Inhibition of MAOB Ameliorated High-Fat-Diet-Induced Atherosclerosis by Inhibiting Endothelial Dysfunction and Modulating Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Treatment
2.3. Serum Biochemical Analysis
2.4. Immunohistology of Atherosclerotic Lesions
2.5. Oxidative Stress Detection
2.6. RNA Isolation and Quantitative Real Time PCR (qPCR)
2.7. Western Blot Analysis
2.8. Luciferase Activity Assay
2.9. RNA-Seq Analysis
2.10. Apoptotic Assay
2.11. 16. S ribosomal RNA (rRNA) Sequencing and Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
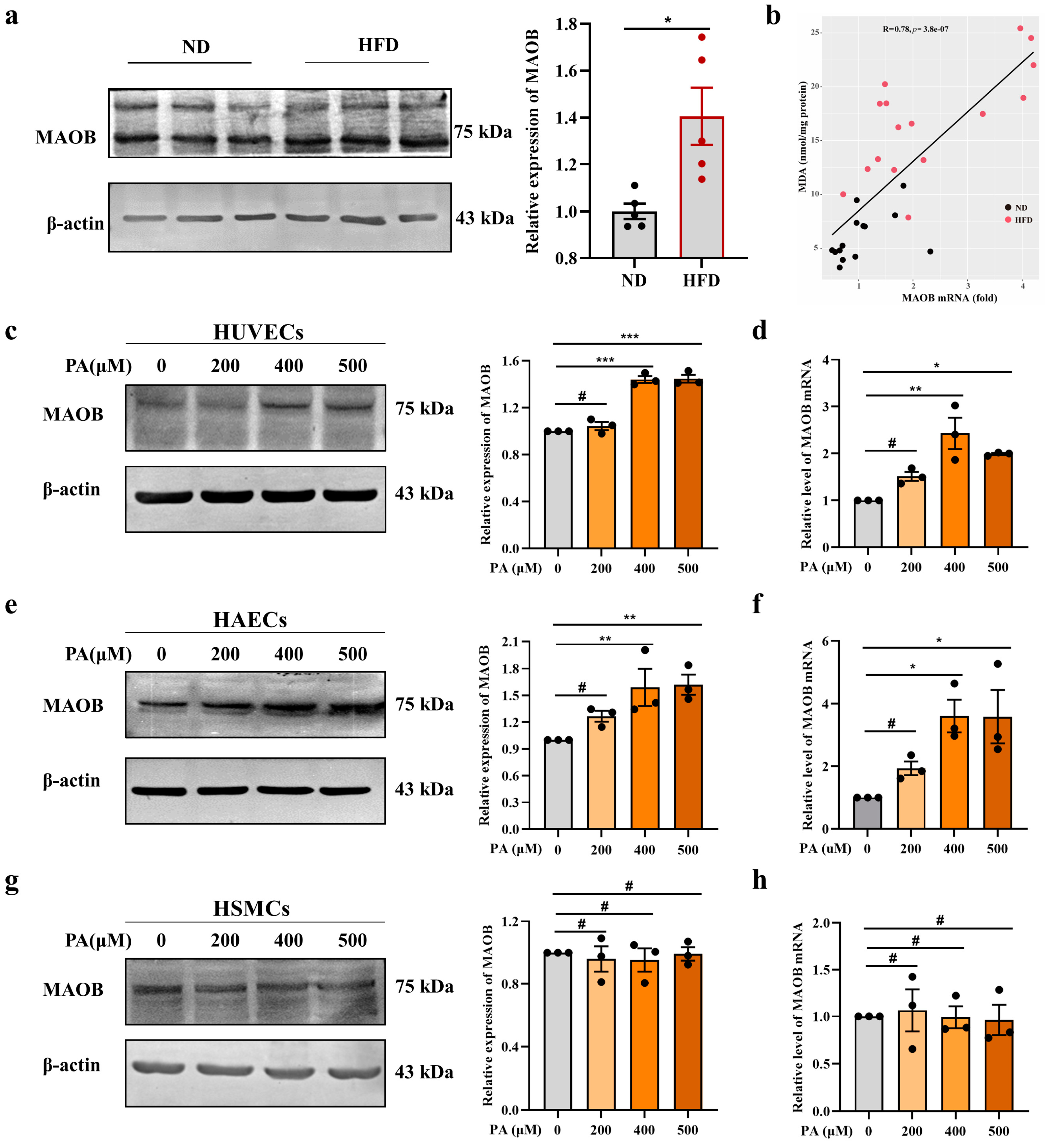
3.1. MAOB Expression Was Activated in HFD-Exposed Vascular Endothelial Cells
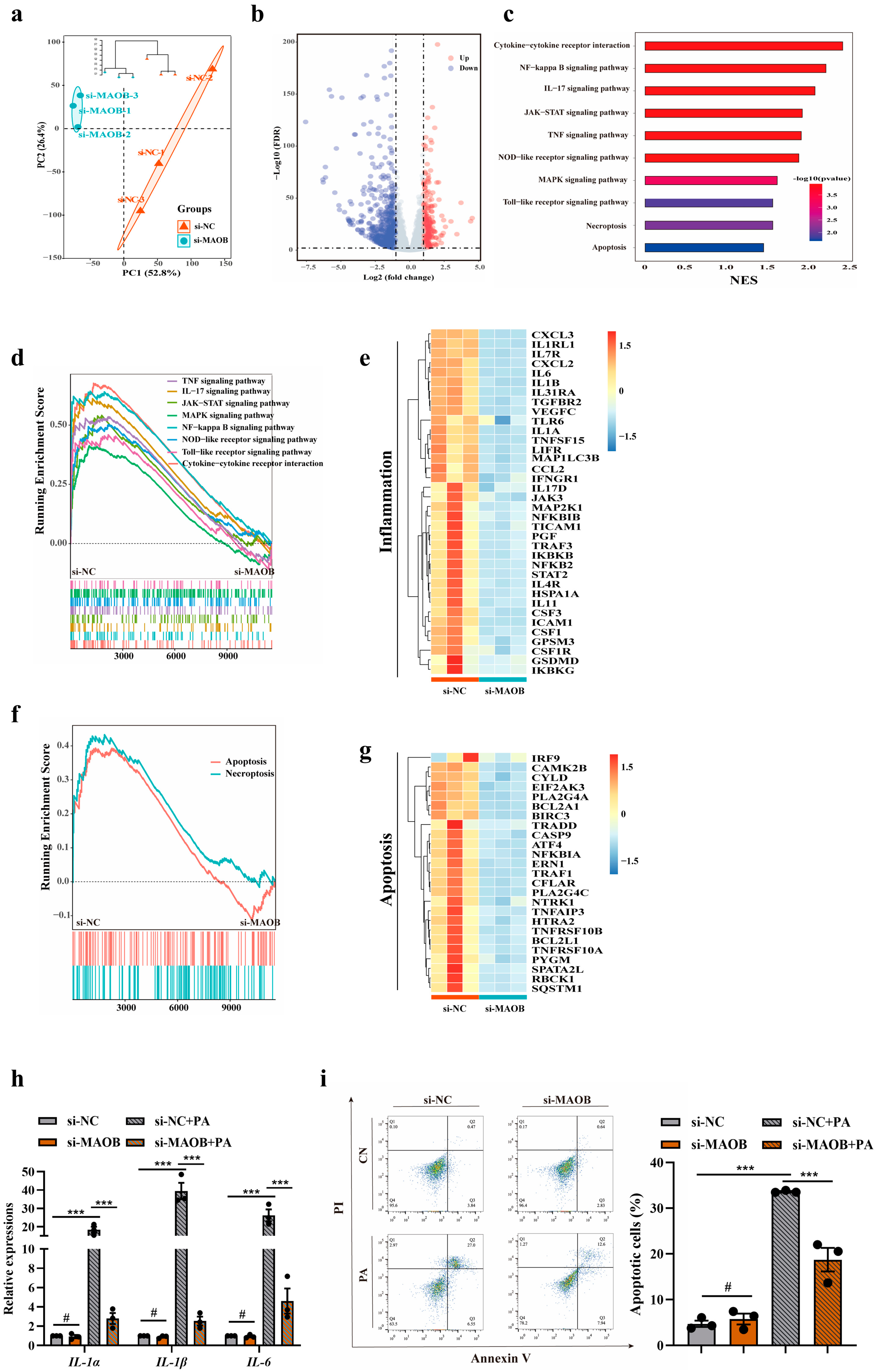
3.2. MAOB Activation Mediated PA-Induced Endothelial Oxidative Stress and Dysfunction In Vitro
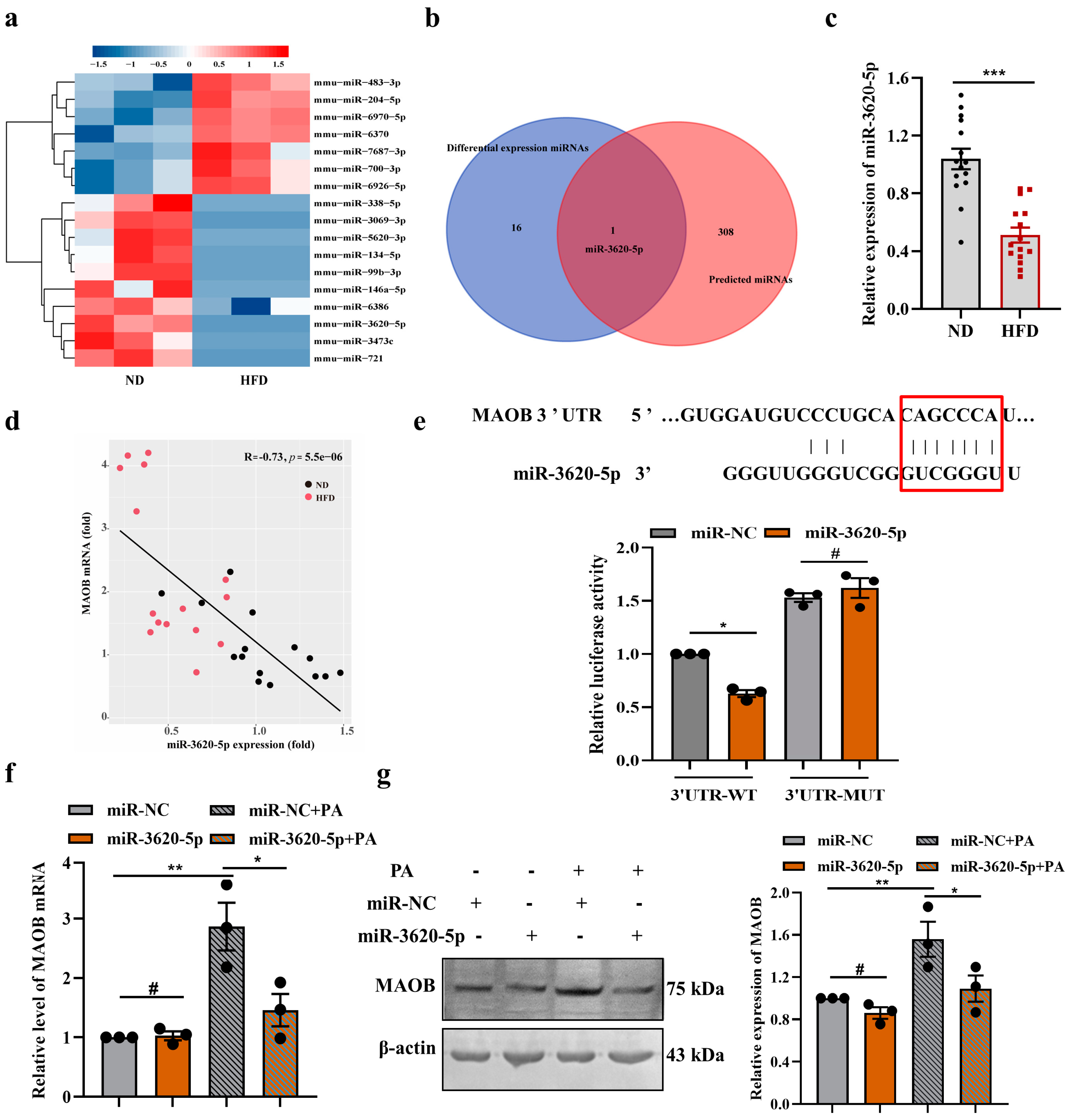
3.3. MAOB Expression Was Regulated by miR-3620-5p in HFD-Exposed Vascular Endothelial Cells
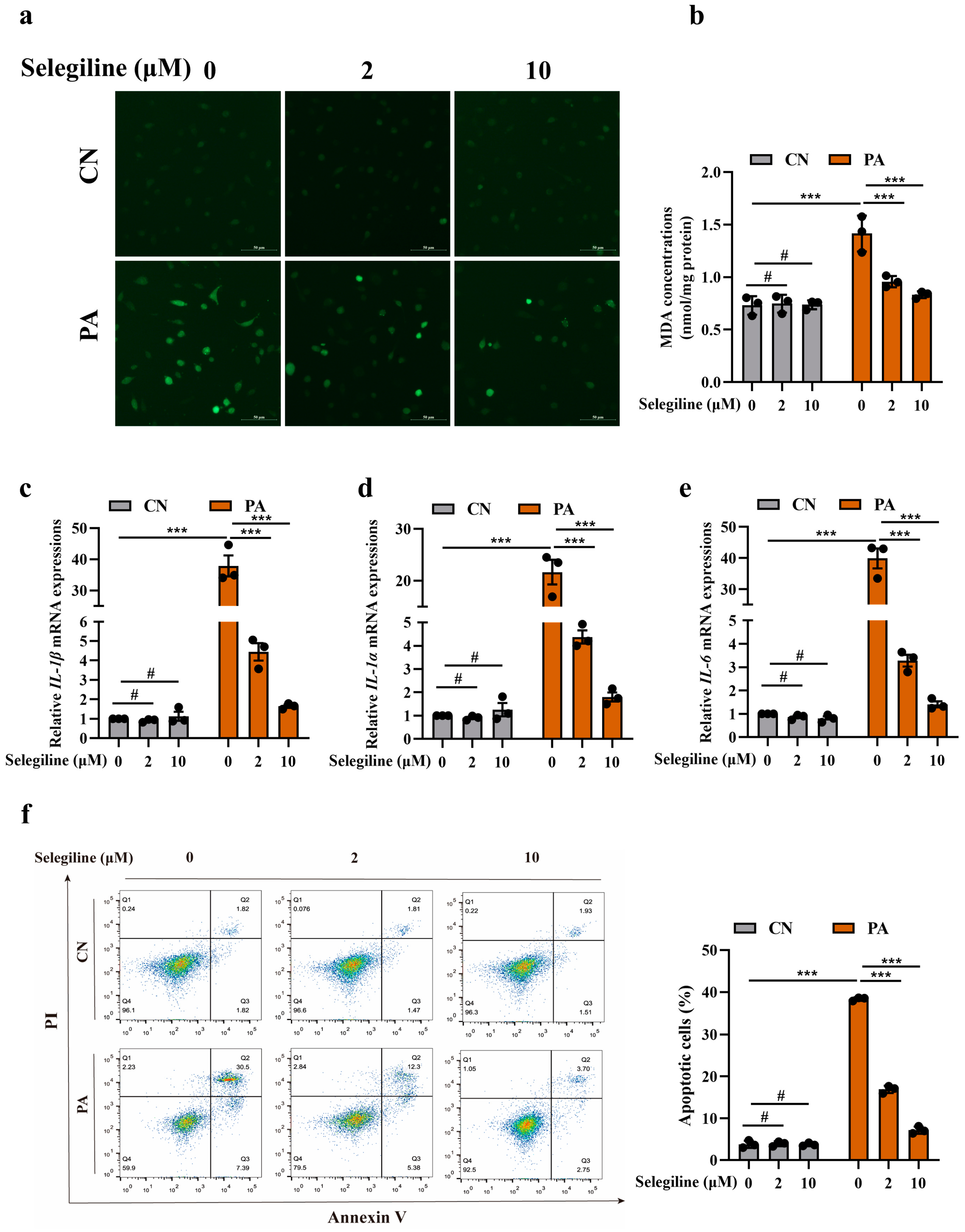
3.4. Selegiline Prevented Atherosclerosis by Attenuating Endothelial Oxidative Stress and Dysfunction
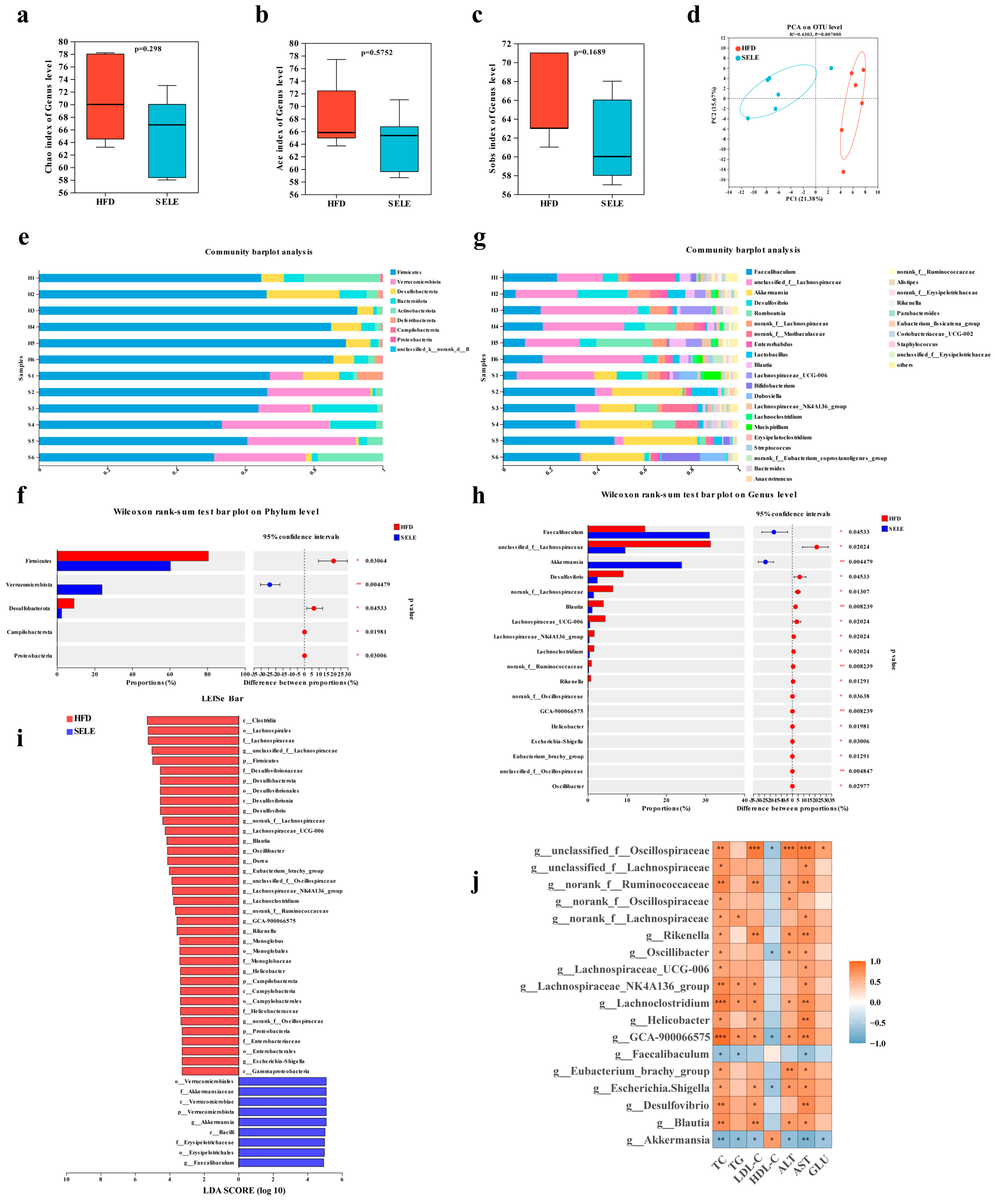
3.5. Selegiline Administration Altered the Composition of Gut Microbiota in ApoE−/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Xia, J.; Cheng, J.; Huang, H.; Zhou, Y.; Yang, X.; Su, X.; Ke, Y.; Ling, W. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Dysfunction via Epigenetic Regulation of p66shc-Mediated Oxidative Stress Pathway. Circulation 2019, 139, 2260–2277. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Yurdagul, A., Jr.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulou, M.; Toutouzas, K.; Michelongona, A.; Tousoulis, D. Statins and vulnerable plaque. Curr. Pharm. Des. 2017, 23, 7069–7085. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27-32. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Alexander, R.W.; Medford, R.M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Investig. 1993, 92, 1866–1874. [Google Scholar] [CrossRef]
- Khan, B.V.; Harrison, D.G.; Olbrych, M.T.; Alexander, R.W.; Medford, R.M. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9114–9119. [Google Scholar] [CrossRef]
- Lenin, R.; Thomas, S.M.; Gangaraju, R. Endothelial Activation and Oxidative Stress in Neurovascular Defects of the Retina. Curr. Pharm. Des. 2018, 24, 4742–4754. [Google Scholar] [CrossRef]
- Lighezan, R.; Sturza, A.; Duicu, O.M.; Ceausu, R.A.; Vaduva, A.; Gaspar, M.; Feier, H.; Vaida, M.; Ivan, V.; Lighezan, D.; et al. Monoamine oxidase inhibition improves vascular function in mammary arteries from nondiabetic and diabetic patients with coronary heart disease. Can. J. Physiol. Pharmacol. 2016, 94, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sturza, A.; Leisegang, M.S.; Babelova, A.; Schroder, K.; Benkhoff, S.; Loot, A.E.; Fleming, I.; Schulz, R.; Muntean, D.M.; Brandes, R.P. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 2013, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.F. 90 years of monoamine oxidase: Some progress and some confusion. J. Neural. Transm. 2018, 125, 1519–1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Tsai, F.C.; Lin, H.H.; Yu, T.Y.; Kuo, C.H.; Li, H.Y.; Lin, M.S. Inhibition of monoamine oxidase B reduces atherosclerosis and fatty liver in mice. Clin. Sci. 2023, 137, 17–30. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Han, T.; Sun, C. Selegiline ameliorated dyslipidemia and hepatic steatosis in high-fat diet mice. Int. Immunopharmacol. 2023, 117, 109901. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Heinonen, E.H.; Anttila, M.I.; Lammintausta, R.A. Pharmacokinetic aspects of l-deprenyl (selegiline) and its metabolites. Clin. Pharmacol. Ther. 1994, 56, 742–749. [Google Scholar] [CrossRef]
- Arige, V.; Agarwal, A.; Khan, A.A.; Kalyani, A.; Natarajan, B.; Gupta, V.; Reddy, S.S.; Barthwal, M.K.; Mahapatra, N.R. Regulation of Monoamine Oxidase B Gene Expression: Key Roles for Transcription Factors Sp1, Egr1 and CREB, and microRNAs miR-300 and miR-1224. J. Mol. Biol. 2019, 431, 1127–1147. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tang, Q.; Nie, J.; Zhang, C.; Zhou, X.; Yu, S.; Sun, J.; Cheng, X.; Dong, N.; Hu, Y.; et al. BMAL1-Downregulation Aggravates Porphyromonas Gingivalis-Induced Atherosclerosis by Encouraging Oxidative Stress. Circ. Res. 2020, 126, e15–e29. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Orso, F.; Olgasi, C.; Taverna, D.; Brizzi, M.F. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1562–1568. [Google Scholar] [CrossRef]
- Chen, Y.; Banda, M.; Speyer, C.L.; Smith, J.S.; Rabson, A.B.; Gorski, D.H. Regulation of the expression and activity of the antiangiogenic homeobox gene GAX/MEOX2 by ZEB2 and microRNA-221. Mol. Cell Biol. 2010, 30, 3902–3913. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bae, W.J.; Ahn, J.M.; Heo, J.H.; Kim, K.M.; Choi, K.W.; Sung, C.O.; Lee, D. MicroRNA signatures associated with lymph node metastasis in intramucosal gastric cancer. Mod. Pathol. 2021, 34, 672–683. [Google Scholar] [CrossRef]
- Figueroa, J.; Phillips, L.M.; Shahar, T.; Hossain, A.; Gumin, J.; Kim, H.; Bean, A.J.; Calin, G.A.; Fueyo, J.; Walters, E.T.; et al. Exosomes from Glioma-Associated Mesenchymal Stem Cells Increase the Tumorigenicity of Glioma Stem-like Cells via Transfer of miR-1587. Cancer. Res. 2017, 77, 5808–5819. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Youdim, M.B.; Riederer, P. Pharmacology of selegiline. Neurology 1996, 47, S137–S145. [Google Scholar] [CrossRef]
- Morningstar, J.E.; Johnson, M.K.; Cecchini, G.; Ackrell, B.A.; Kearney, E.B. The high potential iron-sulfur center in Escherichia coli fumarate reductase is a three-iron cluster. J. Biol. Chem. 1985, 260, 13631–13638. [Google Scholar] [CrossRef]
- Bekesi, G.; Tulassay, Z.; Lengyel, G.; Schaff, Z.; Szombath, D.; Stark, J.; Marczell, I.; Nagy-Repas, P.; Adler, I.; Dinya, E.; et al. The effect of selegiline on total scavenger capacity and liver fat content: A preliminary study in an animal model. J. Neural. Transm. 2012, 119, 25–30. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, D.; Zeng, W.; Chen, Y.; Guo, M.; Lu, B.; Li, H.; Sun, C.; Yang, L.; Jiang, X.; et al. The Role of Intestinal Dysbacteriosis Induced Arachidonic Acid Metabolism Disorder in Inflammaging in Atherosclerosis. Front. Cell. Infect. Microbiol. 2021, 11, 618265. [Google Scholar] [CrossRef]
- Wu, M.; Yang, S.; Wang, S.; Cao, Y.; Zhao, R.; Li, X.; Xing, Y.; Liu, L. Effect of Berberine on Atherosclerosis and Gut Microbiota Modulation and Their Correlation in High-Fat Diet-Fed ApoE−/− Mice. Front. Pharmacol. 2020, 11, 223. [Google Scholar] [CrossRef]
- Huang, K.; Liu, C.; Peng, M.; Su, Q.; Liu, R.; Guo, Z.; Chen, S.; Li, Z.; Chang, G. Glycoursodeoxycholic Acid Ameliorates Atherosclerosis and Alters Gut Microbiota in Apolipoprotein E-Deficient Mice. J. Am. Heart Assoc. 2021, 10, e019820. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, S.; Meng, Y.; Zhao, Q.; Zhang, Y.; Suonan, Z.; Sun, Y.; Shen, Q.; Liao, X.; Xue, Y. Capsaicin Ameliorates High-Fat Diet-Induced Atherosclerosis in ApoE−/− Mice via Remodeling Gut Microbiota. Nutrients 2022, 14, 4334. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Vijay-Kumar, M. Gut microbiome as a novel cardiovascular therapeutic target. Curr. Opin. Pharmacol. 2016, 27, 8–12. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, R.; An, Y.; Gao, W.; Bai, L.; Li, Y.; Zhao, S.; Fan, J.; Liu, E. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis. 2018, 17, 159. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, T.; Hu, H.; Zhang, W.; Hua, X. Association Study of Gut Flora in Coronary Heart Disease through High-Throughput Sequencing. Biomed Res. Int. 2017, 2017, 3796359. [Google Scholar] [CrossRef]
- Yan, S.; Shi, R.; Li, L.; Ma, S.; Zhang, H.; Ye, J.; Wang, J.; Pan, J.; Wang, Q.; Jin, X.; et al. Mannan Oligosaccharide Suppresses Lipid Accumulation and Appetite in Western-Diet-Induced Obese Mice Via Reshaping Gut Microbiome and Enhancing Short-Chain Fatty Acids Production. Mol. Nutr. Food Res. 2019, 63, e1900521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z.; Xue, Z.; Sun, Z.; Zhang, M.; Wang, L.; Wang, G.; Wang, F.; Xu, J.; Cao, H.; et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015, 9, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Wang, Y.; Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Eugenol, A Major Component of Clove Oil, Attenuates Adiposity, and Modulates Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2022, 66, e2200387. [Google Scholar] [CrossRef]
- Li, M.; Kang, S.G.; Huang, K.; Tong, T. Dietary Supplementation of Methyl Cedryl Ether Ameliorates Adiposity in High-Fat Diet-Fed Mice. Nutrients 2023, 15, 788. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe−/− mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Li, J.; Han, X.; Zhu, H.; Yu, G.; Cheng, J. Medium-, long- and medium-chain-type structured lipids ameliorate high-fat diet-induced atherosclerosis by regulating inflammation, adipogenesis, and gut microbiota in ApoE−/− mice. Food Funct 2020, 11, 5142–5155. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence (5′-3′) |
|---|---|
| has-miR-3620-5p | F: GTGGGCTGGGCTGGGC R: AGTGCAGGGTCCGAGGTATT |
| R: AGTGCAGGGTCCGAGGTATT | |
| mmu-miR-3620-5p | F: AACAATCTGTGGGCTGGGCTG |
| R: ATCCAGTGCAGGGTCCGAGG | |
| has-MAOB | F: GAAGAGTGGGACAACATGAC |
| R: CTCCACACTGCTTCACATAC | |
| mmu-MAOB | F: CAACAACCAATGGAGGACAGGAGAG |
| R: CTGAACCCAAAGGCACACGAGAG | |
| β-actin | F: ACTATCGGCAATGAGCG |
| R: GAGCCAGGGCAGTAATCT | |
| has-IL-6 | F: GCCTTCGGTCCAGTTGCCTTC |
| R: GCCTCTTTGCTGCTTTCACACATG | |
| has-IL-1α | F: GACCAACCAGTGCTGCTGAAGG |
| R: CTTAGTGCCGTGAGTTTCCCAGAAG | |
| has-IL-1β | F: GGACAGGATATGGAGCAACAAGTGG |
| R: CAACACGCAGGACAGGTACAGATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Wang, X.; Han, T.; Wang, M.; Ning, H.; Sun, C. Inhibition of MAOB Ameliorated High-Fat-Diet-Induced Atherosclerosis by Inhibiting Endothelial Dysfunction and Modulating Gut Microbiota. Nutrients 2023, 15, 2542. https://doi.org/10.3390/nu15112542
Tian Z, Wang X, Han T, Wang M, Ning H, Sun C. Inhibition of MAOB Ameliorated High-Fat-Diet-Induced Atherosclerosis by Inhibiting Endothelial Dysfunction and Modulating Gut Microbiota. Nutrients. 2023; 15(11):2542. https://doi.org/10.3390/nu15112542
Chicago/Turabian StyleTian, Zhen, Xinyue Wang, Tianshu Han, Maoqing Wang, Hua Ning, and Changhao Sun. 2023. "Inhibition of MAOB Ameliorated High-Fat-Diet-Induced Atherosclerosis by Inhibiting Endothelial Dysfunction and Modulating Gut Microbiota" Nutrients 15, no. 11: 2542. https://doi.org/10.3390/nu15112542
APA StyleTian, Z., Wang, X., Han, T., Wang, M., Ning, H., & Sun, C. (2023). Inhibition of MAOB Ameliorated High-Fat-Diet-Induced Atherosclerosis by Inhibiting Endothelial Dysfunction and Modulating Gut Microbiota. Nutrients, 15(11), 2542. https://doi.org/10.3390/nu15112542





