Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Approval, and Consent
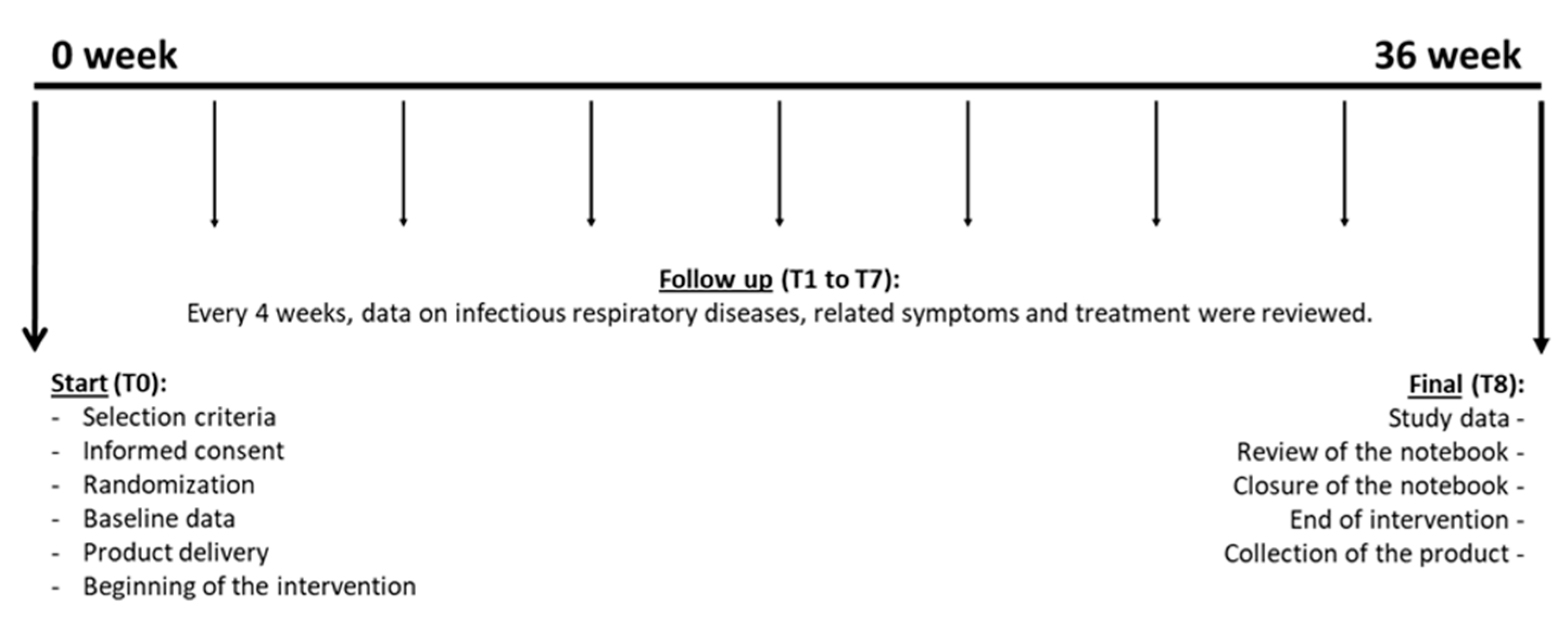
2.2. Subjects and Study Design
2.3. Intervention
2.4. Clinical Parameters
2.5. Statistical Analysis
3. Results
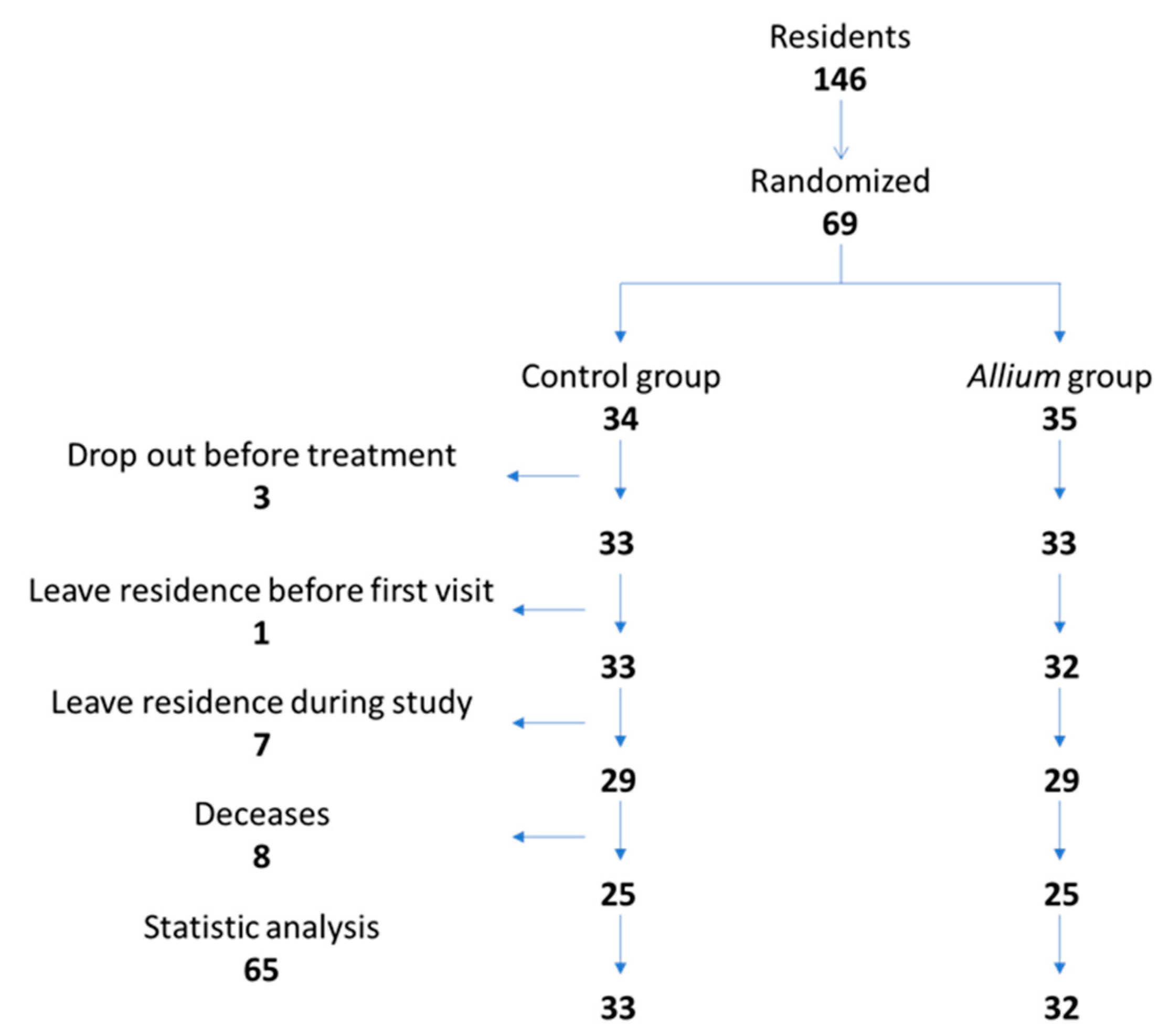
3.1. Study Data, Compliance, and Baseline Characteristics of the Subjects
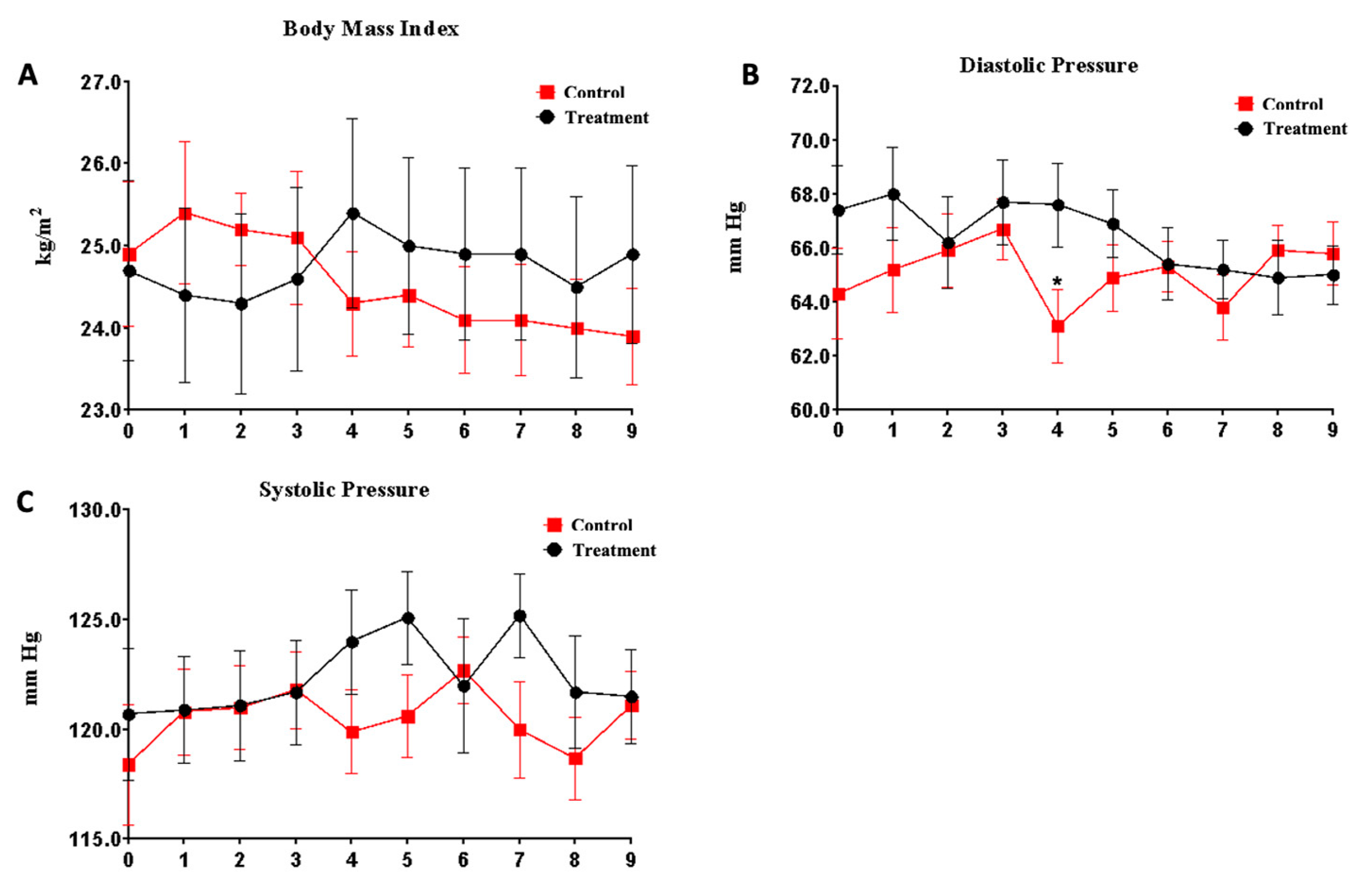
3.2. Security Parameters
3.3. Respiratory Tract Infections Incidence and Related Symptoms
3.4. Symptom Severity
4. Discussion
5. Limitations and Strengths
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Ageing 2019 Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Crighton, E.J.; Elliott, S.J.; Moineddin, R.; Kanaroglou, P.; Upshur, R.E. An exploratory spatial analysis of pneumonia and influenza hospitalizations in Ontario by age and gender. Epidemiol. Infect. 2007, 135, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Viral pneumonia in older adults. Clin. Infect. Dis. 2006, 42, 518–524. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, comorbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.B. Pneumonia in nursing homes and long-term care facilities. Semin. Respir. Crit. Care Med. 2005, 26, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Anne, S.; Gerhard, P. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. S. Afr. J. Bot. 2020, 133, 54–62. [Google Scholar]
- Hafiz Ansar Rasul, S.; Masood Sadiq, B.; Nauman, K.; Saira, S.; Ali, R.; Muhammad, A.; Munawar, A. Garlic (Allium sativum): Diet based therapy of 21st century—A review. Asian Pac. J. Trop. Dis. 2015, 5, 271–278. [Google Scholar]
- Vezza, T.; Garrido-Mesa, J.; Díez-Echave, P.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; García, F.; Sánchez, M.; Toral, M.; Romero, M.; Duarte, J.; et al. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Gálvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, e1800653. [Google Scholar] [CrossRef]
- Cabello-Gómez, J.F.; Aguinaga-Casanas, M.A.; Falcón-Piñeiro, A.; González-Gragera, E.; Márquez-Martín, R.; Agraso, M.D.M.; Bermúdez, L.; Baños, A.; Martínez-Bueno, M. Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies. Molecules 2022, 27, 6900. [Google Scholar] [CrossRef]
- Magrys, A.; Olender, A.; Tchorzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef]
- Liu, Y.; Che, T.M.; Song, M.; Lee, J.J.; Almeida, J.A.; Bravo, D.; Van Alstine, W.G.; Pettigrew, J.E. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2013, 91, 5668–5679. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/all-topics/eu-case-definitions (accessed on 20 November 2022).
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Wu, Y.; Goplen, N.P.; Sun, J. Aging and respiratory viral infection: From acute morbidity to chronic sequelae. Cell. Biosci. 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Evolucíon Pandemia. Available online: https://cnecovid.isciii.es/covid19/#evoluci%C3%B3n-pandemia (accessed on 10 October 2022).
- Mellado-García, P.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Martín-Cameán, A.; Moyano, R.; Blanco, A.; Cameán, A.M. Genotoxicity of a thiosulfonate compound derived from Allium sp. intended to be used in active food packaging: In vivo comet assay and micronucleus test. Mutat. Res. Genet. Toxicol. Env. Mutagen. 2016, 800–801, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mellado-García, P.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Marcos, R.; Pichardo, S.; Cameán, A.M. In vitro toxicological assessment of an organosulfur compound from Allium extract: Cytotoxicity, mutagenicity and genotoxicity studies. Food Chem. Toxicol. 2017, 99, 231–240. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Gutiérrez-Praena, D.; Prieto, A.I.; Pichardo, S.; Jos, A.; Moreno, F.J.; Cameán, A.M. Cytotoxic and mutagenic in vitro assessment of two organosulfur compounds derived from onion to be used in the food industry. Food Chem. 2015, 166, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lira, A.C.; Prieto, A.I.; Baño, S.A.; Guillamón, E.; Moyano; Jos, A.; Cameán, A.M. Safety assessment of propyl-propane-thiosulfonate (PTSO): 90-days oral subchronic toxicity study in rats. Food Chem. Toxicol. 2020, 144, 111612. [Google Scholar] [CrossRef]
- Cascajosa-Lira, A.; Pichardo, S.; Baños, A.; Guillamón, E.; Molina-Hernández, V.; Moyano, R.; Jos, Á.; Cameán, A.M. Acute and subchronic 90-days toxicity assessment of propyl-propane-thiosulfinate (PTS) in rats. Food Chem. Toxicol. 2022, 161, 112827. [Google Scholar] [CrossRef]
- Gracián-Alcaide, C.; Maldonado-Lobón, J.A.; Ortiz-Tikkakoski, E.; Gómez-Vílchez, A.; Fonollá, J.; López-Larramendi, J.L.; Olivares, M.; Blanco-Rojo, R. Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial. Appl. Sci. 2020, 10, 8259. [Google Scholar] [CrossRef]
- Fernández-Ochoa, A.; Borrás-Linares, I.; Baños, A.; García-López, J.D.; Guillamón, E.; Nuñez-Lechado, C.; Quirantes-Piné, R.; Segura, A. A fingerprinting metabolomic approach reveals deregulation of endogenous metabolites after the intake of a bioactive garlic supplement. J. Funct. Foods 2018, 49, 137–145. [Google Scholar] [CrossRef]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136 (Suppl. 3), 816S–820S. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nakanishi, R.; Li, D.; Alani, A.; Rezaeian, P.; Prabhu, S.; Abraham, J.; Fahmy, M.A.; Dailing, C.; Flores, F.; et al. Aged Garlic Extract Reduces Low Attenuation Plaque in Coronary Arteries of Patients with Metabolic Syndrome in a Prospective Randomized Double-Blind Study. J. Nutr. 2016, 146, 427S–432S. [Google Scholar] [CrossRef]
- Josling, P. Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2001, 18, 189–193. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Niktale, H.; Yazdi, A.P.; Ghorani, V.; Rashed, M.M.; Hashemian, A.M. Evaluate the Therapeutic Effect of Allicin (L-cysteine) on Clinical Presentation and Prognosis in Patients with COVID-19. Eur. J. Transl. Myol. 2021, 31, 9518. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; Andreo-Martínez, P.; Mut-Salud, N.; Fonollá, J.; Baños, A. Beneficial Effects of Organosulfur Compounds from Allium cepa on Gut Health: A Systematic Review. Foods 2021, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Keusgen, M.; Schulz, H.; Glodek, J.; Krest, I.; Krüger, H.; Herchert, N.; Keller, J. Characterization of SomeAlliumHybrids by Aroma Precursors, Aroma Profiles, and Alliinase Activity. J. Agric. Food Chem. 2002, 50, 2884–2890. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. Biomed. Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef]
- Guillamón, E.; Mut-Salud, N.; Rodríguez-Sojo, M.J.; Ruiz-Malagón, A.J.; Cuberos-Escobar, A.; Martínez-Férez, A.; Rodríguez-Nogales, A.; Gálvez, J.; Baños, A. In Vitro Antitumor and Anti-Inflammatory Activities of Allium-Derived Compounds Propyl Propane Thiosulfonate (PTSO) and Propyl Propane Thiosulfinate (PTS). Nutrients 2023, 15, 1363. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; García, M.P.; Lara, A.; Rubio, L.A. Garlic derivatives (PTS and PTS-O) differently affect the ecology of swine faecal microbiota in vitro. Vet. Microbiol. 2010, 144, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Ariza-Romero, J.J.; Zurita-González, M.J.; Martín-Platero, A.M.; Baños, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M.; Peralta-Sánchez, J.M. Allium-Based Phytobiotic Enhances Egg Production in Laying Hens through Microbial Composition Changes in Ileum and Cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceutics 2022, 15, 1049. [Google Scholar] [CrossRef]
- Vahid, F.; Rahmani, D. Can an anti-inflammatory diet be effective in preventing or treating viral respiratory diseases? A systematic narrative review. Clin. Nutr. ESPEN 2021, 43, 9–15. [Google Scholar] [CrossRef]
- Marefati, N.; Eftekhar, N.; Kaveh, M.; Boskabadi, J.; Beheshti, F.; Boskabady, M.H. The Effect of Allium cepa Extract on Lung Oxidant, Antioxidant, and Immunological Biomarkers in Ovalbumin-Sensitized Rats. Med. Princ. Pract. 2018, 27, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Myhill, L.J.; Andersen-Civil, A.I.S.; Thamsborg, S.M.; Blanchard, A.; Williams, A.R. Garlic-Derived Organosulfur Compounds Regulate Metabolic and Immune Pathways in Macrophages and Attenuate Intestinal Inflammation in Mice. Mol. Nutr. Food Res. 2022, 66, e2101004. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, W.; Ring, J. Anti-inflammatory substances from onions could be an option for treatment of COVID-19—A hypothesis. Allergo J. 2020, 29, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Haslberger, A.G.; Jakob, U.; Hippe, B.; Karlic, H. Mechanisms of selected functional foods against viral infections with a view on COVID-19, Mini review. Funct. Foods Health Dis. 2020, 5, 195–209. [Google Scholar] [CrossRef]
- Thota, S.M.; Balan, V.; Sivaramakrishnan, V. Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19. Phytother. Res. 2020, 34, 3148–3167. [Google Scholar] [CrossRef]
| Control Group (n = 33) | Treatment Group (n = 32) | p-Value | |
|---|---|---|---|
| Age (years) | 84.5 ± 8.0 | 84.3 ± 10.1 | 0.940 |
| Gender | 0.537 | ||
| Men | 8 (24.2) | 5 (15.6) | |
| Women | 25 (75.8) | 27 (84.4) | |
| Body Mass Index BMI (kg/m2) | 24.9 ± 5.0 | 24.7 ± 6.3 | 0.881 |
| Smoking habits | 0.873 | ||
| Current smoker | 29 (87.9) | 27 (84.4) | |
| Former smoker | 2 (6.1) | 3 (9.4) | |
| No smoker | 2 (6.1) | 2 (6.2) | |
| Alcoholic drinks | 1.000 | ||
| Regular drinker | 30 (90.9) | 30 (93.8) | |
| Former drinker | 2 (6.1) | 1 (3.1) | |
| Non drinker | 1 (3.0) | 1 (3.1) | |
| Physical activity | 0.762 | ||
| Very low | 15 (45.4) | 13 (40.6) | |
| Low | 18 (54.6) | 19 (59.4) | |
| Systolic pressure (mmHg) | 118.4 ± 15.5 | 120.7 ± 17.4 | 0.571 |
| Diastolic pressure (mmHg) | 64.3 ± 9.5 | 67.4 ± 9.4 | 0.191 |
| Group | Number of Events | Statistical Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Ratio ns/s | p-Value | IRR c/t | p-Value | ||
| Influenza | Control | 21 | 9 | 3 | 0 | 0.013 | 0.005 | 3.717 | 0.021 |
| Treatment | 29 | 1 | 2 | 0 | 0.004 | 0.002 | |||
| Common cold | Control | 18 | 8 | 2 | 5 | 0.021 | 0.007 | 4.038 | 0.001 |
| Treatment | 28 | 2 | 0 | 2 | 0.005 | 0.003 | |||
| ORVI | Control | 29 | 4 | 0 | 0 | 0.004 | 0.002 | 2.468 | 0.352 |
| Treatment | 30 | 2 | 0 | 0 | 0.002 | 0.001 | |||
| RBI | Control | 25 | 7 | 1 | 0 | 0.006 | 0.003 | 1.943 | 0.263 |
| Treatment | 29 | 2 | 0 | 1 | 0.003 | 0.002 | |||
| Symptoms | Group | Number of Events | Statistical Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Ratio ns/s | p-Value | IRR c/t | p-Value | ||
| Cough | Control | 18 | 12 | 3 | 0 | 0.014 | 0.005 | 4.792 | 0.005 |
| Treatment | 29 | 2 | 0 | 1 | 0.003 | 0.002 | |||
| Fever | Control | 27 | 5 | 1 | 0 | 0.009 | 0.005 | 6.450 | 0.093 |
| Treatment | 31 | 1 | 0 | 0 | 0.001 | 0.001 | |||
| Nasal congestion | Control | 27 | 6 | 0 | 0 | NC | NC | NC | NC |
| Treatment | 32 | 0 | 0 | 0 | NC | NC | |||
| Throat pain | Control | 30 | 3 | 0 | 0 | 0.001 | 0.001 | 1.719 | 0.599 |
| Treatment | 30 | 1 | 1 | 0 | 0.001 | 0.001 | |||
| Headache | Control | 29 | 4 | 0 | 0 | NC | NC | NC | NC |
| Treatment | 32 | 0 | 0 | 0 | NC | NC | |||
| Bone pain | Control | 28 | 4 | 1 | 0 | 0.002 | 0.002 | 12.465 | 0.033 |
| Treatment | 31 | 0 | 1 | 0 | 0.000 | 0.000 | |||
| Fatigue | Control | 22 | 6 | 3 | 2 | 0.020 | 0.007 | 5.235 | 0.004 |
| Treatment | 29 | 1 | 2 | 0 | 0.004 | 0.002 | |||
| Chest pain | Control | 27 | 4 | 2 | 0 | 0.006 | 0.003 | 2.641 | 0.185 |
| Treatment | 28 | 2 | 1 | 0 | 0.002 | 0.002 | |||
| Difficulty breathing | Control | 20 | 8 | 4 | 1 | 0.016 | 0.006 | 3.751 | 0.007 |
| Treatment | 28 | 2 | 1 | 1 | 0.004 | 0.002 | |||
| Nausea | Control | 32 | 1 | 0 | 0 | 0.000 | 0.000 | 1.407 | 0.818 |
| Treatment | 31 | 1 | 0 | 0 | 0.000 | 0.000 | |||
| Diarrhea | Control | 27 | 4 | 1 | 1 | 0.007 | 0.004 | 1.674 | 0.389 |
| Treatment | 28 | 3 | 1 | 0 | 0.004 | 0.003 | |||
| Lack of appetite | Control | 25 | 5 | 3 | 0 | 0.014 | 0.006 | 3.720 | 0.058 |
| Treatment | 29 | 3 | 0 | 0 | 0.004 | 0.003 | |||
| Sleeping problems | Control | 28 | 5 | 0 | 0 | NC | NC | NC | NC |
| Treatment | 32 | 0 | 0 | 0 | NC | NC | |||
| Group | Number of Symptoms | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | 13 | 17 | |||
| Control | 13 | 5 | 3 | 0 | 1 | 3 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Treatment | 23 | 4 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| Statistical analysis | ||||||||||||||||
| Ratio ns/s | p-value | IRR c/t | p-value | |||||||||||||
| Control | 0.110 | 0.010 | 3.231 | 0.000 | ||||||||||||
| Treatment | 0.034 | 0.006 | ||||||||||||||
| Group | Days | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 7 | 8 | 9 | 10 | 15 | 17 | 18 | 21 | 22 | 24 | 56 | |||
| Control | 13 | 1 | 3 | 1 | 3 | 2 | 1 | 0 | 1 | 3 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | ||
| Treatment | 23 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Statistical analysis | |||||||||||||||||||
| Ratio ns/s | p-value | IRR c/t | p-value | ||||||||||||||||
| Control | 0.168 | 0.019 | 3.508 | 0.000 | |||||||||||||||
| Treatment | 0.048 | 0.008 | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, J.; Gracián, C.; Baños, A.; Guillamón, E.; Gálvez, J.; Rodriguez-Nogales, A.; Fonollá, J. Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers. Nutrients 2023, 15, 2308. https://doi.org/10.3390/nu15102308
García-García J, Gracián C, Baños A, Guillamón E, Gálvez J, Rodriguez-Nogales A, Fonollá J. Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers. Nutrients. 2023; 15(10):2308. https://doi.org/10.3390/nu15102308
Chicago/Turabian StyleGarcía-García, Jorge, Carlos Gracián, Alberto Baños, Enrique Guillamón, Julio Gálvez, Alba Rodriguez-Nogales, and Juristo Fonollá. 2023. "Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers" Nutrients 15, no. 10: 2308. https://doi.org/10.3390/nu15102308
APA StyleGarcía-García, J., Gracián, C., Baños, A., Guillamón, E., Gálvez, J., Rodriguez-Nogales, A., & Fonollá, J. (2023). Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers. Nutrients, 15(10), 2308. https://doi.org/10.3390/nu15102308









