Abstract
Background: Nowadays, there is a growing interest in the relationship among lifestyle, reproductive health, and fertility. Recent investigations highlight the influence of environmental and lifestyle factors such as stress, diet, and nutritional status on reproductive health. The aim of this review was to determine the influence of nutritional status on ovarian reserve in order to improve the reproductive health of women of childbearing age. Methods: A systematic literature review was carried out following the PRISMA method. The quality of the studies was assessed using the Cochrane Collaboration Risk of Bias tool. Data were extracted, and the results were summarized into two blocks: according to the technique used to assess ovarian reserve and nutritional status; according to the results found in the relationship between ovarian reserve and nutritional status. Results: A total of 22 articles involving 5929 women were included. In 12 of the included articles (54.5%), a relationship between nutritional status and ovarian reserve was demonstrated. In seven publications (31.8%), the increased body mass index (BMI) led to a decrease in ovarian reserve, two of them (0.9%) in patients with polycystic ovary syndrome, showing a decrease only if BMI > 25. In two articles (0.9%), there was a negative relationship between ovarian reserve and waist-to-hip ratio, and in one (0.45%), a positive relationship was shown between ovarian reserve and testosterone levels, the latter being related to body mass index. In five articles (22.7%), body mass index was used as a confounder and was negatively related to ovarian reserve, and in another four (18%), no correlation was found. Conclusions: Ovarian reserve appears to be influenced by nutritional status. A high body mass index has a negative impact on the ovary, decreasing antral follicle count and anti-Müllerian hormone. Oocyte quality is compromised, increasing the rate of reproductive problems and the demand for assisted reproductive techniques. Further studies are needed to understand which dietary factors have the greatest effect on ovarian reserve in order to promote reproductive health.
1. Introduction
There is growing interest in the links between lifestyle, reproductive health, and fertility. Against a backdrop of social change, recent years have witnessed an increasing trend towards delayed motherhood, with many women choosing to wait until their 30s or 40s to have their first child. According to the National Statistics Institute, the average age of Spanish mothers in 2021 was 33.05 years [], which was above the European average of 29.5 years []. The data collected since the start of this registry also attest to a growing trend towards delayed motherhood in Spain. Moreover, an increasing number of couples are being diagnosed with infertility or conditions that make it difficult to become pregnant [].
The World Health Organization’s definition of infertility is a “disease of the male or female reproductive system defined by the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse” []. Infertility affects between 15% and 20% of couples of reproductive age in developed countries. In Spain, one in six couples have fertility issues, which is reflected in the growing demand for assisted reproductive technology (ART) procedures in recent years [,]. Concerns about rising infertility rates have intensified research into the environmental causes of infertility, although most research to date has centered on the influence of male infertility and sperm-related issues []. The main causes of female infertility are anovulation, endometriosis, fallopian tube disorders, pelvic adhesions, uterine anomalies, and diminished ovarian reserve [].
Ovarian reserve refers to the number and quality of oocytes and is an indicator of reproductive potential []. It is one of the most important factors for achieving natural pregnancy and a strong predictor of ART success. Ovarian reserve is inversely correlated with maternal chronological age, the main determinant of reproductive capacity and success. Reproductive aging is considered to accelerate after the age of 35 years []. The assessment of ovarian reserve is therefore an important step in both the evaluation and treatment of infertility. Markers of ovarian reserve include antral follicle count (AFC), assessed by transvaginal ultrasound, and serum levels of various biomarkers, such as anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) [,]. While these markers are easy to measure, few studies have investigated the causes of diminished ovarian reserve [].
Well-known factors associated with reduced oocyte quantity and quality are genetic alterations []; gynecological conditions, such as endometriosis, tumors, infections, and ovarian surgery; and eating disorders, such as anorexia nervosa [,]. Recent research has also highlighted the potential effects of environmental and lifestyle factors, such as chronic stress, exposure to certain endocrine disruptors, diet, and nutritional status [,,,].
Nutritional status is assessed using a range of anthropometric parameters, including body mass index (BMI), body fat composition (% of fat), body perimeters, and waist circumference or waist-to-hip ratio. BMI has been used for decades to classify individuals as underweight, normal weight, overweight, or obese []. Obesity has been defined as the pandemic of the 21st century due to its global effects on morbidity, mortality, quality of life, and health expenditure []. In 2020, 61.4% of men and 46.1% of women in Spain were considered to be overweight or obese []. Overweight and obesity are linked to an increased prevalence of reproductive disorders and chronic diseases, such as cardiovascular disease and certain cancers [,].
Several studies have shown that a BMI outside the normal range affects female reproductive capacity. Obesity has been associated with menstrual disorders, anovulation, hirsutism, and higher miscarriage and infertility rates []. Impaired fertility in women with a high BMI is linked to changes in the hypothalamic–pituitary–ovarian (HPO) axis that induces endocrine and metabolic disorders, with adipose tissue acting as a key regulator []. In obese women, the upregulation of enzymes involved in androgen metabolism in adipose tissue can cause hyperandrogenism. The increased peripheral aromatization of androgens to estrogens, combined with the reduced production of sex-hormone-binding globulin (SHBG), exerts negative feedback on the HPO axis in obese women, inhibiting folliculogenesis [].
Another physiological mechanism underlying the effects of obesity on reproductive capacity is the association between obesity and insulin resistance []. Obesity causes adipocyte hyperplasia and hypertrophy, resulting in a greater volume of adipose tissue. As an endocrine organ, adipose tissue secretes multiple proteins known as adipokines. The expansion of this tissue contributes to altered adipokine profiles, with a predominance of proinflammatory cytokines (mainly interleukin-6 and tumor necrosis factor-α), resulting in low-intensity chronic inflammation that can cause muscle and liver insulin resistance []. Obese women are thus prone to chronic low-grade inflammation and insulin resistance, both of which are associated with impaired reproductive function and late spontaneous abortion after ART [,].
Based on the evidence suggesting an important role for optimal nutritional status in fertility, we designed a systematic review to assess the influence of nutritional status on ovarian reserve in women of reproductive age.
2. Materials and Methods
We conducted a systematic review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework []. Study quality was assessed using the Cochrane Collaboration Risk of Bias tool [] (Higgins, J. et al., 2011), which consists of seven items covering six domains of bias. Each item is classified as having a high, low, or unclear risk of bias.
2.1. Data Sources
Electronic searches were carried out in the international databases Medline, ScienceDirect, and the Cochrane Library. Additional articles were identified by a hand search of the reference lists of identified articles.
2.2. Search Strategy
The search strategy was designed to identify full-text, published articles and included MeSH (Medical Subject Heading) terms and the terms title and abstract. The following keywords, transformed into MeSH terms, were used: “ovarian reserve”, “anti-Müllerian hormone”, “nutritional status”, and “body mass index” combined with the Boolean operators AND and OR. The search strategy used in PubMed is shown in Table 1.

Table 1.
Search strategy for PubMed.
2.3. Article Selection
Articles for full-text review were selected by screening the titles and abstracts of all publications yielded by the systematic search of Medline, ScienceDirect, and the Cochrane Library. The articles were independently reviewed by two authors, who checked the inclusion and exclusion criteria. The quality of each study was assessed by the same authors working separately using the Crombie criteria adapted by Petticrew and Roberts. Any discrepancies were resolved by a third author. For cross-sectional studies, the AXIS critical appraisal tool [] was used to assess quality and risk of bias (Table 2). The quality of cohort and case–control studies was assessed using the Newcastle–Ottawa Scale [] (Table 3). Randomized clinical trials were assessed using the PEDro tool [] (Table 4).

Table 2.
Quality of cross-sectional studies assessed using the AXIS critical appraisal tool.

Table 3.
Quality of cohort and case–control studies assessed using the Newcastle–Ottawa Scale.

Table 4.
PEDro Tool for randomized clinical trials.
The first and second authors (L.P.-H. and C.Á.P.-J.) independently scored each article, with discrepancies resolved by agreement with the third author (A.Z.-M.). Cohen’s kappa statistic (κ) was calculated to assess interrater reliability for risk of bias assessments. Assessment of blinding of participants or observers was not performed as all the studies were rated as high risk by both authors based on the overall items. Interrater reliability analyzed using Cohen’s κ yielded an intraclass correlation coefficient of 0.8.
2.4. Inclusion and Exclusion Criteria
The inclusion criteria were (1) open-access articles with an abstract and full text, (2) articles written in English or Spanish, (3) articles published between 2011 and 2021, and (4) articles that included women aged between 18 and 46 years. The exclusion criteria were (I) protocols and articles unrelated to the topic; (II) review articles, systematic reviews, and meta-analyses; (III) conference proceedings; (IV) studies involving women undergoing ART procedures; and (V) studies of women with a serious disease or a condition that could diminish ovarian reserve.
2.5. Data Extraction
The first author extracted all relevant data from the articles, namely, year of publication (2011–2021), study design and objectives, year of study conduct, sample size, mean participant age, country of origin, study results, and conclusions.
2.6. Synthesis of Results
The data extracted from the texts were grouped into two blocks to analyze, including (I) the variables used to assess ovarian reserve and nutritional status and (II) the association between ovarian reserve and nutritional status.
3. Results
The search yielded 103 articles. Five duplicate studies were removed before the selection process. Of the remaining 98 articles, 23 were excluded due to duplication and 55 due to exclusion criteria. Twenty studies were thus included in the systematic review (Figure 1).
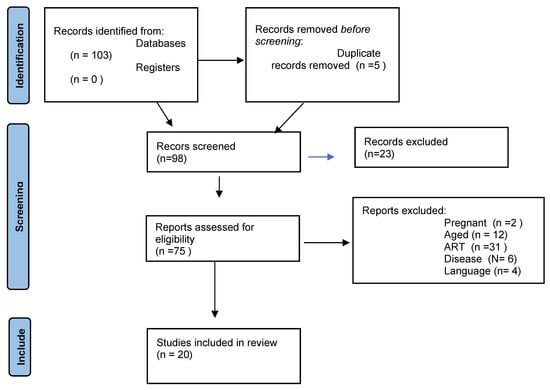
Figure 1.
Selection of the studies.
3.1. Description of Study Characteristics
The characteristics of the articles included in this systematic review are summarized in Table 5. Six articles were conducted in the USA [,,,,,], two in France [,], two in China [,], and one each in Taiwan [], Australia [], Scotland [], Turkey [], the United Arab Emirates [], Israel [], India [], Brazil [], Denmark [], and the Philippines []. The mean age of the study participants was 32.3 years.

Table 5.
Study characteristics.
There were eight cross-sectional studies [,,,,,,,], seven cohort studies [,,,,,,], four case–control studies [,,,], and one randomized clinical trial [].
The total number of participants analyzed in the 20 studies was 5929.
3.2. Description of Study Variables
The study variables used to assess nutritional status and ovarian reserve are summarized in Table 6. BMI was used to assess nutritional status in all the studies. Five studies also measured weight and height [,,,,], and one assessed waist circumference [].

Table 6.
Summary of variables used to assess nutritional status and ovarian reserve.
AMH was used exclusively to assess ovarian reserve in seven studies [,,,,,,]. Six studies used a combination of AMH and other serum biomarkers, namely FSH and LH [,,,,,]. Eleven studies used serum markers and AFC assessed by transvaginal ultrasound [,,,,,,,,,,].
Three studies evaluated biochemical parameters (cholesterol, high-density lipoprotein (HDL), and triglycerides) [,,], and five included smoking as a confounder [,,,,].
3.3. Relationship between BMI and Ovarian Reserve
Table 7 shows the relationship between BMI and AMH, together with the results and conclusions of each study. Five of the studies concluded that a high BMI was associated with diminished ovarian reserve based on below-normal serum AMH levels or AFC [,,,,]. One of these studies [], which divided the sample into three subgroups based on cardiometabolic, psychological, and reproductive profiles, concluded that low ovarian reserve was associated with cardiovascular and psychological factors. Another [] found correlations between a high BMI and diminished ovarian reserve, particularly in Caucasian women. Finally, one study reported that a high BMI had a negative effect on inhibin B levels [].

Table 7.
Summary of the relationship between BMI and ovarian reserve and study results and conclusions.
Two studies [,] indicated a possible positive relationship between a healthy cardiometabolic profile and AMH levels. Bleil et al., who investigated the association between variations in reproductive aging and cardiovascular risk factors, also determined that low and medium AMH levels were associated with a larger waist circumference and higher cholesterol, indicating the need for longitudinal studies to determine whether this association is mediated by BMI []. The same group, in a later study of ovarian reserve, reported that infertile women had a larger waist circumference than fertile women [].
Two studies of women with polycystic ovarian syndrome (PCOS) reported differences in ovarian reserve between women with a high and a normal BMI [,]. Both detected significant differences and found that women with PCOS and a BMI > 25 had lower AMH levels than those with a BMI within the normal range. Yang et al. also investigated associations between iron levels, obesity, and ovarian reserve in women with PCOS, finding evidence of diminished ovarian reserve and higher iron levels. These variables correlated with insulin resistance and reduced menstrual period frequency.
Another study investigating the association between AMH and metabolic syndrome in women with PCOS concluded that AMH was positively correlated with HDL cholesterol and SHBG and negatively correlated with glucose, insulin, blood glucose, BMI, and blood pressure []. Table 8 shows a summary of the relationships between BMI, AMH, and AFC.

Table 8.
Results according to the influence of BMI on AMH and AFC.
4. Discussion
The aim of this systematic review was to evaluate the association between nutritional status and ovarian reserve in women of reproductive age. Eleven of the twenty studies analyzed showed lower AMH levels in obese women [,,,,,,,,]. Five studies included BMI as a confounder [,,,,], and the other six found no evidence that nutritional status influenced ovarian reserve [,,,,,].
The above findings are consistent with previous reports. Vitek et al. studied the relationship between BMI, AMH, and oocyte yield in women undergoing in vitro fertilization; of the 29,895 women studied, 16,579 were obese, and 13,316 had a normal BMI. Compared to normal-weight women, women with a high BMI had lower AMH levels (1.8 ± 2.0 vs. 2.1 ± 2.0, p < 0.001) and a lower oocyte yield (11.9 ± 7.3 vs. 12.8 ± 7.7, p < 0.001) [].
Several publications have reported a negative correlation between BMI and AMH in women of late reproductive age [,]. Freeman et al., in a study of 122 women with a mean age of 45.8 ± 5.2 years, found that AMH levels were 65% lower in obese women than in non-obese women []. Steiner et al., who investigated the effects of oral contraceptive use and AMH levels in obese and normal-weight women, observed a 34% reduction in AMH levels in the former []. Another study observed a correlation between obesity and biochemical and ultrasound markers of ovarian reserve, showing lower AMH levels in obese women compared to normal-weight women of a similar age. AFC, by contrast, was similar in both groups []. Marca et al. found that AMH levels decreased with increasing BMI [].
Ovarian reserve can also be assessed by AFC. We observed lower oocyte quality and AFC in overweight and obese women, confirming previous findings [,,,,,]. Chronic inflammation caused by obesity induces ovarian oxidative stress, which affects the different stages of folliculogenesis (development, maturation, and ovulation). Some authors have indicated that obesity might affect oocyte quality via lipotoxicity, a mechanism marked by the persistent, unregulated release of cytokines from adipose tissue which have even been detected in follicular fluid [,,]. Several authors have suggested that ART could improve the chances of pregnancy in obese women, albeit with an increased risk of spontaneous abortion [,] and implantation failure []. These risks could be reduced by using oocytes from lean donors [,].
This systematic review confirms previous findings showing that metabolic syndrome, understood as a combination of central obesity, elevated blood pressure, elevated triglycerides, elevated fasting glucose, and reduced HDL-cholesterol, has an important role in female fertility. Cardozo et al. suggested that metabolic syndrome might also affect endometrial receptivity as it appears to influence both oocyte and embryo quality []. Similarly, Snider et al. found that obesity-dependent changes in the gut microbiome contributed to lower oocyte quality [].
Several studies have reported contrasting findings on the link between nutritional status and ovarian reserve [,,,,,], possibly because of the use of small samples with few obese women. Dolleman et al., for instance, in a study of 2320 women of reproductive age, found that AMH levels were lower in oral contraceptive users and did not correlate with BMI []. Sahmay et al. suggested that reduced fertility rates in obese women are more likely to be due to impaired endometrial receptivity [].
This systematic review has some limitations. First, we may have missed some evidence as we only analyzed studies published in English and Spanish. Second, we searched just three databases: Medline, Science Direct, and the Cochrane Library. Future studies could target additional databases such as Web of Science and Embase. Third, we excluded studies of women undergoing ART procedures as low ovarian reserve is not the only reason for the use of these treatments. The main strength of this study is that it is one of the few systematic reviews to evaluate the influence of nutritional status on ovarian reserve in women of reproductive age.
5. Conclusions
Nutritional status can influence ovarian reserve in women of reproductive age. Overweight and obesity have a negative impact on ovarian function as women with a high BMI had significantly lower AMH levels and AFC than those with a normal BMI. Overweight and obesity can also affect oocyte quality, leading to higher rates of subfertility and infertility and a greater demand for ART procedures. Suboptimal nutritional status, however, can jeopardize the chances of ART success by inducing a detrimental inflammatory environment in the ovaries.
Future studies are needed to inform the design of prevention and health promotion strategies to improve the nutritional status of women of reproductive age.
Author Contributions
Conceptualization, A.Z.-M., V.S.D.L.C.-D. and M.S.-S.; methodology, L.P.-H. and C.Á.P.-J.; validation, A.Z.-M., V.S.D.L.C.-D. and M.B.G.-V.; formal analysis, L.M.-M.; resources, C.T.-T.; data curation, A.Z.-M.; writing—original draft preparation, A.Z.-M., V.S.D.L.C.-D., M.S.-S., L.P.-H., C.Á.P.-J., M.B.G.-V., L.M.-M. and C.T.-T.; writing—review and editing, A.Z.-M.; visualization, A.Z.-M. and V.S.D.L.C.-D.; supervision, A.Z.-M. and V.S.D.L.C.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data can be found in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Instituto Nacional de Estadística. Indicadores de Fecundidad: Edad Media a la Maternidad Por Orden Del Nacimiento Según Nacionalidad (Española/Extranjera) De La Madre. 2021. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=1579 (accessed on 19 January 2023).
- Sociedad Española de Fertilidad. Libro Blanco Sociosanitario de la SEF: La Infertilidad en España: Situación Actual y Perspectivas. Imago Concept & Image Development, S.L. 2021. Available online: https://www.sefertilidad.net/docs/biblioteca/libros/libroBlanco.pdf (accessed on 19 January 2023).
- World Health Organization. Health topics: Infertility. 2020. Available online: https://www.who.int/es/health-topics/infertility#tab=tab_1 (accessed on 19 January 2023).
- Ministerio de Sanidad. Sociedad Española de Fertilidad. Informe estadístico de Técnicas de Reproducción Asistida 2018. Registro SEF. 2020. Available online: https://cnrha.sanidad.gob.es/registros/pdf/Informe_estadistico_SEF_2018_Version_Final.pdf (accessed on 20 January 2023).
- Cutillas-Tolin, A.; Adoamnei, E.; Navarrete-Muñoz, E.M.; Vioque, J.; Moñino-García, M.; Jorgensen, N.; Chavarro, J.E.; Mendiola, J.; Torres-Cantero, M. Adherence to diet quality índices in relation to semen quality and reproductive hormones in young men. Hum. Reprod. 2019, 34, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Cala, A.; Fajardo, D.; Scott, R. Factores causales de infertilidad. Rev. Inf. Científica 2019, 98, 283–293. [Google Scholar]
- Ruiz-Hoyos, B. Evaluación de la reserva ovárica: Pasado, presente y futuro. RevistaMed 2020, 28, 77–88. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.; Lambalk, C.B. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 2006, 12, 685–718. [Google Scholar] [CrossRef] [PubMed]
- Jirge, P. Poor ovarian reserve. J. Hum. Reprod. Sci. 2016, 9, 63–69. [Google Scholar] [CrossRef]
- Gasparri, M.; Di Micco, R.; Zuber, V.; Taghavi, K.; Bianchini, G.; Bellaminutti, S.; Meani, F.; Graffeo, R.; Candiani, M.; Mueller, M.D.; et al. Ovarian reserve of women with and without BRCA pathogenic variants: A systematic review and meta-analysis. Breast 2021, 60, 155–162. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ahmed, S.M.; El-Gammal, Z.; Shouman, S.; Ahmed, A.; Mansour, R.; El-Badri, N. Oocyte Aging: The Role of Cellular and Environmental Factors and Impact on Female Fertility. Adv. Exp. Med. Biol. 2020, 1247, 109–123. [Google Scholar] [CrossRef]
- Piazza, M.; Urbanetz, A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019, 232, 154–164. [Google Scholar] [CrossRef]
- Moslehi, N.; Shab-Bidar, S.; Ramezani, F.; Mirmiran, P.; Azizi, F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause 2018, 25, 1046–1055. [Google Scholar] [CrossRef]
- World Health Organization Health Topics: Body Mass Index—BMI. 2022. Available online: https://www.euro.who.int/en/healthtopics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bm (accessed on 19 January 2023).
- Ceballos-Macías, J.; Pérez, J.; Flores-Real, J.; Vargas-Sánchez, J.; Ortega-Gutiérrez, G.; Madriz-Prado, R.; Hernández-Moreno, A. Obesidad. Pandemia del siglo XXI. Rev. De Sanid. Mil. 2018, 72, 332–338. [Google Scholar]
- Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Tordecilla, G. Estados hiperandrogénicos: Revisión de la literatura. Rev. Colomb. De Obstet. Y Ginecol. 2009, 60, 357–364. [Google Scholar] [CrossRef]
- Barrios-De-Tomasi, J.; Barrios-De-Tomasi, E.; Vergara-Galicia, J. Efecto de la obesidad en la reproducción femenina. Rev. Mex. Cienc. Farm 2013, 44, 8–18. [Google Scholar]
- Carvajal, C. Tejido adiposo, obesidad e insulino resistencia. Med. Leg. De Costa Rica 2015, 32, 138–144. [Google Scholar]
- Pollak, F.; Araya, V.; Lanas, A.; Sapunar, J.; Arrese, M.; Aylwin, C.; Bezanilla, C.G.; Carrasco, E.; Carrasco, F.; Codner, E.; et al. II Consenso de la Sociedad Chilena de Endocrinología y Diabetes sobre resistencia a la insulina. Rev. Médica De Chile 2015, 143, 627–636. [Google Scholar]
- Yang, T.; Yang, Y.; Zhang, Q.; Liu, D.; Liu, N.; Li, Y.; Yao, Z.; Zhang, Y.; Tian, F.; Zhao, J.; et al. Homeostatic Model Assessment for Insulin Resistance Is Associated With Late Miscarriage in Non-Dyslipidemic Women Undergoing Fresh IVF/ICSI Embryo Transfer. Front. Endocrinol. 2022, 13, 880518. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 34, 103–112. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’ Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 19 January 2023).
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef]
- Yang, J.; Chou, C.; Yang, W.; Ho, H.; Yang, Y.; Chen, M. Iron stores and obesity are negatively associated with ovarian volume and anti-Müllerian hormons levels in women with polycystic ovary syndrome. Taiwan. J. Obstet. Gynecol. 2015, 54, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.; Cedars, M.; Santoro, N.; Eisenberg, E.; Kao, C.; Haisenleder, D.; Diamond, M.P.; Huddleston, H.G. Antimüllerian hormone levels and antral follicle counts are not reduced compared with community controls in patients with rigorously defined unexplained infertility. Fertil. Steril. 2017, 108, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Moy, V.; Jindal, S.; Lieman, H.; Buyuk, E. Obesity adversely affects serum anti-müllerian hormone (AMH) levels in Caucasian women. J. Assist. Reprod. Genet. 2015, 32, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Garrett-Mayer, E.; Mittal, N.; Smith, K.; Shulman, L.; Passaglia, C.; Diamond, M.P.; Huddleston, H.G. Association of BRCA1 mutations with impaired ovarian reserve: Connection between infertility and Breast/Ovarian cancer risk. J. Adolesc. Young Adult Oncol. 2016, 5, 337–343. [Google Scholar] [CrossRef]
- Bleil, M.E.; Gregorich, S.E.; McConnell, D.; Rosen, M.P.; Cedars, M.I. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause 2013, 20, 1139–1146. [Google Scholar] [CrossRef]
- Feldman, R.; O’Neill, K.; Butts, S.; Dokras, A. Antimullerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Ferility Steril. 2017, 107, 276–281. [Google Scholar] [CrossRef]
- Phillips, K.; Collins, I.; Milne, R.; McLachlan, S.; Friedlander, M.; Hickey, M.; Stern, C.; Hopper, J.L.; Fisher, R.; Kannemeyer, G.; et al. Anti-Müllerian hormone serum concentrations of women with germline BRCA I or BRCA 2 mutations. Hum. Reprod. 2016, 31, 1126–1132. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Tsui, K. Serum testosterone levels are positively associated with serum anti-mullerian hormone levels in infertile women. Sci. Rep. 2021, 11, 6336. [Google Scholar] [CrossRef]
- Malhotra, N.; Bahadur, A.; Singh, N.; Kalaivani, M.; Mittal, S. Does obesity compromise ovarian reserve markers? A clinician’s perspective. Arch. Gynecol. Obstet. 2013, 287, 161–166. [Google Scholar] [CrossRef]
- Berwagner da Silva, A.; Da Ré, C.; Dietrich, C.; Fuhrmeister, I.; Pimentel, A.; Von Eye, H. Impact of tubal ligation on ovarian reserve as measured by anti-Müllerian hormone levels: A prospective cohort study. Contraception 2013, 88, 700–705. [Google Scholar] [CrossRef]
- Hvidman, H.; Bentzen, J.; Thuesen, L.; Lauritsen, M.; Forman, J.; Loft, A.; Pinborg, A.; Andersen, A.N. Infertil women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum. Reprod. 2016, 31, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Lambert-Messerlian, G.; Plante, B.; Eklund, E.; Raker, C.; Moore, R. Levels of antimüllerian hormone in serum during the normal menstrual cycle. Fertil. Steril. 2016, 105, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Bragg, J.; Kuzawa, C.; Agustin, S.; Banerjee, M.; McDade, T. Age at menarche and parity are independently associated with Anti-Müllerian hormone, a marker of ovarian reserve, in Filipino young adult women. Am. J. Hum. Biol. 2012, 24, 739–745. [Google Scholar] [CrossRef]
- Tabbalat, A.; Pereira, N.; Klauck, D.; Melhem, C.; Elias, R.; Rosenwaks, Z. Arabian Peninsula ethnicity is associated with lower ovarian reserve and ovarian response in women undergoing fresh ICSI cycles. J. Assist. Reprod. Genet. 2018, 35, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Garnier-Villarreal, M.; McCarthy, D.; Anderson, R.; Reynolds, R. Exploring the Ovarian Reserve within Health Parameters: A Latent Class Analysis. West. J. Nursering Res. 2018, 40, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Makolle, S.; Catteau-Jonard, S.; Robin, G.; Dewailly, D. Revisiting the serum level of anti-Müllerian hormone in patients with functional hypothalamic anovultation. Hum. Reprod. 2021, 36, 1043–1051. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, T.; Song, L.; Yang, M.; Sun, X.; Tian, L. The status and comparison of ovarian reserve between fertile and infertile healthy Chinese women of reproductive age. Medicine 2021, 100, e25361. [Google Scholar] [CrossRef]
- Lefebvre, T.; Dumont, A.; Pigny, P.; Dewailly, D. Effect of obesity and its related metabolic factors on serum anti-Müllerian hormone concentrations in women with and without plycystic ovaries. Reprod. Biomed. Online 2017, 35, 325–330. [Google Scholar] [CrossRef]
- Sahin, A.; Karakus, S.; Durmaz, Y.; Yildiz, Y.; Aydin, H.; Cenglz, A. Ovarian reserve is preserved in Behçet’s disease. Int. J. Rheum. Dis. 2015, 20, 2070–2076. [Google Scholar] [CrossRef]
- Ganer, H.; Gluck, O.; Keidar, R.; Kerner, R.; Kovo, M.; Levran, D.; Bar, J.; Sagiv, R. Ovarian reserve following cesarean section with salpingectomy vs tubal ligation: A randomized trial. Am. J. Obstet. Gynecol. 2017, 217, 472. [Google Scholar] [CrossRef]
- Vitek, W.; Sun, F.; Baker, V.L.; Styer, A.K.; Christianson, M.S.; Stern, J.E.; Zhang, H.; Polotsky, A.J. Lower antimüllerian hormone is associated with lower oocyte yield but not live-birth rate among women with obesity. Am. J. Obstet. Gynecol. 2020, 222, 363.e1–363.e7. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Gracia, C.R.; Sammel, M.D.; Lin, H.; Lim, L.C.; Strauss, J.F. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 2007, 87, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Xu, Y.; Cao, Z.; Wang, Y.; Hao, G.; Bu-Lang, G. High BMI and Insulin Resistance Are Risk Factors for Spontaneous Abortion in Patients With Polycystic Ovary Syndrome Undergoing Assisted Reproductive Treatment: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 592495. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.Z.; Stanczykb, F.Z.; Patelb, S.; Edelmanc, A. Antimullerian hormone and obesity:insights in oral contraceptive users. Contraception 2010, 81, 254–258. [Google Scholar] [CrossRef]
- Su, H.I.; Sammel, M.D.; Freeman, E.W.; Lin, H.; DeBlasis, T.; Gracia, C.R. Body size affects measures of ovarian reserve in late reproductive age women. Menopause 2008, 15, 857–861. [Google Scholar] [CrossRef]
- La Marca, A.; Sighinolfi, G.; Giulini, S.; Traglia, M.; Argento, C.; Sala, C.; Masciullo, C.; Volpe, A.; Toniolo, D. Normal serum concentrations of anti-Müllerian hormone in women with regular menstrual cycles. Reprod. Biomed. Online 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Dolleman, M.; Verschuren, W.M.M.; Eijkemans, M.J.C.; Dollé, M.E.T.; Jansen, E.H.J.M.; Broekmans, F.J.M.; van der Schouw, Y.T. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J. Clin. Endocrinol. Metabol. 2013, 98, 2106–2115. [Google Scholar] [CrossRef]
- Pohlmeier, W.E.; Xie, F.; Kurz, S.G.; Lu, N.; Wood, J.R. Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol. Reprod. Dev. 2014, 81, 735–747. [Google Scholar] [CrossRef]
- Purcell, S.H.; Moley, K.H. The impact of obesity on egg quality. J. Assist. Reprod. Genet. 2011, 28, 517–524. [Google Scholar] [CrossRef]
- Wu, L.L.; Norman, R.J.; Robker, R.L. The impact of obesity on oocytes:evidence for lipotoxicity mechanisms. Reprod. Fertil. Dev. 2011, 24, 29–34. [Google Scholar] [CrossRef]
- Snider, A.P.; Wood, J.R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef] [PubMed]
- Kawwass, J.F.; Kulkarni, A.D.; Hipp, H.S.; Crawford, S.; Kissin, D.M.; Jamieson, D.J. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil. Steril. 2016, 106, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Melo, M.A.B.; Bosch, E.; Serra, V.; Remohí, J.; Pellicer, A. Obesity and poor reproductive outcome: The potential role of the endometrium. Fertil. Steril. 2007, 88, 446–451. [Google Scholar] [CrossRef]
- Levens, E.D.; Skarulis, M.C. Assessing the role of endometrial alteration among obese patients undergoing assisted reproduction. Fertil. Steril. 2008, 89, 1606–1608. [Google Scholar] [CrossRef]
- Cardozo, E.; Pavone, M.E.; Hirshfeld-Cytron, J. Metabolic syndrome and oocyte quality. Trends Endocrinol. Metab. 2011, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sahmay, S.; Usta, T.; Erel, C.T.; Imamoglu, M.; Küçük, M.; Atakul, N.; Seyinsoglu, H. Is there any correlation between AMH and obesity in premenopausal women? Arch. Gynecol. Obstet. 2012, 286, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Halawaty, S.; Elkattan, E.; Azab, H.; ElGhamry, N.; Al-Inany, H. Effects of obesity on parameters of ovarian reserve in premenopausal women. J. Obstet. Gynaecol. Can. 2010, 32, 687–690. [Google Scholar] [CrossRef]
- Nardo, L.G.; Christodoulou, D.; Gould, D.; Roberts, S.A.; Fitzgeral, C.T.; Laing, I. Anti-mullerian hormone levels and antral follicle count in women enrolled in vitro fertilization cycles:relationship to lifestyle factors, chronological age and reproductive history. Gynecol. Endocrinol. 2007, 23, 486–493. [Google Scholar] [CrossRef]
- Park, A.S.; Lawson, M.A.; Chuan, S.S.; Oberfield, S.E.; Hoeger, K.M.; Witchel, S.F.; Chang, R.F. Serum anti-mullerian hormone concentrations are elevated in oligomenorrheic girls without evidence of hyperandrogenism. J. Clin. Endocrinol. Metab. 2010, 95, 1786–1792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).