Ameliorative Effects of Bifidobacterium animalis subsp. lactis J-12 on Hyperglycemia in Pregnancy and Pregnancy Outcomes in a High-Fat-Diet/Streptozotocin-Induced Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Preparation of Bacterial Suspension
2.2. Chemicals and Drugs
2.3. Animal Experimental Design
2.4. Analysis of Relevant Parameters in Rats with HIP
2.4.1. Determination of FBG and HbA1c Levels
2.4.2. Assessment of Serum Biochemistry
2.4.3. Histological Analysis
2.5. Analysis of Relevant Parameters in Fetuses
2.5.1. Experimental Evaluation of Fetuses
2.5.2. Skeletal Assessment of Fetuses
2.5.3. Histological Analysis
2.5.4. Transmission Electron Microscopy (TEM) Analysis
2.5.5. RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.6. Analysis of Relevant Placental Parameters
2.6.1. Placental Sample Collection
2.6.2. Placental Histological
2.6.3. Placental Microbial Diversity
2.7. Statistical Analysis
3. Results
3.1. FBG and HbA1c Levels during Pregnancy in Rats
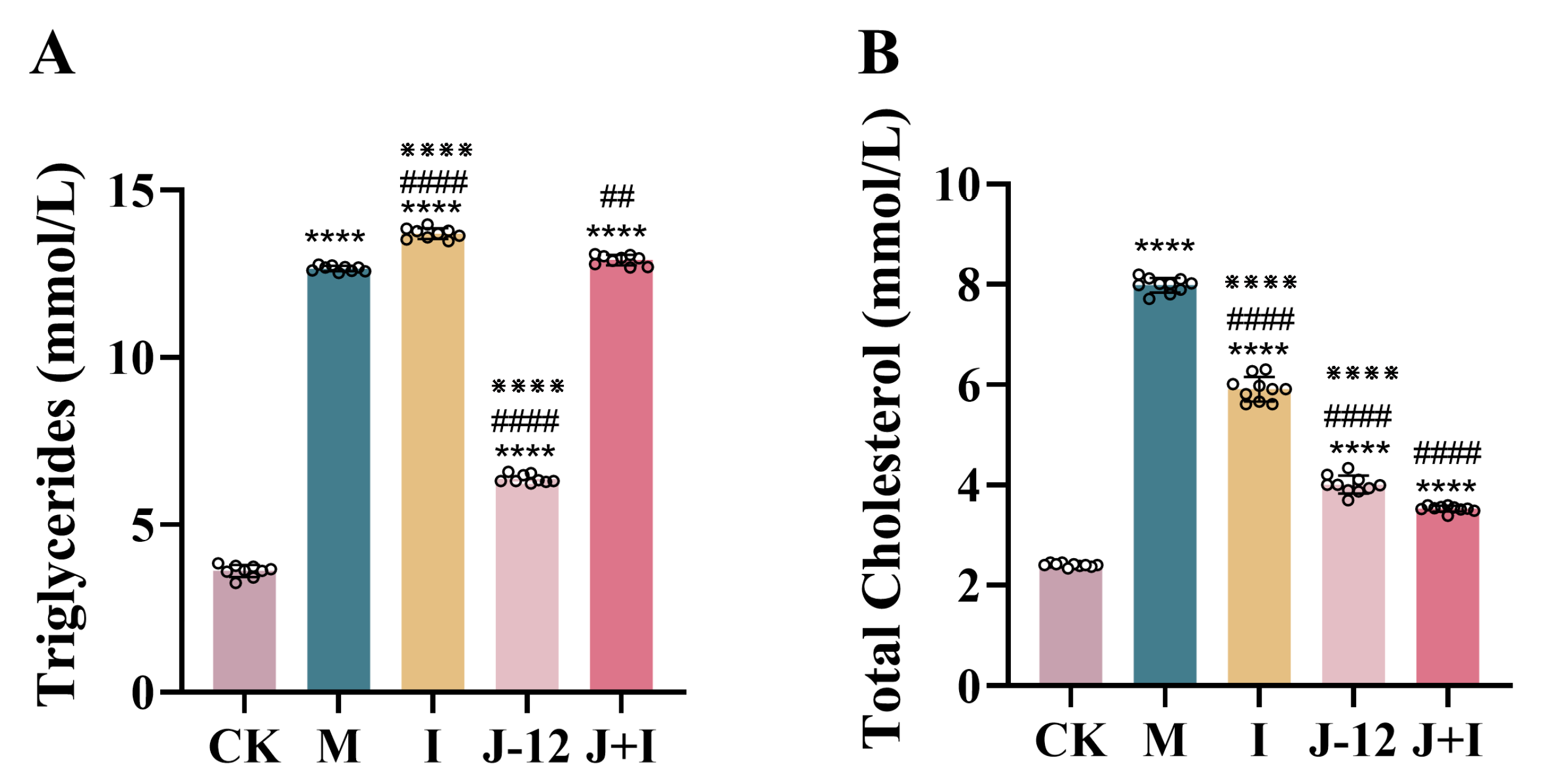
3.2. Effect of J-12 on Dyslipidemia in Rats with HIP
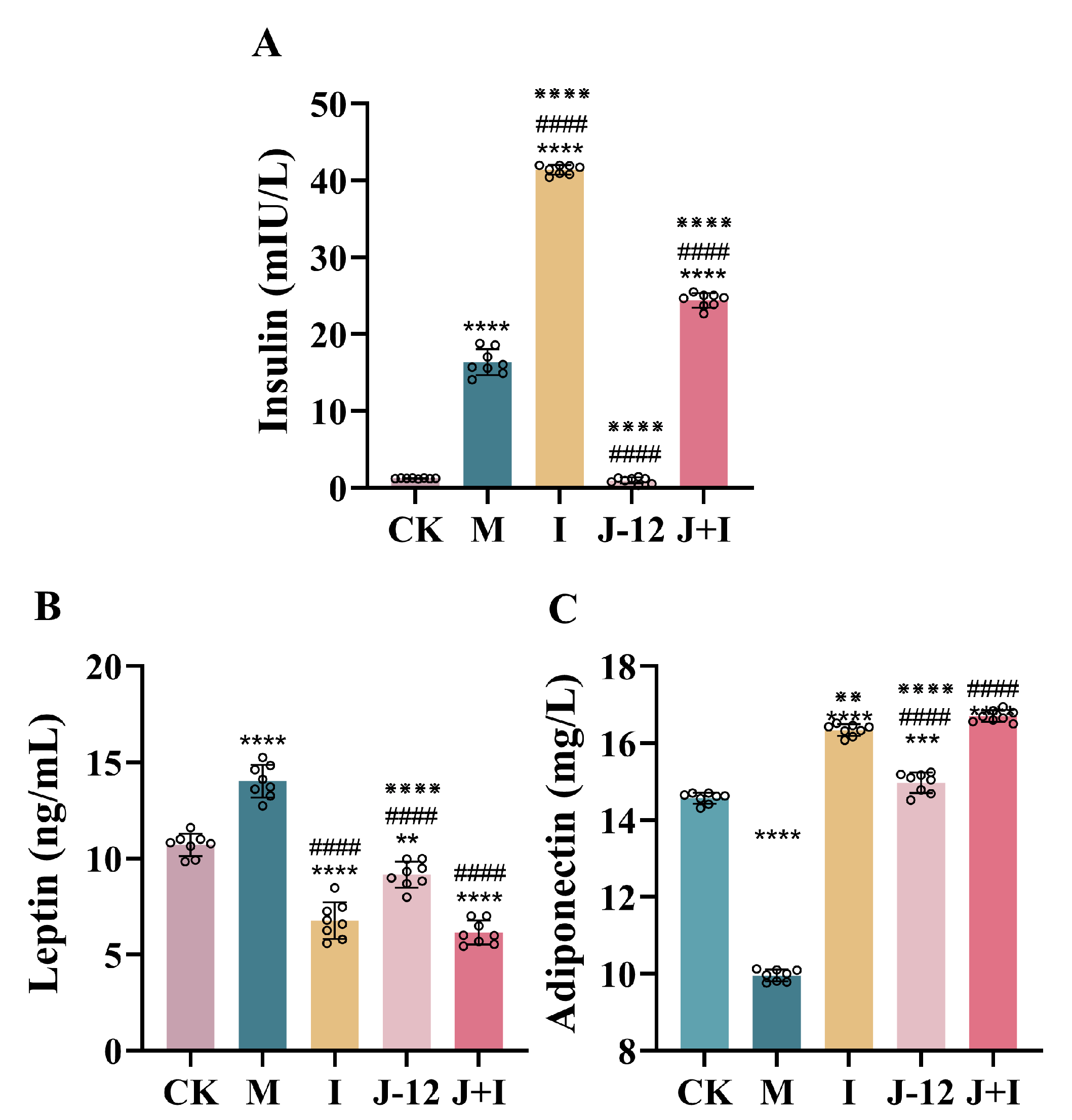
3.3. Effect of J-12 on Insulin, Leptin, and Adiponectin Levels in Rats with HIP
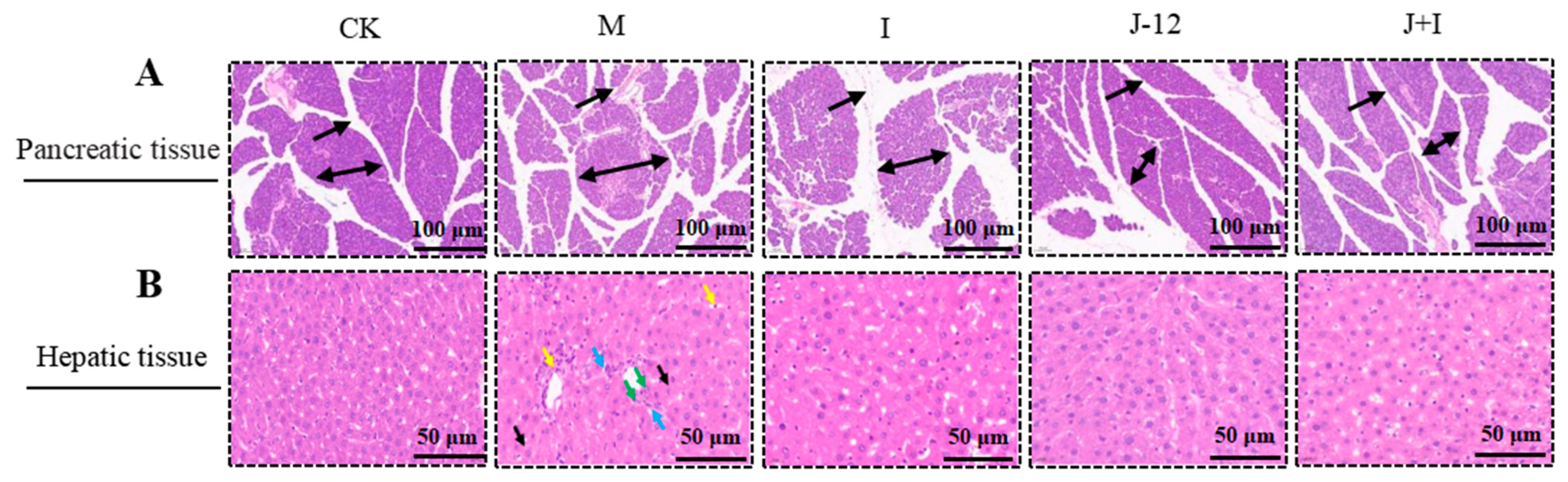
3.4. Effect of J-12 on Pancreatic and Hepatic Tissue Damage in Rats with HIP
3.5. Effect of J-12 on Physiologic Parameters of Fetuses
3.6. Effect of J-12 on Fetal Skeletal Abnormalities
3.7. Effect of J-12 on Fetal Tissue Damage
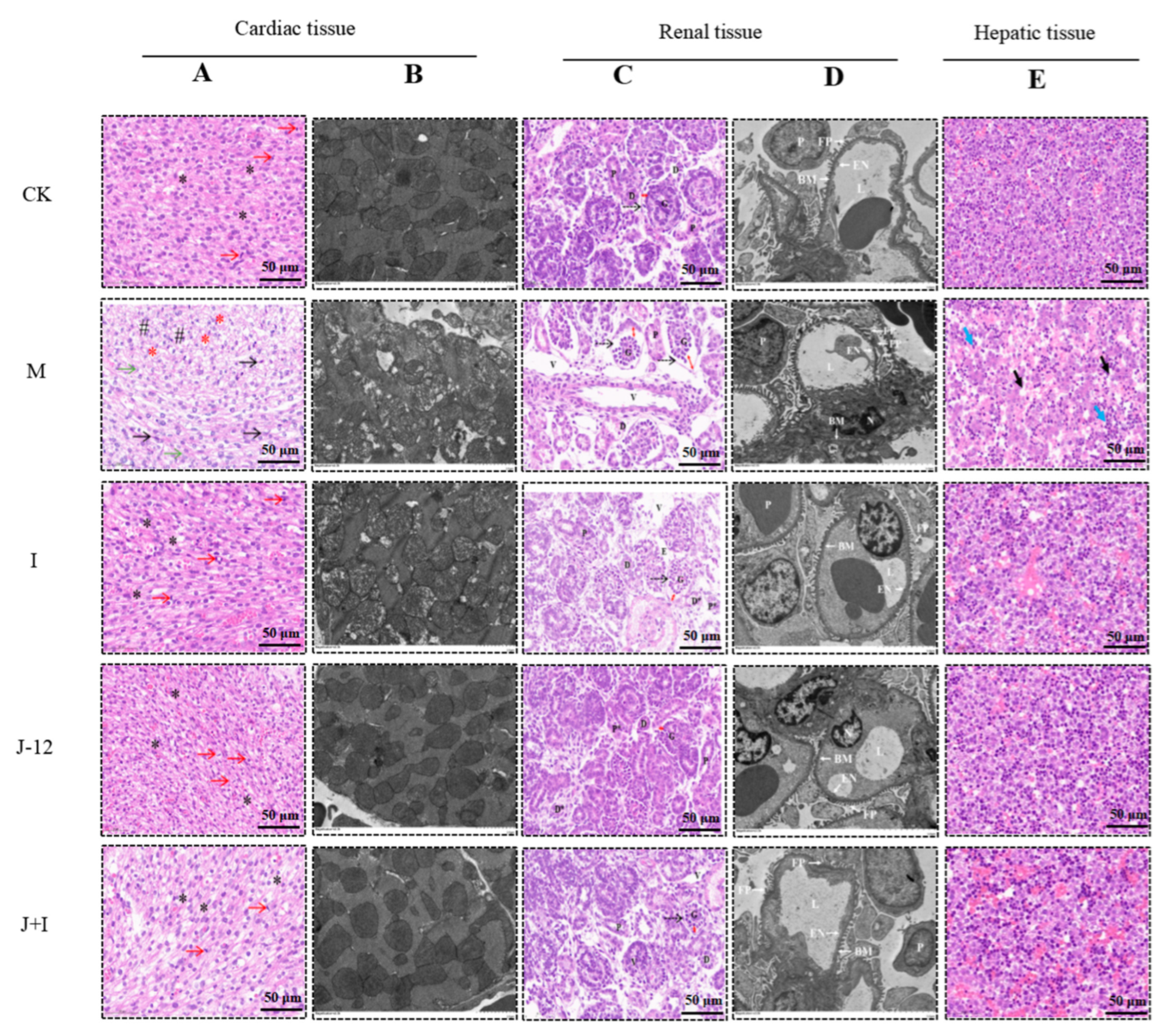
3.7.1. Effect of J-12 on Fetal Cardiac Tissue Damage
3.7.2. Effect of J-12 on Fetal Renal Tissue Damage
3.7.3. Effect of J-12 on Fetal Hepatic Tissue Damage
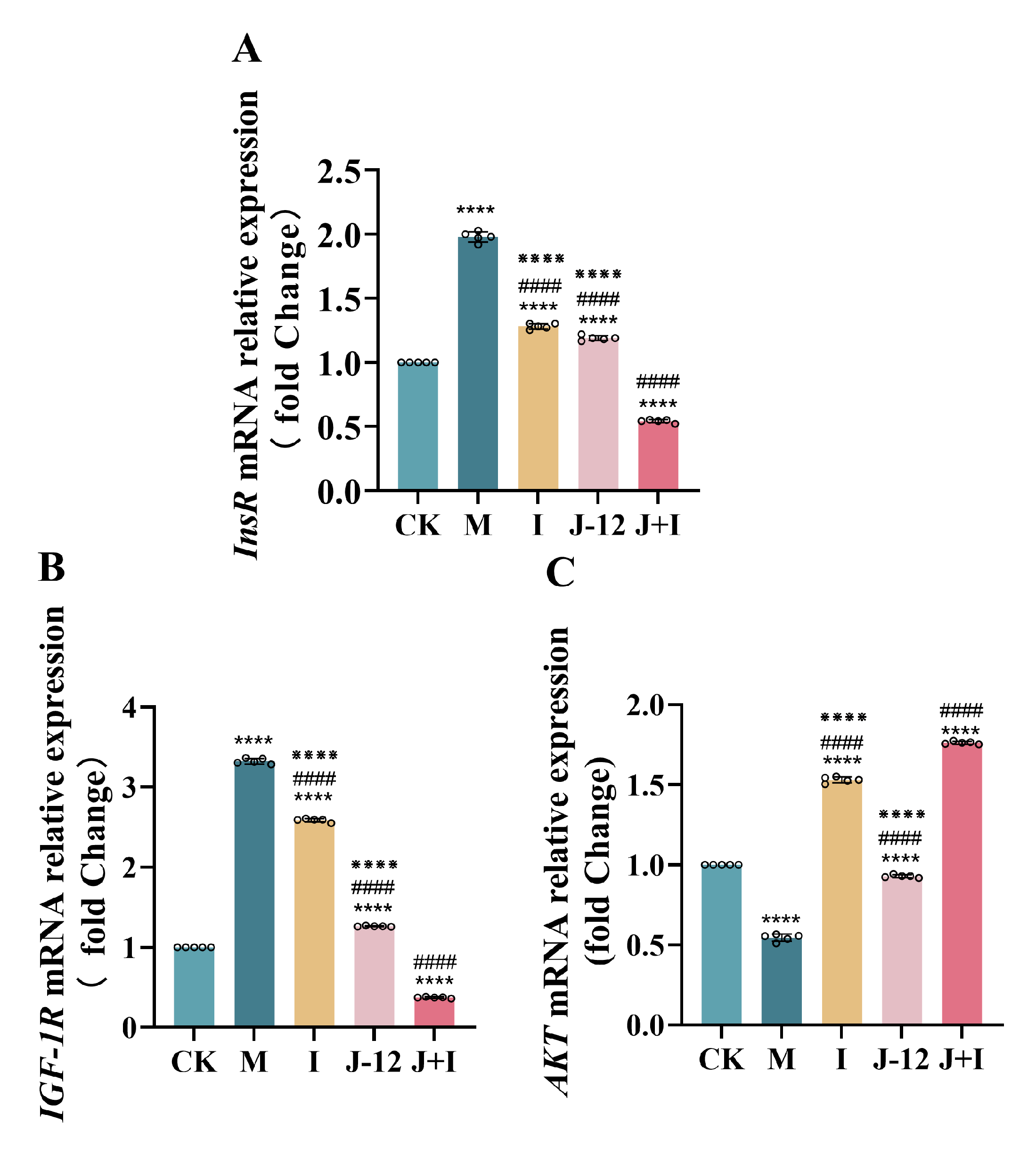
3.8. Effect of J-12 on Gene Expression in the Central Nervous System of Fetuses
3.9. Effect of J-12 on Placental Tissue Damage in Rats with HIP
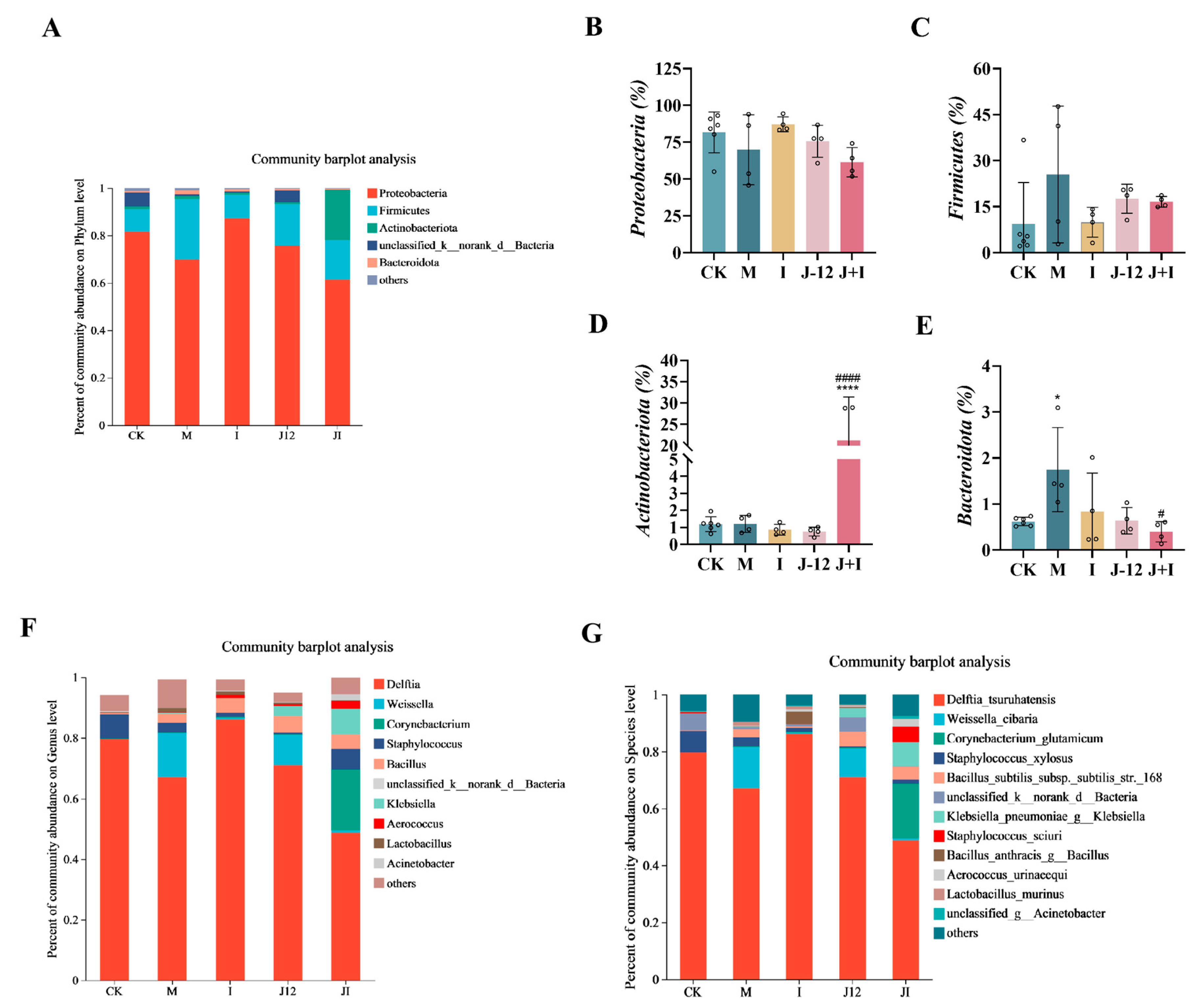
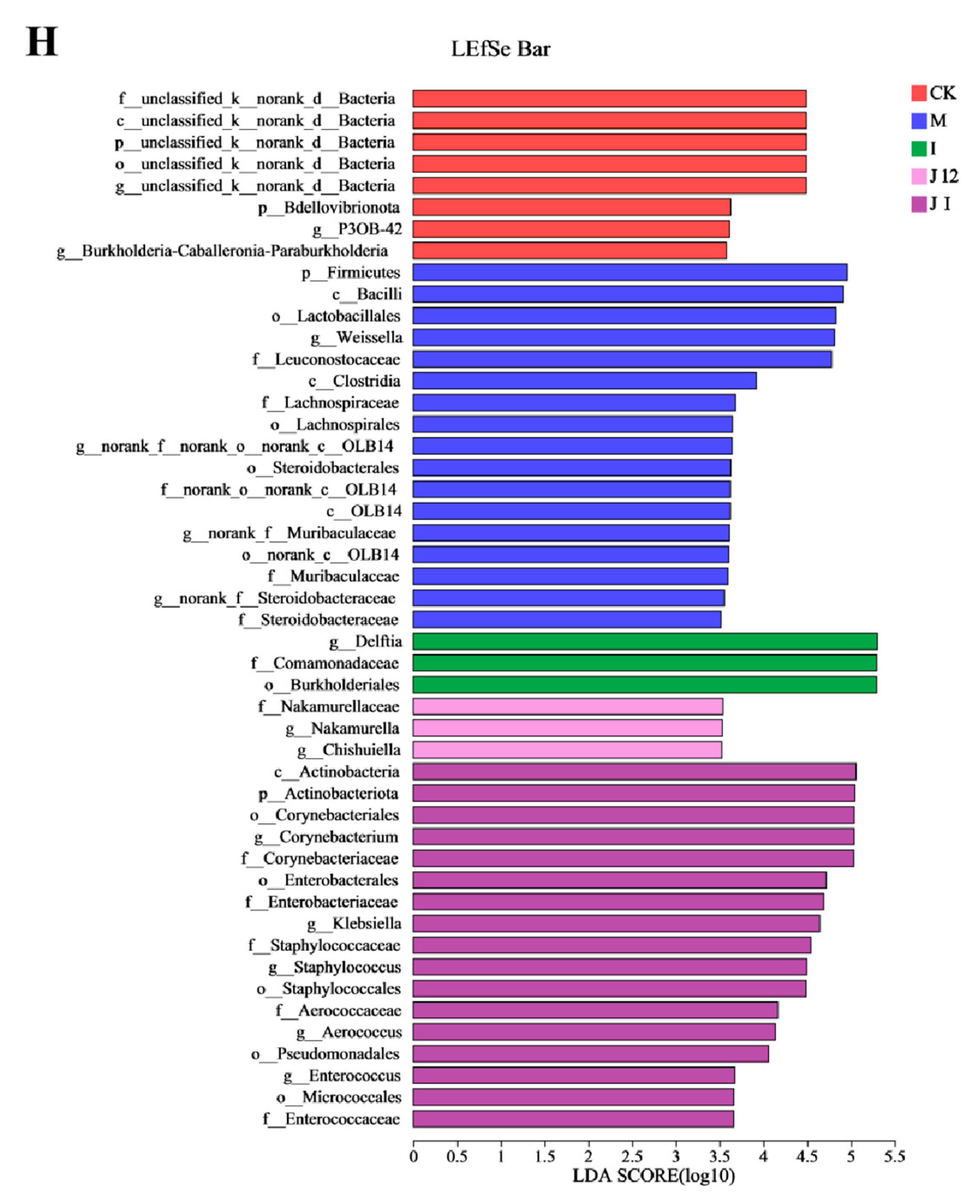
3.10. Effect of J-12 on Placental Community Diversity in Rats with HIP
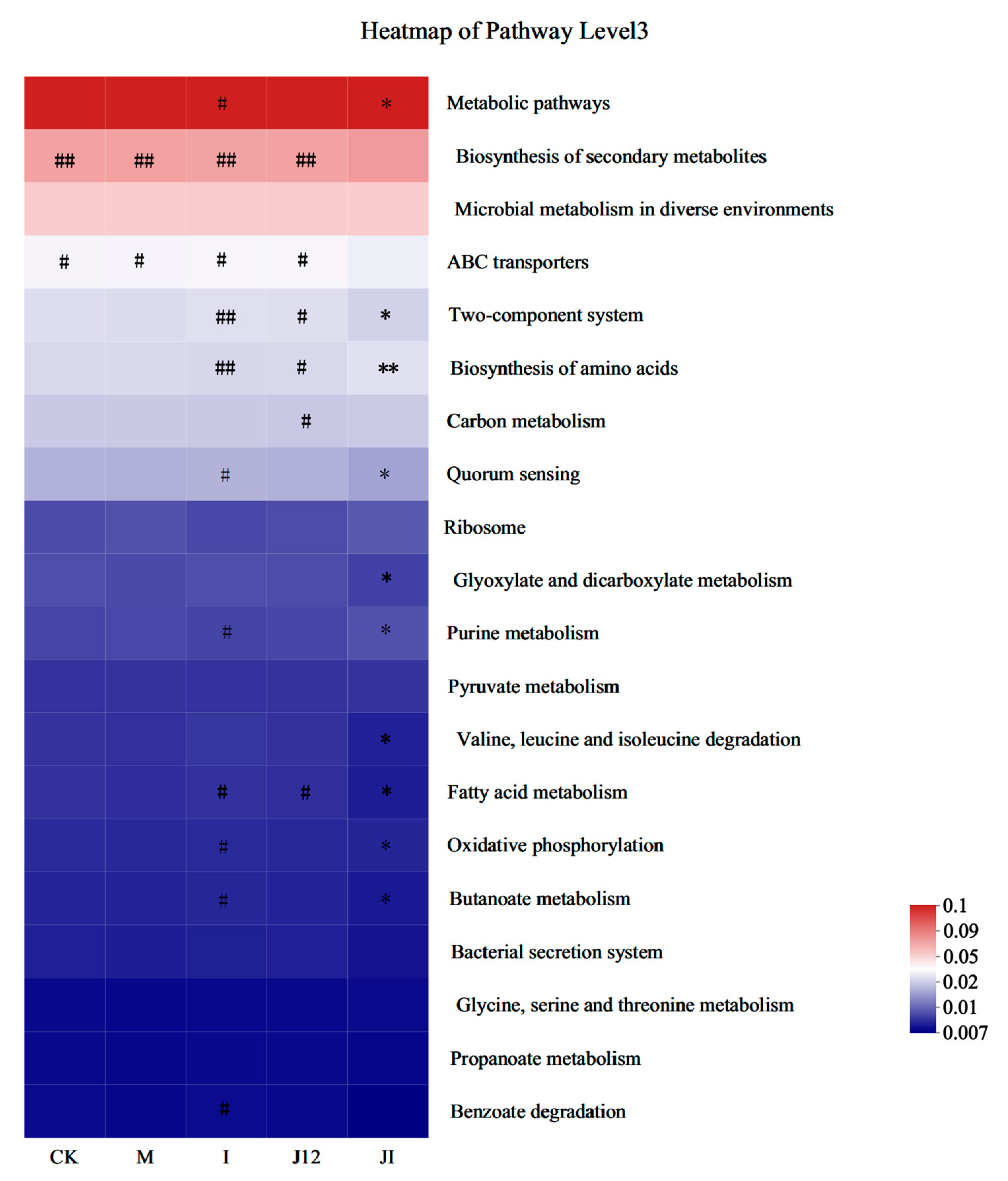
3.11. Predicted Metabolic Profile of the Placental Microbiome after J-12 Supplementation
3.12. Correlation between Overall Placental Microbiota Structure and Maternal and Fetal Rat Parameters
4. Discussion
4.1. J-12 Improved HIP Symptoms, except for FBG, in Mother Rats
4.2. J-12 Improved Physiologic Parameters and Tissue Damage in Fetuses
4.3. J-12 Regulated Gene Expression in the Fetal Central Nervous System
4.4. J-12 Improved Placental Tissue Damage and Placental Community Diversity in Rats with HIP
4.5. Correlation between Overall Placental Microbiota Structure and Maternal and Fetal Rats Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and adverse pregnancy outcomes. Clin. Chem. 2019, 65, 937–938. [Google Scholar] [CrossRef]
- McIntyre, D.; Desoye, G.; Dunne, F.; Simeoni, U.; Visser, G.H.; Kapur, A.; Hod, M. FIGO analysis of research priorities in hyperglycemia in pregnancy. Diabetes Res. Clin. Pract. 2018, 145, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Kemkem, Y.; Nasteska, D.; De Bray, A.; Bargi-Souza, P.; Peliciari-Garcia, R.A.; Guillou, A.; Mollard, P.; Hodson, D.J.; Schaeffer, M. Maternal hypothyroidism in mice influences glucose metabolism in adult offspring. Diabetologia 2020, 63, 1822–1835. [Google Scholar] [CrossRef] [PubMed]
- Zawiejska, A.; Wróblewska-Seniuk, K.; Gutaj, P.; Kippen, J.; Gomulska, A.; Wender-Ozegowska, E. Markers of Maternal Insulin Resistance and Lipid Ratios Measured in Early Pregnancy Are Related to Adverse Fetomaternal Outcomes in Women Treated for Hyperglycemia Detected in Early Pregnancy—Data from a Retrospective Cohort Study. J. Clin. Med. 2022, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, P.; Schäffer, L.; Sørensen, A.; Kristensen, C.; Jonassen, I.; Schmid, C.; Trüb, T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000, 49, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Kitwitee, P.; Limwattananon, S.; Limwattananon, C.; Waleekachonlert, O.; Ratanachotpanich, T.; Phimphilai, M.; Nguyen, T.V.; Pongchaiyakul, C. Metformin for the treatment of gestational diabetes: An updated meta-analysis. Diabetes Res. Clin. Pract. 2015, 109, 521–532. [Google Scholar] [CrossRef]
- Castillo, W.C.; Boggess, K.; Stürmer, T.; Brookhart, M.A.; Benjamin, D.K.; Funk, M.J. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr. 2015, 169, 452–458. [Google Scholar] [CrossRef]
- Campieri, M.; Gionchetti, P. Bacteria as the cause of ulcerative colitis. Gut 2001, 48, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Basti, A.A.; Ansari, F. Stability and efficiency of double-coated beads containing Lactobacillus acidophilus obtained from the calcium alginate-chitosan and eudragit S100 nanoparticles microencapsulation. Int. J. Probiotics Prebiotics 2018, 13, 77–84. [Google Scholar]
- Reid, J.N.; Bisanz, J.E.; Monachese, M.; Burton, J.P.; Reid, G. The rationale for probiotics improving reproductive health and pregnancy outcome. Am. J. Reprod. Immunol. 2013, 69, 558–566. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi Rad, M.; Rahimi Foroushani, A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.X.; Jiang, X.M.; Wang, H.W.; Ge, L.; Lai, Y.T.; Jiang, X.Y.; Chen, F.; Huang, P.P. Probiotic supplements alleviate gestational diabetes mellitus by restoring the diversity of gut microbiota: A study based on 16S rRNA sequencing. J. Microbiol. 2021, 59, 827–839. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forhead, A.J.; Coan, P.M.; Burton, G.J. The placenta and intrauterine programming. J. Neuroendocrinol. 2008, 20, 439–450. [Google Scholar] [CrossRef]
- Leach, L.; Taylor, A.; Sciota, F. Vascular dysfunction in the diabetic placenta: Causes and consequences. J. Anat. 2009, 215, 69–76. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Panigrahi, P. Is a foetus developing in a sterile environment? Lett. Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef]
- Dantas, A.; Verruck, S.; Canella, M.H.M.; Hernandez, E.; Prudencio, E.S. Encapsulated Bifidobacterium BB-12 addition in a concentrated lactose-free yogurt: Its survival during storage and effects on the product’s properties. Food Res. Int. 2021, 150, 110742. [Google Scholar] [CrossRef]
- Cedran, M.F.; Rodrigues, F.J.; Bicas, J.L. Encapsulation of Bifidobacterium BB12® in alginate-jaboticaba peel blend increases encapsulation efficiency and bacterial survival under adverse conditions. Appl. Microbiol. Biotechnol. 2021, 105, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, J.; Zhang, H.; Xie, Y.; Jin, J. Bifidobacterium from breastfed infant faeces prevent high-fat-diet-induced glucose tolerance impairment, mediated by the modulation of glucose intake and the incretin hormone secretion axis. J. Sci. Food Agric. 2020, 100, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ren, P.; Ren, Y.; Liu, H. Study on intestinal dynamic distribution of Bifidobacterium lactis A12 and its effect on constipation relief. J. Beijing Agric. Univ. 2021, 36, 114–120. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Z.; Chen, M.; Zou, J.; Jiang, S.; Qian, D.; Duan, J. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J. Ethnopharmacol. 2019, 241, 112032. [Google Scholar] [CrossRef]
- Hosni, A.; Abd El-twab, S.; Abdul-Hamid, M.; Prinsen, E.; AbdElgawad, H.; Abdel-Moneim, A.; Beemster, G.T. Cinnamaldehyde mitigates placental vascular dysfunction of gestational diabetes and protects from the associated fetal hypoxia by modulating placental angiogenesis, metabolic activity and oxidative stress. Pharmacol. Res. 2021, 165, 105426. [Google Scholar] [CrossRef] [PubMed]
- Staples, R. Refinements in rapid clearing technic in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964, 39, 61–63. [Google Scholar]
- Kassab, B.M.; Hussein, H.H.; Mahmoud, O.M.; Abdel-Alrahman, G. Effects of insulin and metformin on fetal kidney development of streptozotocin-induced gestational diabetic albino rats. Anat. Cell Biol. 2019, 52, 161–175. [Google Scholar] [CrossRef]
- Wei, S.H.; Chen, Y.P.; Chen, M.J. Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; GOEBEL, B.M. Taxonomic notea: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.; Tess, E.R.; Schanzer, A.S.; Coutifaris, C.; Su, E.J.; Parry, S.; Huh, D. A microphysiological model of the human placental barrier. Lab Chip 2016, 16, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2008, 101, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Karamali, M.A.R.Y.A.M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Plows, J.F.; Nieves, J.R.; Budin, F.; Mace, K.; Reynolds, C.M.; Vickers, M.H.; Silva-Zolezzi, I.; Stanley, J.L. The effects of myo-inositol and probiotic supplementation in a high-fat-fed preclinical model of glucose intolerance in pregnancy. Br. J. Nutr. 2020, 123, 516–528. [Google Scholar] [CrossRef]
- Li, T.; Yang, J.; Zhang, H.; Xie, Y.; Liu, H.; Ren, J.; Ren, F.; Jin, J. Bifidobacterium animalis subsp. lactis A12 prevents obesity-associated dyslipidemia by modulating gut microbiota-derived short-chain fatty acid production and energy metabolism in high-fat diet-fed mice. Food Nutr. Res. 2022, 66, 8670. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Wang, J.; Huang, H.; Huang, Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015, 117, 63–69. [Google Scholar] [CrossRef]
- Qinna, N.A.; Badwan, A.A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. DrugDes. Dev. Ther. 2015, 9, 2515. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Hosseini, S.M.; Amini, N. Launaea acanthodes (Boiss) O. Kuntze mediates hepatic glucose metabolism and ameliorates impaired pancreatic function in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 268, 113577. [Google Scholar] [CrossRef] [PubMed]
- Fakharzadeh, S.; Kalanaky, S.; Hafizi, M.; Nazaran, M.H.; Zardooz, H. DIBc, a nanochelating-based nano metal-organic framework, shows anti-diabetic effects in high-fat diet and streptozotocin-induced diabetic rats. Int. J. Nanomed. 2019, 14, 2145. [Google Scholar] [CrossRef]
- Owens, D.R. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol. Ther. 2013, 15, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Abdelmageed, M.E.; Shehatou, G.S.; Abdelsalam, R.A.; Suddek, G.M.; Salem, H.A. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; He, Y.; Ji, L.L.; Wang, K.Y.; Wang, Y.L.; Chen, D.F.; Geng, Y.; OuYang, P.; Lai, W.M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017, 8, 101545. [Google Scholar] [CrossRef]
- Xu, W.; Tang, M.; Wang, J.; Wang, L. Anti-inflammatory activities of puerarin in high-fat diet-fed rats with streptozotocin-induced gestational diabetes mellitus. Mol. Biol. Rep. 2020, 47, 7537–7546. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, D.C.; Volpato, G.T.; Sinzato, Y.K.; Lima, P.H.O.; Souza, M.S.S.; Iessi, I.L.; Kiss, A.C.; Takaku, M.; Rudge, M.V.; Calderon, I.M. Genotoxicity and fetal abnormality in streptozotocin-induced diabetic rats exposed to cigarette smoke prior to and during pregnancy. Exp. Clin. Endocrinol. Diabetes 2011, 119, 549–553. [Google Scholar] [CrossRef]
- Gat-Yablonski, G.; De Luca, F. Effect of nutrition on statural growth. Horm. Res. Paediatr. 2017, 88, 46–62. [Google Scholar] [CrossRef]
- El-Mansi, A.A.; Al-Kahtani, M.A. Calcitriol and punica granatum extract concomitantly attenuate cardiomyopathy of diabetic mother rats and their neonates via activation of Raf/MEK/ERK signalling and mitigation of apoptotic pathways. Folia Biol. 2019, 65, 65–87. [Google Scholar]
- Ma, R.J.; Qiu, X.Q.; Zhan, Y.K. Effect of vitamin E on fetal heart apoptosis of gestational diabetes mellitus rat. Mod. Prev. Med. 2015, 42, 235–238+254. [Google Scholar]
- Guariguata, L.; Linnenkamp, U.; Makaroff, L.E.; Ogurtsova, K.; Colagiuri, S. Global estimates of hyperglycaemia in pregnancy: Determinants and trends. In Nutrition and Diet in Maternal Diabetes; Humana Press: Chams, Switzerland, 2018; pp. 3–15. [Google Scholar] [CrossRef]
- El-Beeh, M.E.; Aljabri, M.; Orabi, H.F.; Qari, S.H.; Ramadan, M.F. Ameliorative impact of cold-pressed Rosmarinus officinalis oil against liver toxicity and genotoxic effects in streptozotocin-induced diabetic rats and their offspring. J. Food Biochem. 2019, 43, e12905. [Google Scholar] [CrossRef]
- DeCarolis, N.A.; Eisch, A.J. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology 2010, 58, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Förster, E.; Zhao, S.; Frotscher, M. Laminating the hippocampus. Nat. Rev. Neurosci. 2006, 7, 259–268. [Google Scholar] [CrossRef] [PubMed]
- McNay, E.C.; Gold, P.E. Extracellular Glucose Concentrations in the Rat Hippocampus Measured byZero-Net-Flux: Effects of Microdialysis Flow Rate, Strain, andAge. J. Neurochem. 1999, 72, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Hami, J.; Sadr-Nabavi, A.; Sankian, M.; Balali-Mood, M.; Haghir, H. The effects of maternal diabetes on expression of insulin-like growth factor-1 and insulin receptors in male developing rat hippocampus. Brain Struct. Funct. 2013, 218, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Hami, J.; Kerachian, M.A.; Karimi, R.; Haghir, H.; Sadr-Nabavi, A. Effects of streptozotocin-induced type 1 maternal diabetes on PI3K/AKT signaling pathway in the hippocampus of rat neonates. J. Recept. Signal Transduct. 2016, 36, 254–260. [Google Scholar] [CrossRef]
- Tehranipour, M.; Khakzad, M.R. Effect of maternal diabetes on hippocampus neuronal density in neonatal rats. J. Biol. Sci. 2008, 6, 1027–1032. [Google Scholar] [CrossRef]
- Stenvers, K.L.; Lund, P.K.; Gallagher, M. Increased expression of type 1 insulin-like growth factor receptor messenger RNA in rat hippocampal formation is associated with aging and behavioral impairment. Neuroscience 1996, 72, 505–518. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Dysregulation of PI3K-Akt-mTOR pathway in brain of streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Lipids Health Dis. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Cho, H.; Mu, J.; Kim, J.K.; Thorvaldsen, J.L.; Chu, Q.; Crenshaw, E.B., III; Kaestner, K.H.; Bartolomei, M.S.; Shulman, G.I.; Birnbaum, M.J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 2001, 292, 1728–1731. [Google Scholar] [CrossRef]
- Bathina, S.; Gundala, N.K.; Rhenghachar, P.; Polavarapu, S.; Hari, A.D.; Sadananda, M.; Das, U.N. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martín, S. Placental structure in gestational diabetes mellitus. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef] [PubMed]
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am. J. Obstet. Gynecol. 2015, 212, 653-e1. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.H.; Zhang, Q.; Mao, L.L.; Yu, M.; Xu, J.P.; Wang, T. Correlation of placental microbiota with fetal macrosomia and clinical characteristics in mothers and newborns. Oncotarget 2017, 8, 82314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef]
- Bassols, J.; Serino, M.; Carreras-Badosa, G.; Burcelin, R.; Blasco-Baque, V.; Lopez-Bermejo, A.; Fernandez-Real, J.M. Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatr. Res. 2016, 80, 777–784. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J.; Wang, T. The placental microbiota is altered among subjects with gestational diabetes mellitus: A pilot study. Front. Physiol. 2017, 8, 675. [Google Scholar] [CrossRef]
- Tang, N.; Luo, Z.C.; Zhang, L.; Zheng, T.; Fan, P.; Tao, Y.; Ouyang, F. The association between gestational diabetes and microbiota in placenta and cord blood. Front. Endocrinol. 2020, 11, 550319. [Google Scholar] [CrossRef]
- Ranc, A.; Dubourg, G.; Fournier, P.E.; Raoult, D.; Fenollar, F. Delftia tsuruhatensis, an emergent opportunistic healthcare-associated pathogen. Emerg. Infect. Dis. 2018, 24, 594. [Google Scholar] [CrossRef]
- John, C.M.; Ramasamy, R.; Al Naqeeb, G.; Al-Nuaimi, A.H.D.; Adam, A. Nicotinamide supplementation protects gestational diabetic rats by reducing oxidative stress and enhancing immune responses. Curr. Med. Chem. 2012, 19, 5181–5186. [Google Scholar] [CrossRef]
- Lee, J.S.; Hyun, I.K.; Seo, H.J.; Song, D.; Kim, M.Y.; Kang, S.S. Biotransformation of whey by Weissella cibaria suppresses 3T3-L1 adipocyte differentiation. J. Dairy Sci. 2021, 104, 3876–3887. [Google Scholar] [CrossRef]
- Nakayama, Y. Corynebacterium glutamicum mechanosensing: From osmoregulation to L-glutamate secretion for the avian microbiota-gut-brain axis. Microorganisms. 2021, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Ayers, W.A. Phosphorylation of cellobiose and glucose by Ruminococcus flavefaciens. J. Bacteriol. 1958, 76, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Hugerth, L.W.; Hases, L.; Saxena, A.; Seifert, M.; Thomas, Q.; Williams, C. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int. J. Cancer 2019, 144, 3086–3098. [Google Scholar] [CrossRef]
- Tao, Y.W.; Gu, Y.L.; Mao, X.Q.; Zhang, L.; Pei, Y.F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, L.; Xi, Y.; Li, Y.; Xie, X.; Fan, C.; Yang, L.; Yang, S.; Chenx, X.; Zhang, J.; et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 1670–1688. [Google Scholar] [CrossRef] [PubMed]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef]



| Primers Name | Oligonucleotide (5′-3′) | Oligonucleotide (3′-5′) | Tm (°C) |
|---|---|---|---|
| InsR | GGCCCGATGCTGAGAACA | CGTCATTCCACGTCATTCCA | 60 |
| IGF-1R | GCCGTGCTGTGCCTGTCCTAAAAC | GCTACCGTGGTGTTCCTGCTTCG | 60 |
| AKT | GAAGCTGAGCCCACCTTTCA | CATCTTGATCAGGCGGTGTG | 56 |
| GADPH | AACTCCCATTCTTCCACCTTTG | CTGTAGCCATATTCATTGTCATACCAG | 60 |
| β-actin | TATCGGCAATGAGCGGTTCC | ACTGTGTTGGCATAGAGG | 56 |
| Variables | Live Fetuses (%) | Dead Fetuses (%) | Resorptions (%) | Body Weight (g) |
|---|---|---|---|---|
| CK | 100 a | 0 b | 0 b | 3.85 ± 0.09 |
| M | 73.8 b | 26.2 a | 0 b | 3.49 ± 0.11 |
| I | 97.4 a | 0 b | 2.6 a | 3.39 ± 0.04 |
| J-12 | 100 a | 0 b | 0 b | 3.64 ± 0.14 |
| J + I | 95.2 a | 0 b | 4.8 a | 3.42 ± 0.09 |
| Variables | CK | M | I | J-12 | J + I |
|---|---|---|---|---|---|
| Absence of the xiphoid process of the sternum (%) | 0 c | 100 a | 100 a | 25 b | 80 a |
| Sternum fractur (%) | 0 b | 0 b | 50 a | 0 b | 0 b |
| Unossified coccyx (%) | 0 b | 0 b | 0 b | 0 b | 80 a |
| Incomplete ossification of the bottom vertebra (%) | 0 b | 0 b | 0 b | 0 b | 20 a |
| Variables | CK | M | I | J-12 | J + I | p-Value (CK-M) | p-Value (M-J-12) | p-Value (J-12-J + I) |
|---|---|---|---|---|---|---|---|---|
| sobs | 202 ± 23.82 | 227 ± 30.90 | 177 ± 14.73 | 136 ± 26.15 | 96 ± 41.69 | 0.215 | 0.004 ** | 0.153 |
| shannon | 0.84 ± 0.21 | 1.37 ± 0.74 | 0.74 ± 0.25 | 1.16 ± 0.33 | 1.64 ± 0.27 | 0.167 | 0.624 | 0.066 |
| simpson | 0.73 ± 0.09 | 0.52 ± 0.29 | 0.75 ± 0.09 | 0.54 ± 0.14 | 0.34 ± 0.13 | 0.192 | 0.968 | 0.079 |
| ace | 223 ± 25.89 | 272 ± 40.37 | 217 ± 22.57 | 159 ± 39.58 | 106 ± 44.20 | 0.060 | 0.007 ** | 0.120 |
| chao | 219 ± 25.27 | 272 ± 38.62 | 215 ± 18.44 | 161 ± 46.82 | 104 ± 44.37 | 0.041 * | 0.012 * | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ma, Y.; Li, T.; Pang, Y.; Zhang, H.; Xie, Y.; Liu, H.; Sun, Y.; Ren, J.; Jin, J. Ameliorative Effects of Bifidobacterium animalis subsp. lactis J-12 on Hyperglycemia in Pregnancy and Pregnancy Outcomes in a High-Fat-Diet/Streptozotocin-Induced Rat Model. Nutrients 2023, 15, 170. https://doi.org/10.3390/nu15010170
Yang J, Ma Y, Li T, Pang Y, Zhang H, Xie Y, Liu H, Sun Y, Ren J, Jin J. Ameliorative Effects of Bifidobacterium animalis subsp. lactis J-12 on Hyperglycemia in Pregnancy and Pregnancy Outcomes in a High-Fat-Diet/Streptozotocin-Induced Rat Model. Nutrients. 2023; 15(1):170. https://doi.org/10.3390/nu15010170
Chicago/Turabian StyleYang, Jianjun, Yumeng Ma, Tong Li, Yuanxiang Pang, Hongxing Zhang, Yuanhong Xie, Hui Liu, Yanfang Sun, Jianhua Ren, and Junhua Jin. 2023. "Ameliorative Effects of Bifidobacterium animalis subsp. lactis J-12 on Hyperglycemia in Pregnancy and Pregnancy Outcomes in a High-Fat-Diet/Streptozotocin-Induced Rat Model" Nutrients 15, no. 1: 170. https://doi.org/10.3390/nu15010170
APA StyleYang, J., Ma, Y., Li, T., Pang, Y., Zhang, H., Xie, Y., Liu, H., Sun, Y., Ren, J., & Jin, J. (2023). Ameliorative Effects of Bifidobacterium animalis subsp. lactis J-12 on Hyperglycemia in Pregnancy and Pregnancy Outcomes in a High-Fat-Diet/Streptozotocin-Induced Rat Model. Nutrients, 15(1), 170. https://doi.org/10.3390/nu15010170







