver enzymes and serum lipids compared with db/db mice that were not fed RJ. (A) Administration of RJ (0.2%, 1%, and 5% per feed weight) was initiated at 8 weeks of age. (B) Changes in the body weight (n = 6). (C) Spontaneous locomotor activity in light and dark phases (n = 6). (D) Results of intraperitoneal glucose tolerance test (2 g/kg body weight) for 15-week-old mice and AUC analysis (n = 6). (E) Results of insulin tolerance test (0.75 U/kg body weight) for 15-week-old mice and AUC analysis (n = 6). (F) Serum ALT, T-Chol, TG, and NEFA levels (n = 6). Data are presented as the mean ± standard deviation. Data were analyzed using a one-way analysis of variance and Holm–Šídák’s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared to db/db mice. ALT, alanine aminotransferase; AUC, the area under the curve; NEFA, non-esterified fatty acid; RJ, royal jelly; T-Chol, total cholesterol; TG, triglycerides.
2.2. Momentum Measurement
The mice were kept individually in cages with a running wheel (MK-713; Muromachi Kikai, Tokyo, Japan). Before being euthanized, we recorded the count of rotations made by the running wheel in each individual cage every 12-h night cycle for 5 days. We used CompACT AMS ver. 3 software (Muromachi Kikai), which was connected to the running wheels to record the number of rotations.
2.3. Glucose and Insulin Tolerance Tests
Due to the association of dysbiosis with inflammation, NAFLD, diabetes, and insulin resistance, we conducted glucose and insulin tolerance tests. At 15 weeks of age, mice underwent intraperitoneal glucose tolerance testing (iPGTT) with a dose of 2 g/kg body weight after a fasting period of 16 h. Additionally, they underwent insulin tolerance testing (ITT) with a dose of 0.5 U/kg body weight following a fasting period of 5 h. These tests were performed as described previously [20].
2.4. Biochemical Analysis
Fasting mice were used to collect blood samples, and the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (T-Chol), triglycerides (TG), and non-esterified fatty acids (NEFA) were evaluated. Biochemical analysis was conducted at Fujifilm Wako Pure Chemical Corporation (Osaka, Japan).
2.5. Liver Histological Analysis
Liver tissues were taken from all mice and subjected to Masson’s trichrome and hematoxylin and eosin (HE) staining. The tissues were immersed in a 10% buffered formaldehyde solution for fixation. For the purpose of oil red O staining, liver tissues were additionally subjected to fixation in a 4% paraformaldehyde phosphate buffer solution. The severity of NAFLD and fibrosis stage were determined as per the NAFLD activity score [21]. Experiments were performed as described previously [22].
2.6. Isolation of Mononuclear Cells from the Liver and the Small Intestine
Before harvesting or washing the tissues with phosphate-buffered salts (PBS), systemic perfusion was performed using heparinized saline to minimize the risk of blood contamination. Experiments were performed as described previously [23].
2.7. Histological Analysis of the Small and Large Intestines
The colon and jejunum were excised from the mice and promptly immersed in 10% buffered formaldehyde at 22 °C for 24 h. Histological analysis was described as described previously [22,24,25].
2.8. Flow Cytometry
The analysis of stained cells was conducted using a FACS Canto II system, and the resulting data were evaluated by FlowJo ver. 10 software (TreeStar, Ashland, OR, USA). The evaluation procedures were carried out following previously described methods. Analyses were performed as described previously [23].
2.9. Gene Expression Analysis
Following a 16-h fasting period, the livers and small intestines of the mice were removed and promptly frozen using liquid nitrogen. Gene expression analysis was performed as described previously [20].
2.10. 16S rRNA Sequencing
For the bacterial flora analysis, the 16S rRNA could not be classified in detail at the species level, so the analysis was performed at the genus level. To conduct 16S rRNA sequencing, we adhered to established protocols [23,24,25]. Frozen appendicular fecal samples were used, and microbial DNA was extracted using the QIAamp DNA Feces Mini Kit (Qiagen, Venlo, The Netherlands), following the protocol outlined by the manufacturer. A bacterial universal primer set (341F and 806R) was utilized to amplify the V3–V4 region of the 16S rRNA gene from the DNA samples. PCR was conducted using EF-Taq (Solgent, Korea) with a 30 µL reaction mixture that included 20 ng of genomic DNA as the template. The thermocycling parameters consisted of an initial activation step at 95 °C for 2 min, followed by 35 cycles at 95 °C, 55 °C, and 72 °C for 1 min each, and a final step at 72 °C for 10 min. The amplified products underwent purification using a multiscreen filter plate (Millipore Corp., Billerica, MA, USA). For 16S rRNA sequencing, a MiSeq sequencer (Illumina, CA, USA) was utilized following the protocol outlined by the manufacturer (Macrogen, Seoul, Korea). QIIME version 1.9 was employed for quality filtering of the sequences, and barcodes or primers with scores below 75% were excluded from the analysis. The number of operational taxonomic units (OTUs) was calculated utilizing the UCLUST algorithm with a similarity threshold of 97%. Additionally, for the taxonomic classification of 16S rRNAs, BLAST (UNITE, 2017) was employed with the UNITE sequence set of the Greengenes core set aligned with UCLUST and ITS.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog abundance predictions were obtained using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) software (version 2.4.1).
The relative abundance of phyla within the groups was assessed using a one-way ANOVA with the Holm–Šídák multiple-comparison test. To analyze the alpha diversity, which refers to the diversity within individual samples, we employed the Chao1, Shannon, and Gini–Simpson indices.
We employed linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe) to assess the relative abundance of bacterial genera among the groups (available online: http://huttenhower.sph.harvard.edu/lefse/ (accessed on 15 May 2022).
Using a normalized relative abundance matrix, LEfSe detected taxa exhibiting statistically significant differences in abundance, and the effect size of each feature was assessed using LDA. A significance threshold of 0.05 (Wilcoxon rank-sum test) and an effect size threshold of 2 were employed for all biomarkers examined.
Furthermore, we conducted a principal component analysis (PCA) to evaluate the effectiveness of RJ. Additionally, nonhierarchical K-means cluster analysis was performed, with the number of clusters to be generated prespecified as 2, using Tinn-R Gui version 1.19.4.7 and R version 1.36.
2.11. Measurement of Palmitic Acid, Short-Chain Fatty Acid (SCFA), and Middle-Chain Fatty Acid Concentrations
The levels of palmitic acid in the serum, liver, and feces, as well as SCFAs in the serum and feces and MCFAs in the serum and liver, were determined by gas chromatography-mass spectrometry (GC/MS) using an Agilent 7890B/7000D system (Agilent Technologies, Santa Clara, CA, USA). Measurements were conducted according to the previously outlined procedure [23].
2.12. Human Hepatoma Cell Culture
The HepG2 cell line was obtained from KAC Company (Kyoto, Japan). The cells were incubated in a minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin–streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate. To maintain the cells, they were cultured in a humidified incubator with 5% CO2 at 37 °C. Twenty-four to forty-eight hours prior to treatments, cells were seeded in a 96-well plate and allowed to reach 70% confluence. Twenty-four hours after changing the medium, the cells underwent treatment with ethanol (Ctrl), 200 μM palmitic acid (PA), or 200 μM PA and 100 μg/mL of 10 H2DA (PA + 10 H2DA), 100 μg/mL of 10 HDAA (PA + 10 HDAA), 100 μg/mL of 2-decenoic acid (PA + 2-DA), or 100 μg/mL of SA (PA + SA) (Day 1). We performed oil red O staining and real-time reverse transcription-polymerase chain reaction (RT-PCR) 24 h following treatment (day 2).
2.13. Oil Red O Staining
HepG2 cells were subjected to 30-min fixation in 4% paraformaldehyde, followed by PBS washing. Experiments were performed as described previously [18].
2.14. Gene Expression Analysis of HepG2 Cells
RT-PCR was performed to assess gene expression. Experiments were performed as described previously [18].
2.15. Statistical Analysis
The data underwent analysis utilizing JMP ver. 13.0 software. (SAS, Cary, NC, USA). To compare the results among different groups, one-way analysis of variance and Holm-Šídák’s multiple comparisons test were employed. An unpaired t-test was utilized to compare the results between the two groups. We determined statistical significance at a threshold of p < 0.05. GraphPad Prism ver. 9.0 software (San Diego, CA, USA) was utilized for generating figures.
3. Results
3.1. Administration of RJ Improved Locomotor Activity, Glucose Metabolism, and Lipid Metabolism
The body weight of db/db mice was higher compared with RJ-fed db/db mice (Figure 1B). Locomotor activity in RJ-fed db/db mice was higher than that in db/db mice (Figure 1C). Moreover, iPGTT and ITT revealed that RJ improved glucose tolerance and insulin sensitivity (Figure 1D). Serum ALT, T-chol, TG, and NEFA levels in db/db mice were higher than those in db/db mice fed RJ (Figure 1E,F).
3.2. Administration of RJ Improved NAFLD
The absolute liver weight in db/db mice was lower than that in RJ-fed mice, whereas the differences in relative liver weights between the two groups were not significant (Figure 2A). The NAFLD activity scores, which were utilized to evaluate the severity of NAFLD and stage of fibrosis, in db/db mice were higher than those in RJ-fed db/db mice (Figure 2B). Additionally, fat deposition in the liver of db/db mice was greater than that in RJ-fed mice (Figure 2C,D).
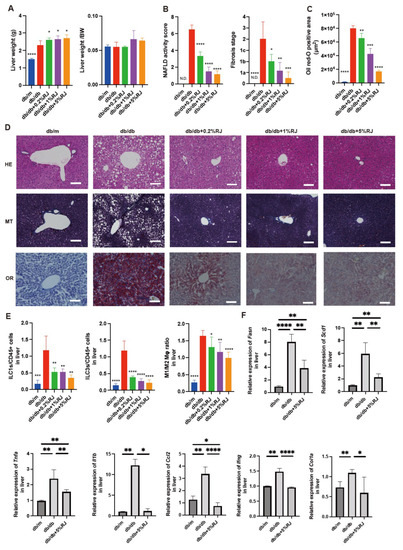
Figure 2.
RJ-fed db/db mice had lower intrahepatic fat accumulation, inflammatory cells, and gene expression associated with inflammation compared with db/db mice that were not fed RJ. (A) Absolute and relative weights of the liver (n = 6). (B) NAFLD activity scores and the fibrosis stage (n = 6). (C) Oil red O staining positive area (μm3) (n = 6). (D) Representative images of HE-, MT-, and OR-stained liver sections. Liver tissues were obtained from 16-week-old mice. Scale bar = 100 μm. (E) The ratio of ILC1s, ILC3s, and M1 to M2 macrophages to CD45-positive cells in the liver (n = 6 in each case). (F) Relative mRNA expression of Fasn, Scd1, Tnfa, Il1b, Ccl2, Ifng, and Col1a in the liver, normalized to the expression of Gapdh (n = 6). Data are presented as the mean ± standard deviation. Data were analyzed using a one-way analysis of variance with Holm–Šídák’s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared to db/db mice. HE, hematoxylin and eosin; MT, Masson trichrome; OR, oil red O; Mφ, macrophages; N.D., not-detected; NAFLD, non-alcoholic fatty liver disease; RJ, royal jelly; ILC, innate lymphoid cell.
3.3. Administration of RJ Improved Visceral Fat, Obesity, and Skeletal Muscle Loss
The epididymal fat weight of db/db mice and epididymal fat weight/BW of db/db mice were higher than those of RJ-fed db/db mice (Supplementary Figure S1A,B). Histological images of epididymal fat tissues are shown in Supplementary Figure S1C. The adipocyte size in db/db mice was larger than that in RJ-fed db/db mice (Supplementary Figure S1D). In addition, the soleus muscle weight of db/db mice was lower than that of RJ-fed db/db mice (Supplementary Figure S1E,F).
3.4. Administration of RJ Improved Inflammation and Decreased the Expression of Genes Related to Fatty Acid Metabolism, Inflammation, and Fibrosis in the Liver
The ratios of the innate lymphoid cell (ILC) 1 or ILC3, which are associated with inflammation, to CD45-positive cells in db/db mice, were higher than those in db/m mice and db/db mice fed RJ. The ratio of M1 macrophages (pro-inflammatory) to M2 macrophages (anti-inflammatory) in db/db mice was higher than that in db/m mice and db/db mice fed RJ (Figure 2E). The relative expression of genes associated with fatty acid metabolism-related enzymes (Fasn and Scd1) and inflammation and fibrosis (Tnfa, ll1b, Ccl2, Ifng, and Col1a) in db/db mice was higher than that in db/m mice and db/db mice fed 5% RJ (Figure 2F).
3.5. Administration of RJ Improved Atrophy of the Small and Large Intestinal Mucosa
Figure 3A displays the histological images of the jejunum and colon. Compared with db/m mice and db/db mice fed RJ, db/db mice exhibited lower villus height and width and atrophic jejunal mucosa. In contrast, the crypt depth/villus height ratio in db/db mice was notably higher than that in the other four groups. The number of goblet cells/crypts in db/db mice was lower than that in db/m mice and db/db mice fed RJ (Figure 3B).
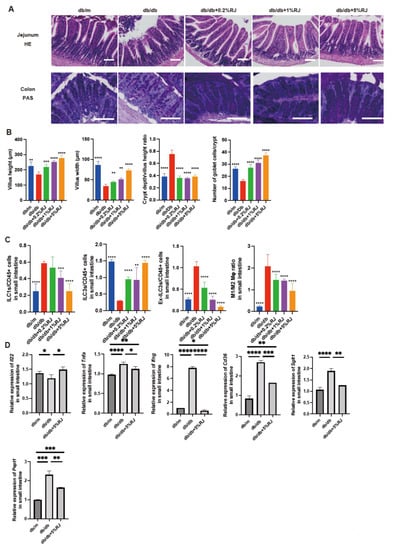
Figure 3.
RJ-fed db/db mice showed lower intestinal inflammation compared with db/db mice that were not fed RJ. (A) Representative images of HE- and PAS-stained jejunum (HE) and colon (PAS) sections. Intestinal tissues were obtained from 16-week-old mice. (B) Villus height of the jejunum (μm), villus width of the jejunum (μm), crypt depth/villus height ratio, and the number of goblet cells/crypt. (C) The ratio of ILC1s, ILC3s, ex-ILC3s, and M1 to M2 macrophages to CD45-positive cells in the small intestine (n = 6 in each case). (D) Relative mRNA expression of Il22, Tnfa, Ifng, Cd36, Sglt1, and Pept1 in the small intestine, normalized to the expression of Gapdh (n = 6). Data are presented as the mean ± standard deviation. Data were analyzed using a one-way analysis of variance and Holm–Šídák’s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared to db/db mice in (A–C) and among three groups in (D). RJ, royal jelly; HE, hematoxylin and eosin; PAS, periodic acid Schiff; ILC, innate lymphoid cell.
3.6. Administration of RJ Regulated Inflammatory Responses in Innate Immunity in the Small Intestine
In the small intestine, ILC3 cells produce antimicrobial peptides. Ex-ILC3 cells are associated with inflammation by acting similarly to ICL1 cells. The ratio of ILC1 and ex-ILC3 cells to CD45-positive cells in the small intestine of db/db mice was higher than that in db/m mice and db/db mice fed RJ. The ratio of ILC3 cells to CD45-positive cells in db/db mice was lower than that in db/m mice and db/db mice fed RJ. Moreover, the ratio of M1 macrophages to M2 macrophages in db/db mice was higher than that in db/m mice and db/db mice fed RJ (Figure 3C).
3.7. Administration of RJ Increased the Expression of Genes Related to Mucin Secretion in the Small Intestine and Decreased the Expression of Genes Related to Inflammation and Nutrient Absorption Transporters
Il22 is associated with enhanced mucin secretion, which acts as a barrier and supports the symbiosis between the human body and gut microbiota [26]. The relative expression of Il22 was lower in db/db mice than in db/m mice, whereas RJ increased the expression of this gene. The expression of inflammation-related genes (Tnfa and Ifng) and nutrient transporter genes (Cd36, Sglt1, and Pept1) was higher in db/db mice than that in db/m mice; however, this expression was significantly decreased upon RJ administration (Figure 3D).
3.8. Administration of RJ Improved Dysbiosis
OTUs in db/db mice were lower than those in db/m mice and db/db mice fed RJ. The Chao1 and Shannon indices, which are indicators of the α-diversity of gut microbiota, were lower in db/db mice than those in db/m mice. However, these indices improved after RJ administration (Figure 4A). In db/db mice, the most abundant phylum was Firmicutes, which is associated with diabetes and obesity, and in db/m and db/db mice fed 5% RJ, the most abundant phylum was Bacteroidetes (Figure 4B). Seven taxa, including Firmicutes, were observed to be overexpressed in db/db mice, whereas seven taxa, including SCFA-producing bacteria, such as the family Ruminococcaceae, genus Butyricicocus, and genus Acetivibrio, were overexpressed in db/db mice fed 5% RJ (Figure 4C). In addition, PCA showed that db/m and db/db mice fed 5% RJ belonged to the same cluster (Figure 4D).
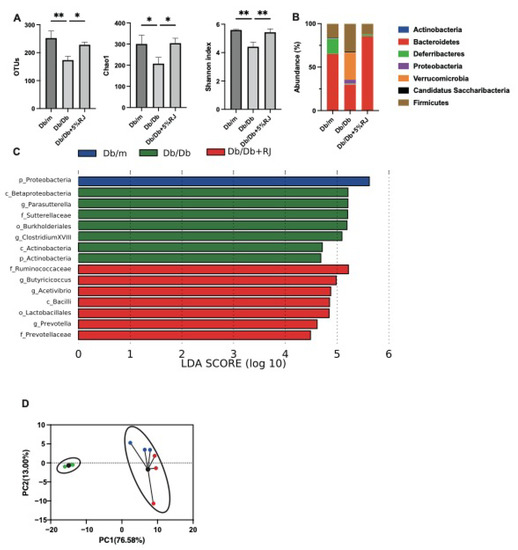
Figure 4.
RJ-fed db/db mice showed a higher diversity of gut microbiota and a higher abundance of short-chain fatty acid-producing gut microbiota compared with db/db mice that were not fed RJ. (A) OTUs (n = 3), Chao1 index (n = 3), and Shannon index (n = 3). (B) Relative abundance of gut microbiota at the phylum level (n = 3). (C) LDA scores of gut microbiota of db/m, db/db, and db/db mice fed 5% RJ (n = 3). db/m mice (blue); db/db mice (green); db/db mice fed 5% RJ (red). (D) PCA and k-means clustering for gut microbiota (n = 3). Data were analyzed using a one-way analysis of variance and Holm–Šídák’s multiple comparison test. * p < 0.05 and ** p < 0.01 among the three groups. LDA, linear discriminant analysis; OTU, operational taxonomic units; PCA, principal component analysis.
3.9. Administration of RJ Increased Palmitic acid Excretion and SCFA Production in Feces and Increased MCFAs in the Blood and Liver
Next, we measured the concentration of palmitic acid in the feces, serum, and liver. Palmitic acid concentrations in the liver and serum of db/db mice were higher than those in db/m mice, and these levels decreased upon administration of RJ. In contrast, the concentration of palmitic acid in the feces was lower in db/db mice than that in db/m mice, and these levels increased upon RJ administration (Figure 5A). Levels of SCFAs, such as acetic, butyric, and propionic acids, were significantly higher in the feces and serum of db/db mice fed RJ than those observed for db/db mice (Figure 5B,C). We also measured the concentrations of RJ-specific MCFAs, such as 10H2DA, 10HDAA, SA, and 2-DA, in the serum and liver of mice (Figure 5D,E). RJ increased the concentration of MCFAs in db/db mice.
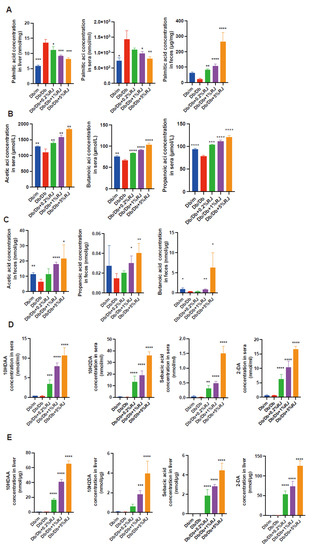
Figure 5.
Metabolites in the liver, sera, and feces. (A) Concentrations of palmitic acid in the liver, sera, and feces (n = 6). (B,C) Concentrations of acetic acid, propanoic acid, and butanoic acid in the sera and feces (n = 6). (D,E) Concentrations of 10HDAA, 10H2DA, SA, and 2-DA in the sera and liver (n = 6). Data are presented as the mean ± standard deviation. Data were analyzed using a one-way analysis of variance and Holm–Šídák’s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared to db/db mice. 10HDAA, 10-hydroxydecanoic acid; 10H2DA, 10-hidroxy-2-decenoic acid; 2-DA, SA, sebacic acid, 2-decenedioic acid.
3.10. RJ-Related MCFAs Decreased Saturated Fatty Acid Deposition in HepG2 Cells and Decreased the Expression of Genes Related to Fatty Acid Metabolism and Fibrosis
We treated HepG2 cells with palmitic acid and RJ-related MCFAs (10H2DA, 10HDAA, SA, or 2-DA) and analyzed the cells for fat deposition and gene expression. The oil red O-positive area in HepG2 cells corresponding to RJ-related MCFAs was smaller than that with palmitic acid alone (Figure 6A). The relative expression of FASN and SCD1, which are genes associated with fatty acid metabolism, and COLA1A, which is a gene associated with fibrosis, was lower in the presence of RJ-related MCFAs than in the presence of only palmitic acid (Figure 6B–D). In particular, the relative expression of these genes decreased following treatment with 10H2DA or SA (Supplementary Table S1).
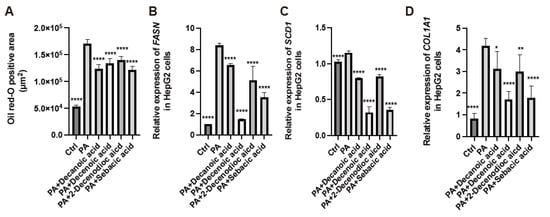
Figure 6.
Administration of medium-chain fatty acids from royal jelly decreased the accumulation of saturated fatty acids and gene expression related to fatty acid synthesis and fibrosis in HepG2 cells. (A) Oil red-O positive area (n = 4). Relative mRNA expression of (B) FASN, (C) SCD1, and (D) COL1A1 in HepG2 cells treated with ethanol (Ctrl), 200 µM PA, 200 µM PA, and 0.5 mM 10 HDAA acid, 200 µM PA and 0.5 mM 10 H2DA acid, 200 µM PA and 0.5 mM 2-DA, and 200 µM PA and 0.5 mM SA (n = 4). Data are represented as the mean ± SD values. Data were analyzed using a 1-way ANOVA with Holm-Šídák’s multiple-comparisons test. * p < 0.05, ** p < 0.01, **** p < 0.0001 compared to db/db mice. PA, palmitic acid; 10 HDAA, 10-hydroxydecanoic acid; 10 H2DA, 10-hidroxy-2-decenoic acid; 2-DA, 2-decenedioic acid; SA, sebacic acid.
4. Discussion
In this study, RJ improved dysbiosis and inflammation of the intestinal mucosa, increased saturated palmitic acid excretion in the feces and SCFA-producing bacteria in the intestine, decreased the concentration of palmitic acid in the blood and liver, and increased MCFAs in the liver, thereby resulting in an improvement in NAFLD. This study is the first to elucidate that RJ regulates the expression of nutrient absorption transporter genes in the small intestine in vivo, measure the concentration of RJ-related MCFAs in the liver, and show that RJ and RJ-related MCFAs may improve NAFLD.
In our study, RJ administration improved dysbiosis. In particular, SCFA-producing bacteria were overexpressed in the intestine of RJ-fed mice. SCFAs are used as a major source of nutrition by the mucosal cells in the large intestine and increase mucin expression in goblet cells, increase mucus production, and strengthen tight junctions between the epithelial cells of the intestine [20,27]. Impairment of the intestinal barrier results in systemic microinflammation due to lipopolysaccharides produced by gram-negative rods [28], and a previous study has demonstrated increased serum endotoxin levels in patients with non-alcoholic steatohepatitis [29]. In addition, SCFAs bind to the free fatty acid receptor and suppress the activation of nuclear factor-kappa B [30], resulting in the suppression of the secretion of cytokines such as IL1, tumor necrosis factor-α (TNF-α), and IL12 [31,32]. ILC1 cells produce TNF-α and interferon-gamma (IFN-γ) in response to IL12 [33], and ILC3 cells produce IL17 and IL22 in response to IL1β [34], resulting in cytotoxicity. In this study, RJ increased the excretion of palmitic acid in the feces and decreased its concentration in the serum and liver. SCFAs increase the secretion of GLP1, which slows the excretion of gastric contents [35] and delays nutrient absorption. High-fat diets have been reported to increase the expression of fat absorption transporters [36], and high-protein diets have been reported to increase the expression of Pept1, which is a protein absorption transporter found in the small intestine [37]. Other research has reported that high glucose conditions increase the expression of SGLT1 in the small intestine [38]. We envisage that the expression of nutrient absorption transporters in db/db mice increased because of overeating, whereas the administration of RJ decreased the expression of these transporters in the small intestine and increased GLP1, resulting in decreased nutrient absorption, including that of saturated fatty acids. RJ has been shown to improve serum lipid profiles in mice [16], which is consistent with our results. Saturated fatty acids in the diet have been reported to be related to increased IL12 secretion from macrophages, mitochondrial damage, chronic low-grade inflammation, metabolic disorders, and cardiovascular diseases [39,40,41,42]. Saturated fatty acids have also been implicated in muscle atrophy and progressive inflammation in the liver [20]. We envisage that the administration of RJ maintained intestinal barrier function, regulated inflammation, and decreased saturated fatty acid levels in the liver by improving gut microbiota dysbiosis, increasing SCFAs, and regulating the expression of nutrient absorption transporters in the small intestine. As a result, NAFLD improved. Further, the improvement in liver inflammation and decreased absorption of nutrients are thought to improve glucose intolerance, lipid metabolism, visceral fat accumulation, and muscle mass loss. In this study, RJ increased the liver size without causing fatty liver disease, possibly because RJ inhibited steatohepatitis in db/db mice because of the aforementioned effects. However, db/db mice are known to consume more food than db/m mice, resulting in fat deposition and hypertrophy of the liver and other organs. In addition, it is possible that the livers of db/db mice that were not fed RJ were atrophied because of inflammation and fibrosis.
In our study, the concentrations of 10H2DA, 10HDAA, 2-DA, and SA in the serum and liver increased after RJ administration. These RJ-related MCFAs decreased saturated fatty acid deposition and decreased gene expression related to fatty acid metabolism and fibrosis in HepG2 cells. MCFAs are absorbed through the portal system and quickly provide energy to the liver, thereby inhibiting hepatic fat accumulation as well as the development of dyslipidemia [16]. MCFAs have also been reported to activate energy metabolism by themselves, and their metabolites modify histone acetylation [43,44]. RJ contains several characteristic MCFAs. Particularly, 10H2DA, which is an unsaturated fatty acid unique to RJ, has been reported to have antimicrobial, anticancer, epidermal protective, lifespan-prolonging, and immunomodulatory effects [9,45,46,47,48,49,50]. RJ also contains 10HDAA (a saturated fatty acid of 10H2DA), 2-DA, and SA (a saturated fatty acid of 2-DA) [9]. 10HDAA has been reported to inhibit the synthesis of nitric oxide associated with inflammation [51]. Moreover, SA exerts a suppressive effect on inflammation by decreasing the level of TNF-α [52]. RJ-related MCFAs may regulate gene expression, improve liver inflammation, and contribute to the improvement of NAFLD. Previous studies have reported that major RJ proteins (MRJPs) improve obesity, fatty liver, and insulin resistance in mice, as well as fat accumulation in vitro [14,15]. However, MRJPs are known to get digested during the RJ powder production process [19]. A study showed that the concentrations of serum 2-DA and SA were increased by RJ or enzyme-treated RJ [17]. Our study revealed that among the components of RJ, i.e., not only MRJPs but also RJ-related MCFAs, some have anti-fat accumulation effects.
This study has a limitation. HepG2 cells have been used in this study, and we have not used primary hepatocytes. More precise experiments might have been possible if we had used primary hepatocytes.
5. Conclusions
RJ improved dysbiosis and regulated nutrient absorption-related gene expression in the small intestine, resulting in increased excretion of saturated fatty acids in feces, an increase in SCFA-producing intestinal bacteria, an improvement in inflammation in the intestine and liver, and an improvement in atrophy of the small and large intestinal mucosa. This study represents the initial evidence to demonstrate that RJ regulates the expression of nutrient absorption transporter genes in the small intestine in vivo, to measure RJ-related MCFA concentrations in the liver, and to show that RJ and RJ-related MCFA may improve NAFLD. Further clinical trials are required to validate our findings for the treatment of NAFLD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15112580/s1, Supplementary Figure S1A–F: db/db mice fed with royal jelly showed lower visceral fat accumulation and higher skeletal muscle mass; Table S1: The comparison among six groups.
Author Contributions
G.K. interpreted the data, contributed to the discussion, and wrote the manuscript. T.O. conceived and designed the study, acquired, analyzed, and interpreted the data, contributed to the discussion, and wrote the manuscript. S.M., T.S., H.O. (Hiroshi Okada), E.U., N.N., Y.N., T.Y., H.O. (Hideto Okamoto), N.O. and R.S. contributed to the discussion. M.H. and M.F. conceived and designed the work, interpreted the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Yamada Bee Company, Inc.
Institutional Review Board Statement
All animal experiments were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine, Kyoto, Japan (approval number: M2020-48).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets of the study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Yamada Bee Company, Inc., Metabologenomics, Inc., and all members of the Kyoto Prefectural University of Medicine.
Conflicts of Interest
G.K., T.O., S.M., and R.S. have no conflicts of interest. T.S. received personal fees from Eli Lilly Japan K.K., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Takeda Pharma Co., Ltd., Ltd., Astellas Pharma Inc., Terumo Corporation, Taisho Toyama Pharma Co., Sanofi K.K., Kyowa Kirin Co., Ltd., Kowa Pharma Co., Ltd., MSD K.K., Ono Pharma Co., Ltd., Abbott Japan Co., Ltd., Kissei Pharma Co., Ltd., Novo Nordisk Pharma Ltd., and Mochida Pharma Co., Ltd. Okada Hiroshi received personal fees from Teijin Pharma Ltd. and Kyowa Hakko Kirin Company Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Mochida Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., AstraZeneca K.K., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Sanofi K.K., MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. Emi Ushigome received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure, the Astellas Foundation for Research on Metabolic Disorders, the Mishima Kaiun Memorial Foundation, and received personal fees from MSD K.K., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Novo Nordisk Pharma Ltd. The Donated Fund Laboratory of Diabetes Therapeutics is an endowment department supported by an unrestricted grant provided by Taiyo Kagaku Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. Nakanishi Naoko received personal fees from Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co., Ltd., and Terumo Corporation. Y.N. and T.Y. were affiliated with Metabologenomics Inc. as employees. Hideto Okamoto and N.O. were affiliated with Yamada Bee Company, Inc. as employees. Hiroshi Takakuwa was affiliated with Agilent Technologies as an employee. M.H. received grants from AstraZeneca K.K., Kowa Pharma Co., Ltd., and Ono Pharma Co., Ltd., and received personal fees from Daiichi Sankyo Co., Ltd., Ono Pharma Co., Ltd., Eli Lilly, Japan K.K, Mitsubishi Tanabe Pharma Corp., Kowa Pharma Co., Ltd., Sumitomo Dainippon Pharma Co., Sanofi K.K., and AstraZeneca K.K. M.F. received grants from Oishi Kenko Inc., Yamada Bee Farm, Takeda Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Ono Pharma Co., Ltd., Sanofi K.K., Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corp., MSD K.K., Sanwa Kagagu Kenkyusho Co., Astellas Pharma Inc., Kyowa Kirin Co., Ltd., Kissei Pharma Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Terumo Corp., Kowa Pharma Co., Ltd., Taisho Pharma Co., Ltd., Tejin Pharma Ltd., Eli Lilly, Japan, K.K., Nippon Chemiphar Co., Ltd., Johnson & Johnson K.K. Medical Co., Novo Nordisk Pharma Ltd., TERUMO CORPORATION, and Abbott Japan Co., Ltd., and was remunerated with personal fees by TERUMO CORPORATION, Eli Lilly Japan K.K., Kissei Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., Sanofi K.K., Takeda Pharma Co., Ltd., Abbott Japan Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Novo Nordisk Pharma Ltd., Medtronic Japan Co., Ltd., MSD K.K., Kyowa Kirin Co., Ltd., Teijin Pharma Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corp., Mochida Pharma Co., Ltd., Ono Pharma Co., Ltd., Taisho Pharma Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Arkray Inc., Nippon Boehringer Ingelheim Co., Ltd., and Nipro Corp.
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Hamaguchi, M.; Hashimoto, Y.; Okamura, T.; Nakanishi, N.; Obora, A.; Kojima, T.; Fukui, M. Obesity and Metabolic Abnormalities as Risks of Alcoholic Fatty Liver in Men: NAGALA Study. BMC Gastroenterol. 2021, 21, 321. [Google Scholar] [CrossRef] [PubMed]
- Argo, C.K.; Caldwell, S.H. Epidemiology and Natural History of Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 511–531. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St. Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The Natural History of Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal Jelly Ameliorates Diet-Induced Obesity and Glucose Intolerance by Promoting Brown Adipose Tissue Thermogenesis in Mice. Obes. Res. Clin. Pract. 2018, 12, 127–137. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal Jelly Causes Hypotension and Vasodilation Induced by Increasing Nitric Oxide Production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal Jelly Supplementation Improves Lipoprotein Metabolism in Humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef]
- Takaki-Doi, S.; Hashimoto, K.; Yamamura, M.; Kamei, C. Antihypertensive Activities of Royal Jelly Protein Hydrolysate and Its Fractions in Spontaneously Hypertensive Rats. Acta Med. Okayama 2009, 63, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Meng, X.C.; Zhou, Y.J.; Zhu, J.X.; Chang, Y.N. Major Royal Jelly Proteins Alleviate Non-Alcoholic Fatty Liver Disease in Mice Model by Regulating Disordered Metabolic Pathways. J. Food Biochem. 2022, 46, e14214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.; Zhou, Y.; Guo, X.; Chang, Y. Major Royal Jelly Proteins Prevents NAFLD by Improving Mitochondrial Function and Lipid Accumulation through Activating the AMPK/SIRT3 Pathway in Vitro. J. Food Sci. 2021, 86, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal Jelly Attenuates Nonalcoholic Fatty Liver Disease by Inhibiting Oxidative Stress and Regulating the Expression of Circadian Genes in Ovariectomized Rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef]
- Yamaga, M.; Tani, H.; Yamaki, A.; Tatefuji, T.; Hashimoto, K. Metabolism and Pharmacokinetics of Medium Chain Fatty Acids after Oral Administration of Royal Jelly to Healthy Subjects. RSC Adv. 2019, 9, 15392–15401. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Nishida, K.; Hashimoto, Y.; Fukuda, T.; Senmaru, T.; et al. Immune Modulating Effects of Additional Supplementation of Estradiol Combined with Testosterone in Murine Testosterone-Deficient NAFLD Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G989–G999. [Google Scholar] [CrossRef]
- Moriyama, T.; Yanagihara, M.; Yano, E.; Kimura, G.; Seishima, M.; Tani, H.; Kanno, T.; Nakamura-Hirota, T.; Hashimoto, K.; Tatefuji, T.; et al. Hypoallergenicity and Immunological Characterization of Enzyme-Treated Royal Jelly from Apis Mellifera. Biosci. Biotechnol. Biochem. 2013, 77, 789–795. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian Green Propolis Improves Gut Microbiota Dysbiosis and Protects against Sarcopenic Obesity. J. Cachexia Sarcopenia Muscle 2022, 13, 3028–3047. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Hasegawa, Y.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ. Health Perspect. 2023, 131, 027006. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Nakajima, H.; Kitagawa, N.; Majima, S.; Senmaru, T.; Okada, H.; Ushigome, E.; Nakanishi, N.; Sasano, R.; et al. Milk Protects against Sarcopenic Obesity Due to Increase in the Genus Akkermansia in Faeces of Db/Db Mice. J. Cachexia Sarcopenia Muscle 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, S.; Qian, L.; Jiang, S.; Tang, Y.; Han, T. DHA-Enriched Phosphatidylserine Ameliorates Non-Alcoholic Fatty Liver Disease and Intestinal Dysbacteriosis in Mice Induced by a High-Fat Diet. Food Funct. 2021, 12, 4021–4033. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, Y.; Qian, L.; Jiang, S.; Tang, Y.; Han, T. DHA-Enriched Phosphatidylserine Alleviates High Fat Diet-Induced Jejunum Injury in Mice by Modulating Gut Microbiota. Food Funct. 2023, 14, 1415–1429. [Google Scholar] [CrossRef]
- Jinnohara, T.; Kanaya, T.; Hase, K.; Sakakibara, S.; Kato, T.; Tachibana, N.; Sasaki, T.; Hashimoto, Y.; Sato, T.; Watarai, H.; et al. IL-22BP Dictates Characteristics of Peyer’s Patch Follicle-Associated Epithelium for Antigen Uptake. J. Exp. Med. 2017, 214, 1607–1618. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. Conference on Diet and Digestive Disease Symposium 2: Sensing and Signalling of the Gut Environment: Scfa: Mechanisms and Functional Importance in the Gut. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2021; Volume 80, pp. 37–49. [Google Scholar]
- Nagpal, R.; Yadav, H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab. 2017, 71, 11–16. [Google Scholar] [CrossRef]
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated Endotoxin Levels in Non-Alcoholic Fatty Liver Disease. J. Inflamm. 2010, 7, 15. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Milligan, G. Metabolism Meets Immunity: The Role of Free Fatty Acid Receptors in the Immune System. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef]
- Koch, P.D.; Pittet, M.J.; Weissleder, R. The Chemical Biology of IL-12 Production: Via the Non-Canonical NFkB Pathway. RSC Chem. Biol. 2020, 1, 166–176. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-KB) Signaling in Cancer Development and Immune Diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Vermi, W.; Lee, J.S.; Lonardi, S.; Gilfillan, S.; Newberry, R.D.; Cella, M.; Colonna, M. Intraepithelial Type 1 Innate Lymphoid Cells Are a Unique Subset of IL-12- and IL-15-Responsive IFN-γ-Producing Cells. Immunity 2013, 38, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Gamini, R.; Sécca, C.; Collins, P.L.; Zhao, S.; Peng, V.; Robinette, M.L.; Schettini, J.; Zaitsev, K.; Gordon, W.; et al. Subsets of ILC3−ILC1-like Cells Generate a Diversity Spectrum of Innate Lymphoid Cells in Human Mucosal Tissues. Nat. Immunol. 2019, 20, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Poirier, H.; Degrace, P.; Niot, I.; Bernard, A.; Besnard, P. Localization and Regulation of the Putative Membrane Fatty-Acid Transporter (FAT) in the Small Intestine Comparison with Fatty Acid-Binding Proteins (FABP). Eur. J. Biochem. 1996, 238, 368–373. [Google Scholar] [CrossRef]
- Spanier, B. Transcriptional and Functional Regulation of the Intestinal Peptide Transporter PEPT1. J. Physiol. 2014, 592, 871–879. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose Transporters in the Small Intestine in Health and Disease. Pflugers. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Hernández, E.A.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute Dietary Fat Intake Initiates Alterations in Energy Metabolism and Insulin Resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fat, Carbohydrate, and Cardiovascular Disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Hamaguchi, M.; et al. Trans Fatty Acid Intake Induces Intestinal Inflammation and Impaired Glucose Tolerance. Front. Immunol. 2021, 12, 669672. [Google Scholar] [CrossRef]
- Nobuhara, M.; Saotome, M.; Watanabe, T.; Urushida, T.; Katoh, H.; Satoh, H.; Funaki, M.; Hayashi, H. Mitochondrial Dysfunction Caused by Saturated Fatty Acid Loading Induces Myocardial Insulin-Resistance in Differentiated H9c2 Myocytes: A Novel Ex Vivo Myocardial Insulin-Resistance Model. Exp. Cell Res. 2013, 319, 955–966. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Mochizuki, K.; Kimura, M.; Kawamura, M.; Hariya, N.; Goda, T. The Mechanism of Ameliorating the Metabolism by the Medium-Chain Fatty Acid via Pathways Related to Energy Production and the Epigenetics. Oleoscience 2018, 18, 375–381. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; Binmowyna, M.N. Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules 2021, 26, 7021. [Google Scholar] [CrossRef] [PubMed]
- Dzopalic, T.; Vucevic, D.; Tomic, S.; Djokic, J.; Chinou, I.; Colic, M. 3,10-Dihydroxy-Decanoic Acid, Isolated from Royal Jelly, Stimulates Th1 Polarising Capability of Human Monocyte-Derived Dendritic Cells. Food Chem. 2011, 126, 1211–1217. [Google Scholar] [CrossRef]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The Functional Property of Royal Jelly 10-Hydroxy-2-Decenoic Acid as a Melanogenesis Inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Ito, T.; Degawa, T.; Moriyama, M.; Moriyama, H. Royal Jelly Protects against Epidermal Stress through Upregulation of the NQO1 Expression. Int. J. Mol. Sci. 2021, 22, 12973. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-Decenoic Acid, a Major Fatty Acid from Royal Jelly, Inhibits VEGF-Induced Angiogenesis in Human Umbilical Vein Endothelial Cells. Evid. Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-Decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis Elegans through Dietary Restriction and Target of Rapamycin Signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef]
- Takahashi, K.; Sugiyama, T.; Tokoro, S.; Neri, P.; Mori, H. Inhibitory Effect of 10-Hydroxydecanoic Acid on Lipopolysaccharide-Induced Nitric Oxide Production via Translational Downregulation of Interferon Regula-Tory Factor-1 in RAW264 Murine Macrophages. Biomed. Res. 2013, 34, 205–214. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, K.; Zhang, Y.-Z.; Zheng, Y.-F.; Hu, F.-L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).