Low Serum Vitamin D Status Is Associated with Incident Alzheimer’s Dementia in the Oldest Old
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
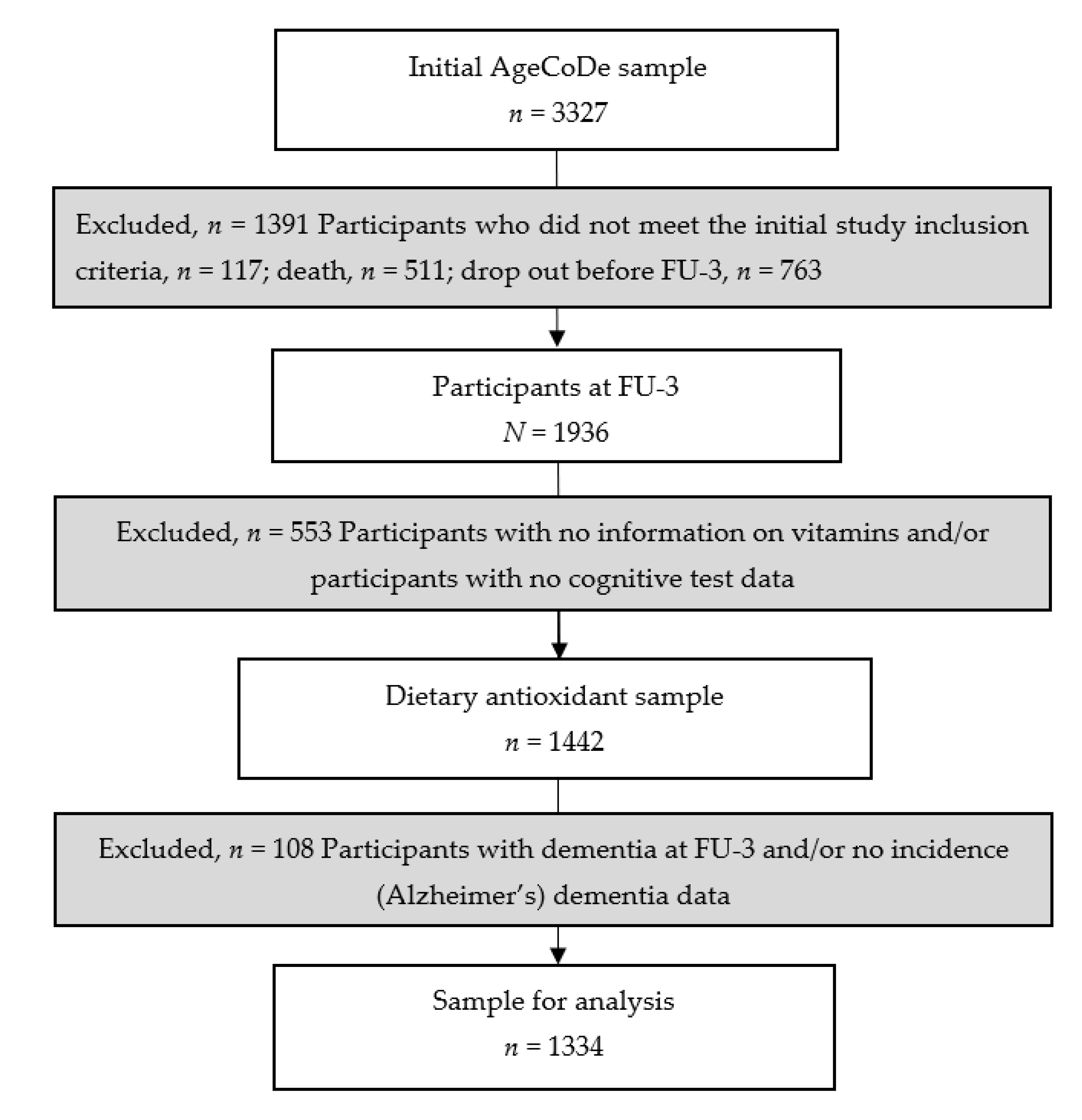
2.2. Study Participants
2.3. Blood Concentrations of Vitamins A, D, and E, Beta-Carotene, Creatinine, Total Cholesterol and Triglycerides
2.4. Assessment and Diagnosis of Dementia
2.5. Confounders
2.6. Statistical Analysis
3. Results
3.1. Longitudinal Associations between Vitamins and Beta-Carotene and Incidence of All-Cause Dementia
3.2. Longitudinal Associations between Vitamins and Beta-Carotene and Incident AD
3.3. Effect Modification
3.4. Longitudinal Associations between Vitamins and Incidence of Vascular Dementia
4. Discussion
4.1. Findings in Other Studies
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Jicha, G.A.; Carr, S.A. Conceptual evolution in Alzheimer’s disease: Implications for understanding the clinical phenotype of progressive neurodegenerative disease. J. Alzheimers Dis. 2010, 19, 253–272. [Google Scholar] [CrossRef]
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernandez-Tajes, J.; Pasaro, E.; Valdiglesias, V.; Laffon, B. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2286. [Google Scholar] [CrossRef]
- Welch, A. Micronutrient malnutrition across the life course, sarcopenia and frailty. Proc. Nutr. Soc. 2021, 80, 279–282. [Google Scholar] [CrossRef]
- Grimm, M.O.; Mett, J.; Hartmann, T. The Impact of Vitamin E and Other Fat-Soluble Vitamins on Alzheimers Disease. Int. J. Mol. Sci. 2016, 17, 1785. [Google Scholar] [CrossRef]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef]
- Nesaretnam, K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008, 269, 388–395. [Google Scholar] [CrossRef]
- Galli, F.; Polidori, M.C.; Stahl, W.; Mecocci, P.; Kelly, F.J. Vitamin E biotransformation in humans. Vitam. Horm. 2007, 76, 263–280. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. Geriatr. J. 2015, 18, 231–245. [Google Scholar] [CrossRef]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; Melo van Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases-An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef]
- Mangialasche, F.; Kivipelto, M.; Mecocci, P.; Rizzuto, D.; Palmer, K.; Winblad, B.; Fratiglioni, L. High plasma levels of vitamin E forms and reduced Alzheimer’s disease risk in advanced age. J. Alzheimers Dis. 2010, 20, 1029–1037. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Kareholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Lucicesare, A.; Pisacane, N.; Rietti, E.; Mangialasche, F.; Cecchetti, R.; Patterson, C.; Mecocci, P. Plasma tocopherols and risk of cognitive impairment in an elderly Italian cohort. Am. J. Clin. Nutr. 2008, 87, 1306–1313. [Google Scholar] [CrossRef]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 1999, 92, 39–45. [Google Scholar] [CrossRef]
- Luck, T.; Riedel-Heller, S.G.; Kaduszkiewicz, H.; Bickel, H.; Jessen, F.; Pentzek, M.; Wiese, B.; Koelsch, H.; van den Bussche, H.; Abholz, H.H.; et al. Mild cognitive impairment in general practice: Age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe). Dement. Geriatr. Cogn. Disord. 2007, 24, 307–316. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bickel, H.; Eifflander-Gorfer, S.; Fuchs, A.; Kaduszkiewicz, H.; Kohler, M.; Luck, T.; Mosch, E.; Pentzek, M.; et al. Prediction of dementia in primary care patients. PLoS ONE 2011, 6, e16852. [Google Scholar] [CrossRef]
- Erhardt, J.G.; Heinrich, F.; Biesalski, H.K. Determination of retinol, antioxidant vitamins and homocysteine in skin puncture blood. Int. J. Vitam. Nutr. Res. 1999, 69, 309–314. [Google Scholar] [CrossRef]
- Rili-BÄK. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen-Rili-BÄK. Deutsches Ärzteblatt 2014, 111, 19. [Google Scholar]
- Zaudig, M.; Mittelhammer, J.; Hiller, W.; Pauls, A.; Thora, C.; Morinigo, A.; Mombour, W. SIDAM--A structured interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia and dementias of other aetiology according to ICD-10 and DSM-III-R. Psychol. Med. 1991, 21, 225–236. [Google Scholar] [CrossRef]
- Zaudig, M.; Hiller, W. SIDAM-Handbuch Strukturiertes Interview Für Die Diagnose Einer Demenz Vom Alzheimer Typ, der Multiinfarkt-(Oder Vaskulären) Demenz und Demenzen Anderer Ätiologie Nach DSM-III-R, DSM-IV, ICD-10; Hans Huber: Bern, Switzerland, 1996. [Google Scholar]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Roman, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Blessed, G.; Tomlinson, B.E.; Roth, M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychiatry 1968, 114, 797–811. [Google Scholar] [CrossRef]
- Brauns, H.; Steinmann, S. Educational reform in France, West-Germany and the United Kingdom: Updating the CASMIN educational classification. ZUMA Nachr. 1999, 44, 7–44. [Google Scholar]
- Hixson, J.E.; Vernier, D.T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990, 31, 545–548. [Google Scholar] [CrossRef]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef]
- Thalmann, B.; Monsch, A.U.; Schneitter, M.; Bernasconi, F.; Aebi, C.; Camachova-Davet, Z.; Staehelin, H.B. The cerad neuropsychological assessment battery (Cerad-NAB)—A minimal data set as a common tool for German-speaking Europe. Neurobiol. Aging 2000, 21, 30. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014, 10, 296–302. [Google Scholar] [CrossRef]
- Annweiler, C.; Rolland, Y.; Schott, A.M.; Blain, H.; Vellas, B.; Beauchet, O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: A 7-year longitudinal study. Dement. Geriatr. Cogn. Disord. 2011, 32, 273–278. [Google Scholar] [CrossRef]
- Knekt, P.; Saaksjarvi, K.; Jarvinen, R.; Marniemi, J.; Mannisto, S.; Kanerva, N.; Heliovaara, M. Serum 25-hydroxyvitamin d concentration and risk of dementia. Epidemiology 2014, 25, 799–804. [Google Scholar] [CrossRef]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement. 2017, 13, 1207–1216. [Google Scholar] [CrossRef]
- Licher, S.; de Bruijn, R.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.; Ikram, M.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimers Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef]
- Graf, C.E.; Rossi, C.; Giannelli, S.V.; Nobari, B.H.; Gold, G.; Herrmann, F.R.; Zekry, D. Vitamin D is not associated with cognitive status in a cohort of very old hospitalized patients. J. Alzheimers Dis. 2014, 42 (Suppl. 3), S53–S61. [Google Scholar] [CrossRef]
- Schneider, A.L.; Lutsey, P.L.; Alonso, A.; Gottesman, R.F.; Sharrett, A.R.; Carson, K.A.; Gross, M.; Post, W.S.; Knopman, D.S.; Mosley, T.H.; et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC Brain MRI Study. Eur. J. Neurol. 2014, 21, 1211-e70. [Google Scholar] [CrossRef] [PubMed]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of Serum Vitamin D with the Risk of Incident Dementia and Subclinical Indices of Brain Aging: The Framingham Heart Study. J. Alzheimers Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Byberg, L.; Karlstrom, B.; Cederholm, T.; Melhus, H.; Sjogren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. J. Alzheimers Dis. 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Lloret, A.; Giraldo, E.; Badia, M.C.; Alonso, M.D. Antioxidant pathways in Alzheimer’s disease: Possibilities of intervention. Curr. Pharm. Des. 2011, 17, 3861–3864. [Google Scholar] [CrossRef] [PubMed]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Feron, F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimers Dis. 2016, 53, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 16. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; de Groot, L.C. Vitamin D and cognition in older adults: An update of recent findings. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 11–16. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008, 22, 982–1001. [Google Scholar] [CrossRef]
- Cui, X.; Pelekanos, M.; Liu, P.Y.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 2013, 236, 77–87. [Google Scholar] [CrossRef]
- Eyles, D.W.; Liu, P.Y.; Josh, P.; Cui, X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience 2014, 268, 1–9. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Yilmazer, S.; Dursun, E. Why vitamin D in Alzheimer’s disease? The hypothesis. J. Alzheimers Dis. 2014, 40, 257–269. [Google Scholar] [CrossRef]
- Kang, J.H.; Grodstein, F. Plasma carotenoids and tocopherols and cognitive function: A prospective study. Neurobiol. Aging 2008, 29, 1394–1403. [Google Scholar] [CrossRef]
- Schmidt, R.; Hayn, M.; Reinhart, B.; Roob, G.; Schmidt, H.; Schumacher, M.; Watzinger, N.; Launer, L.J. Plasma antioxidants and cognitive performance in middle-aged and older adults: Results of the Austrian Stroke Prevention Study. J. Am. Geriatr. Soc. 1998, 46, 1407–1410. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Ruitenberg, A.; Meijer, J.; Kiliaan, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Plasma levels of antioxidants are not associated with Alzheimer’s disease or cognitive decline. Dement. Geriatr. Cogn. Disord. 2005, 19, 134–139. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids, alpha-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mecocci, P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J. Alzheimers Dis. 2002, 4, 517–522. [Google Scholar] [CrossRef]
- Schippling, S.; Kontush, A.; Arlt, S.; Buhmann, C.; Sturenburg, H.J.; Mann, U.; Muller-Thomsen, T.; Beisiegel, U. Increased lipoprotein oxidation in Alzheimer’s disease. Free Radic. Biol. Med. 2000, 28, 351–360. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, F.J.; Molina, J.A.; de Bustos, F.; Garcia-Redondo, A.; Gomez-Escalonilla, C.; Martinez-Salio, A.; Berbel, A.; Camacho, A.; Zurdo, M.; Barcenilla, B.; et al. Serum levels of coenzyme Q10 in patients with Parkinson’s disease. J. Neural Transm. (Vienna) 2000, 107, 177–181. [Google Scholar] [CrossRef]
- von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G.; Acti, F.E.U.S.G. Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef]
- Hu, P.; Bretsky, P.; Crimmins, E.M.; Guralnik, J.M.; Reuben, D.B.; Seeman, T.E. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 616–620. [Google Scholar] [CrossRef]
- Cherubini, A.; Martin, A.; Andres-Lacueva, C.; Di Iorio, A.; Lamponi, M.; Mecocci, P.; Bartali, B.; Corsi, A.; Senin, U.; Ferrucci, L. Vitamin E levels, cognitive impairment and dementia in older persons: The InCHIANTI study. Neurobiol. Aging 2005, 26, 987–994. [Google Scholar] [CrossRef]
- Farina, N.; Llewellyn, D.; Isaac, M.G.; Tabet, N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 1, CD002854. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martinez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Dysken, M.W.; Guarino, P.D.; Vertrees, J.E.; Asthana, S.; Sano, M.; Llorente, M.; Pallaki, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; et al. Vitamin E and memantine in Alzheimer’s disease: Clinical trial methods and baseline data. Alzheimers Dement. 2014, 10, 36–44. [Google Scholar] [CrossRef]
- Lloret, A.; Badia, M.C.; Mora, N.J.; Pallardo, F.V.; Alonso, M.D.; Vina, J. Vitamin E paradox in Alzheimer’s disease: It does not prevent loss of cognition and may even be detrimental. J. Alzheimers Dis. 2009, 17, 143–149. [Google Scholar] [CrossRef]
- Comstock, G.W.; Burke, A.E.; Hoffman, S.C.; Norkus, E.P.; Gross, M.; Helzlsouer, K.J. The repeatability of serum carotenoid, retinoid, and tocopherol concentrations in specimens of blood collected 15 years apart. Cancer Epidemiol. Biomark. Prev. 2001, 10, 65–68. [Google Scholar]
- Holvoet, P.; Macy, E.; Landeloos, M.; Jones, D.; Jenny, N.S.; Van de Werf, F.; Tracy, R.P. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin. Chem. 2006, 52, 760–764. [Google Scholar] [CrossRef]
| Characteristics | Main Sample |
|---|---|
| n = 1334 | |
| Age (years) | 84 ± 3 |
| Female (n, %) | 861 (64.5) |
| BMI (kg/m2) | 25.9 ± 3.7 |
| APOE ε4 allele (n, %) | 257 (19.3) |
| Time to develop all-cause dementia (years) | 3 ± 2 |
| Time to censoring (years) | 5 ± 2 |
| Serum/plasma analyses | |
| Vitamin A (mg/L) | 0.54 ± 0.23 |
| Beta-carotene (mg/L) | 0.32 (IQR: 0.22–0.48) |
| Vitamin D (nmol/L) | 37.0 (IQR: 24.8–58.3) |
| Vitamin E (mg/L) | 15.73 ± 6.33 |
| Creatinine (mg/dL) | 1.00 (IQR: 0.83–1.22) |
| Total cholesterol (g/L) | 2.21 ± 0.48 |
| Triglycerides (g/L) | 1.11 (IQR: 0.87–1.49) |
| Education (n, %) | |
| Lower | 779 (58.4) |
| Middle | 396 (29.7) |
| High | 159 (11.9) |
| Physical activity (n, %) | |
| Low (0–1) | 114 (8.5) |
| Middle (2) | 906 (67.9) |
| High (3–5) | 314 (23.5) |
| Smoking (n, %) | |
| Never | 681 (51.0) |
| Past | 569 (42.7) |
| Current | 84 (6.3) |
| Vitamin supplement intake (n, %) | 84 (6.3) |
| Systolic blood pressure (mmHg) | 136 ± 16 |
| Diastolic blood pressure (mmHg) | 80 (IQR: 70–80) |
| ACE inhibitors usage (n, %) | 47 (3.5) |
| Calcium channel blockers usage (n, %) | 5 (0.4) |
| Ginkgo biloba usage (n, %) | 10 (0.8) |
| Laxative usage (n, %) | 9 (0.9) |
| Incident All-Cause Dementia (n = 250 Cases) | p-Values for Interaction | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Sex | APOE ε4 | |||
| Vitamins | HR (95% CI) | p | HR (95% CI) | p | ||
| Vitamin A (mg/L) | 1.22 (0.72; 2.07) | 0.453 | 1.22 (0.68; 2.18) | 0.508 | - | - |
| Beta-carotene (mg/L) | 0.96 (0.64; 1.44) | 0.850 | 1.02 (0.67; 1.57) | 0.928 | 0.03 | - |
| Vitamin E (mg/L) | 1.01 (0.99; 1.03) | 0.378 | 1.01 (0.99; 1.03) | 0.500 | - | - |
| Vitamin D (mmol/L) | 0.99 (0.98; 0.99) | 0.008 | 0.99 (0.98; 0.99) | 0.015 | - | - |
| Vitamin D cut-offs | ||||||
| ≥50 (mmol/L) (n = 449) | Reference | Reference | ||||
| ≥25–<50 (mmol/L) (n = 548) | 1.10 (0.80; 1.49) | 0.568 | 1.19 (0.84; 1.67) | 0.323 | ||
| <25 (mmol/L) (n = 337) | 1.75 (1.28; 2.40) | 0.001 | 1.91 (1.30; 2.81) | 0.001 | ||
| Incidence of AD (n = 209 Cases) | p-Values for Interaction | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Sex | APOE ε4 | |||
| Vitamins | HR (95% CI) | p | HR (95% CI) | p | ||
| Vitamin A (mg/L) | 1.11 (0.61; 2.01) | 0.735 | 1.02 (0.52; 2.00) | 0.950 | - | - |
| Beta-carotene (mg/L) | 0.96 (0.61; 1.49) | 0.838 | 0.98 (0.61; 1.57) | 0.939 | 0.01 | - |
| Vitamin E (mg/L) | 1.01 (0.99; 1.03) | 0.173 | 1.01 (0.99; 1.04) | 0.270 | - | - |
| Vitamin D (mmol/L) | 0.98 (0.98; 0.99) | 0.001 | 0.99 (0.98; 0.99) | 0.003 | - | - |
| Vitamin D cut-offs | ||||||
| ≥50 (mmol/L) (n = 449) | Reference | Reference | ||||
| ≥25–<50 (mmol/L) (n = 548) | 1.41 (0.99; 1.99) | 0.056 | 1.52 (1.04; 2.22) | 0.031 | ||
| <25 (mmol/L) (n = 337) | 2.06 (1.44; 2.96) | <0.001 | 2.28 (1.47; 3.53) | <0.001 | ||
| Incidence All-Cause Dementia (n = 250 Cases) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| By sex | ||||
| Beta-carotene (mg/L) | ||||
| Men (n = 473) | 1.59 (0.79; 3.19) | 0.195 | 1.32 (0.63; 2.77) | 0.458 |
| Women (n = 861) | 0.81 (0.49; 1.34) | 0.414 | 0.93 (0.55; 1.58) | 0.790 |
| Incidence of AD (n = 209 Cases) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| By sex | ||||
| Beta-carotene (mg/L) | ||||
| Men (n = 473) | 1.87 (0.92; 3.63) | 0.086 | 1.33 (0.64; 2.78) | 0.444 |
| Women (n = 861) | 0.73 (0.41; 1.31) | 0.293 | 0.83 (0.45; 1.54) | 0.555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo van Lent, D.; Egert, S.; Wolfsgruber, S.; Kleineidam, L.; Weinhold, L.; Wagner-Thelen, H.; Stoffel-Wagner, B.; Bickel, H.; Wiese, B.; Weyerer, S.; et al. Low Serum Vitamin D Status Is Associated with Incident Alzheimer’s Dementia in the Oldest Old. Nutrients 2023, 15, 61. https://doi.org/10.3390/nu15010061
Melo van Lent D, Egert S, Wolfsgruber S, Kleineidam L, Weinhold L, Wagner-Thelen H, Stoffel-Wagner B, Bickel H, Wiese B, Weyerer S, et al. Low Serum Vitamin D Status Is Associated with Incident Alzheimer’s Dementia in the Oldest Old. Nutrients. 2023; 15(1):61. https://doi.org/10.3390/nu15010061
Chicago/Turabian StyleMelo van Lent, Debora, Sarah Egert, Steffen Wolfsgruber, Luca Kleineidam, Leonie Weinhold, Holger Wagner-Thelen, Birgit Stoffel-Wagner, Horst Bickel, Birgitt Wiese, Siegfried Weyerer, and et al. 2023. "Low Serum Vitamin D Status Is Associated with Incident Alzheimer’s Dementia in the Oldest Old" Nutrients 15, no. 1: 61. https://doi.org/10.3390/nu15010061
APA StyleMelo van Lent, D., Egert, S., Wolfsgruber, S., Kleineidam, L., Weinhold, L., Wagner-Thelen, H., Stoffel-Wagner, B., Bickel, H., Wiese, B., Weyerer, S., Pentzek, M., Jessen, F., Schmid, M., Maier, W., Scherer, M., Riedel-Heller, S. G., Ramirez, A., & Wagner, M. (2023). Low Serum Vitamin D Status Is Associated with Incident Alzheimer’s Dementia in the Oldest Old. Nutrients, 15(1), 61. https://doi.org/10.3390/nu15010061





