Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Patients
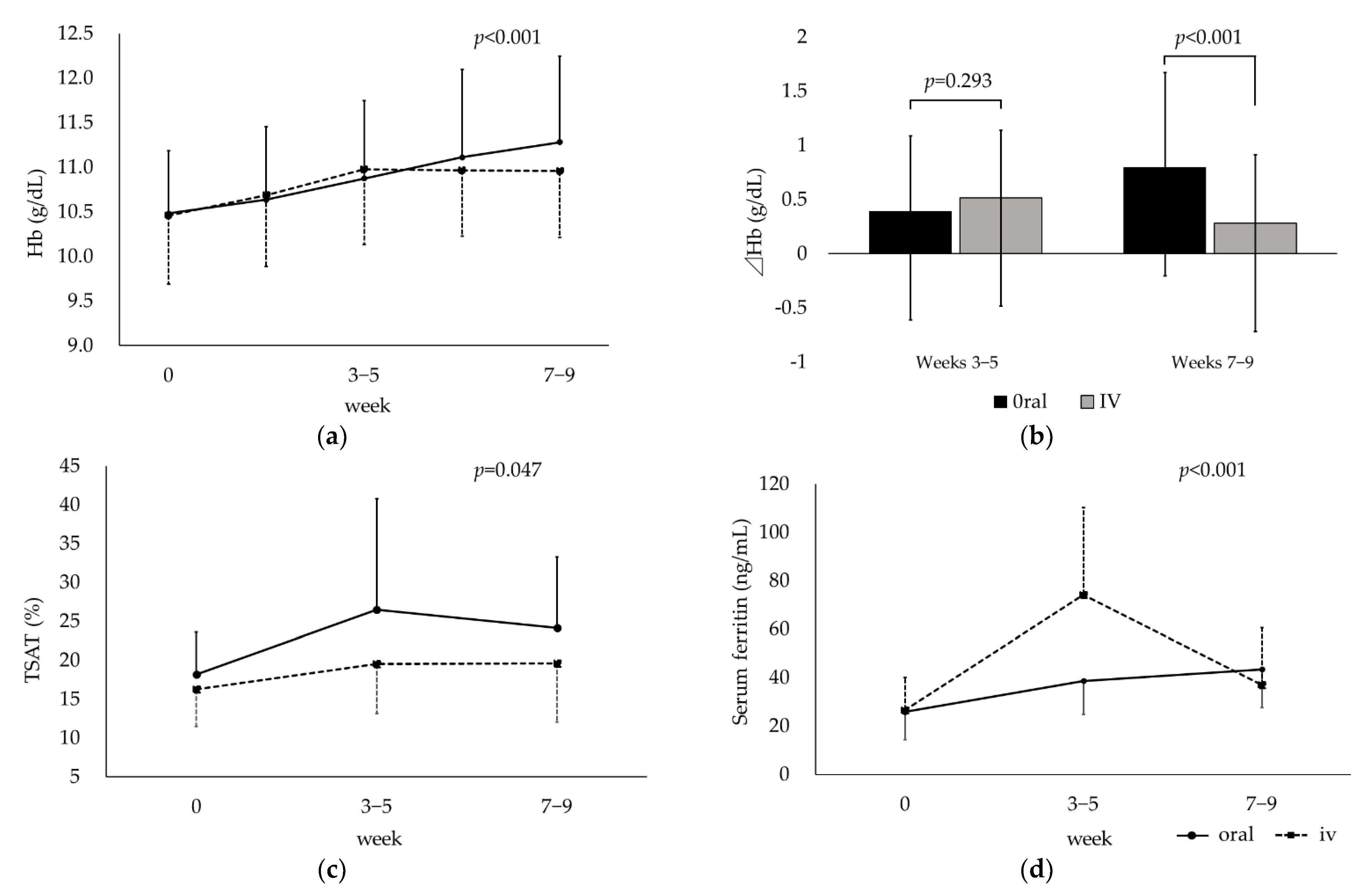
3.2. Compared between Oral and Intravenous Iron Replacement Therapy
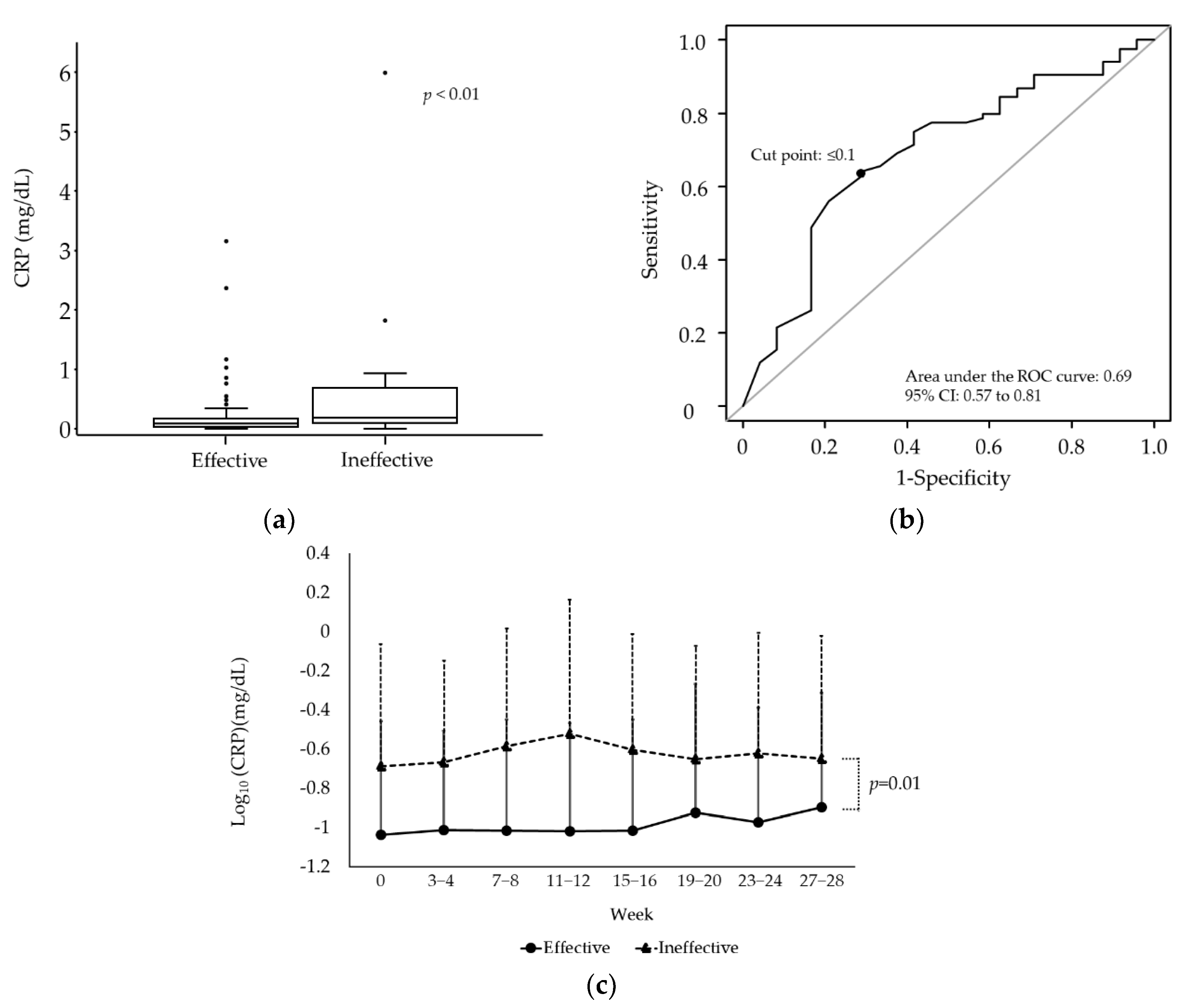
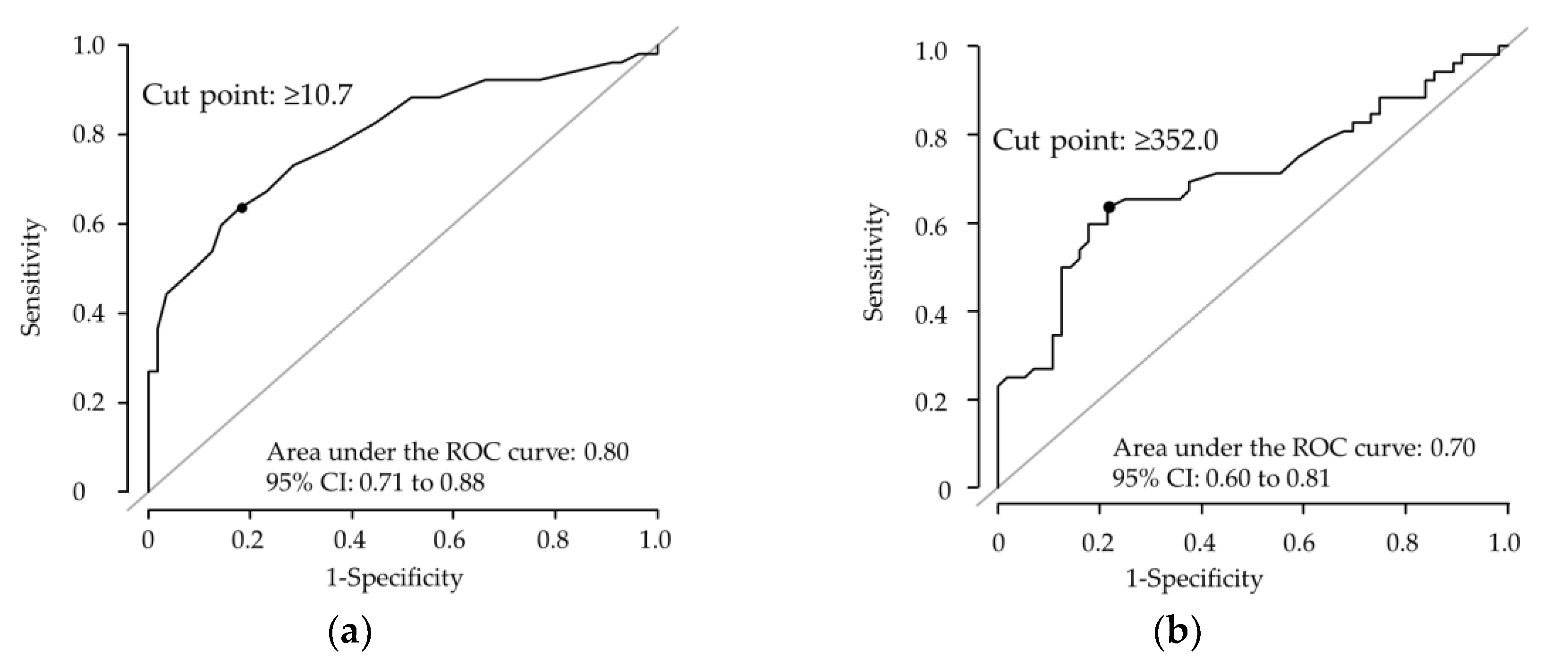
3.3. Identification of Factors Associated with the Efficacy of Oral Iron Supplementation
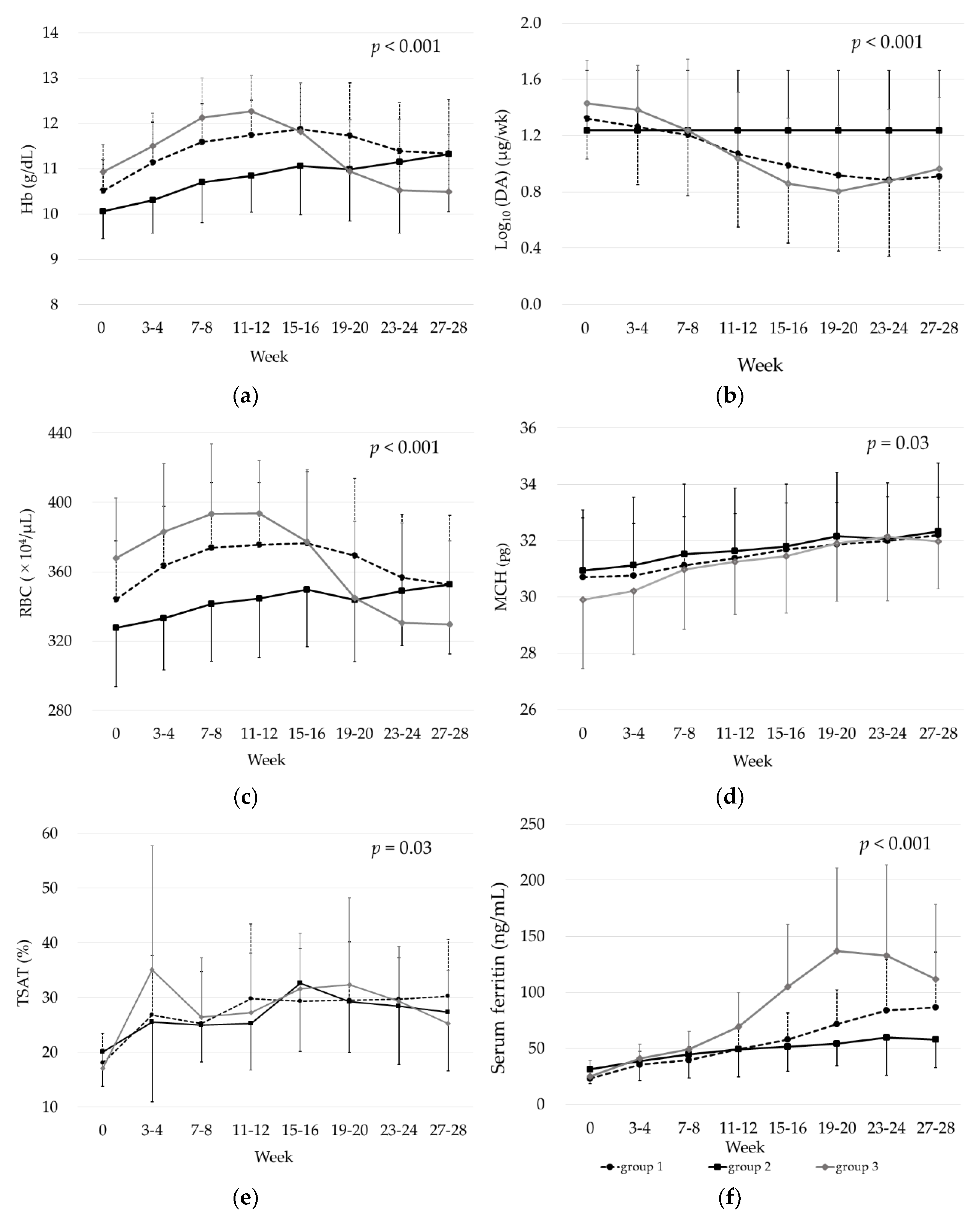
3.4. Erythrocyte- and Iron-Related Parameters and Change in DA Dose over Time
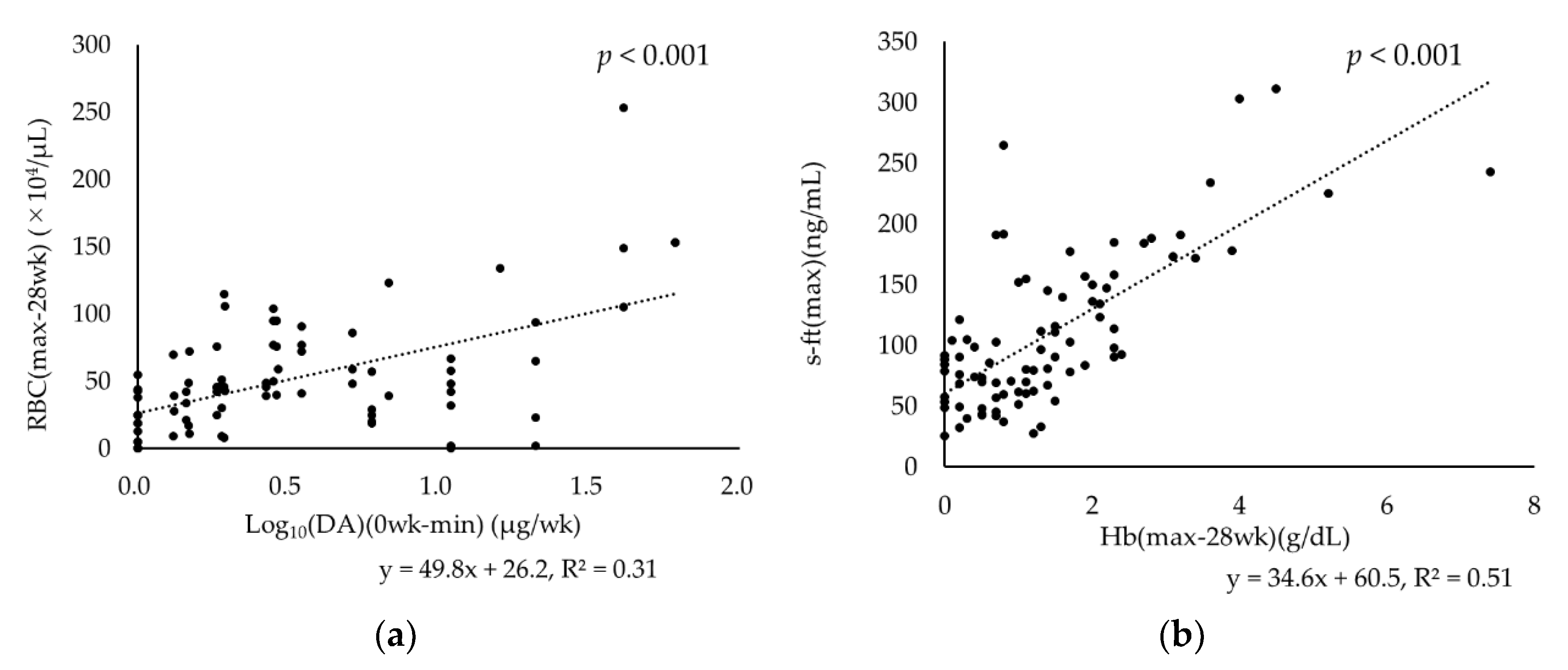
3.5. Relationships between RBC Fluctuation and DA Dose Reduction and between s-ft and Hb Fluctuation
3.6. Identification of Erythrocyte Parameters at the Start of Iron Supplementation That Was Associated with Hb > 12 g/dL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenga, M.F.; Nolte, I.M.; van der Meer, P.; Bakker, S.J.L.; Gaillard, C.A.J.M. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T.; Joki, N.; Hase, H.; Kitamura, K.; Murata, T.; Fujimoto, S.; Fukatsu, A.; Inoue, T.; Itakura, Y.; Nakanishi, T. Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS ONE 2020, 15, e0236277. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Parfrey, P.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Locatelli, F.; Bárány, P.; Covic, A.; De Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; Van Biesen, W.; et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol. Dial. Transplant. 2013, 28, 1346–1359. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin-a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract. Res. Clin. Heamatol. 2006, 108, 1381–1387. [Google Scholar] [CrossRef]
- Ogawa, C.; Tsuchiya, K.; Tomosugi, N.; Shimada, K.; Kanda, F.; Maeda, K. The target hemoglobin content values of reticulocytes for efficient anemia improvement are achieved by low ferritin levels and moderate transferrin saturation: A retrospective observational study. Hematology 2020, 25, 71–78. [Google Scholar] [CrossRef]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.; Murray, H.; Tomson, C.R.; Wheeler, D.C.; et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bhandari, S.; White, C.; Anker, S.D.; Farrington, K.; Kalra, P.A.; Mark, P.B.; McMurray, J.J.; Reid, C.; Robertson, M.; et al. Intravenous Iron Dosing and Infection Risk in Patients on Hemodialysis: A Prespecified Secondary Analysis of the PIVOTAL Trial. J. Am. Soc. Nephrol. 2020, 31, 1118–1127. [Google Scholar] [CrossRef]
- Reis, K.A.; Guz, G.; Ozdemir, H.; Erten, Y.; Atalay, V.; Bicik, Z.; Ozkurt, Z.N.; Bali, M.; Sindel, S. Intravenous iron therapy as a possible risk factor for atherosclerosis in end stage renal disease. Int. Heart J. 2005, 46, 255–264. [Google Scholar] [CrossRef]
- Dűeke, T.; Witko-Sarsat, V.; Massy, Z.; Descamps-Latscha, B.; Guerin, A.P.; Marchais, S.J.; Gausson, V.; London, G.M. Iron Therapy, Advanced Oxidation Protein Products and Carotid Artery Intima-Media Thickness in End-Stage Renal Disease. Circulation 2002, 106, 2212–2217. [Google Scholar] [CrossRef]
- Shah, A.A.; Donovan, K.; Seeley, C.; Dickson, E.A.; Palmer, A.J.R.; Doree, C.; Brunskill, S.; Reid, J.; Acheson, A.G.; Sugavanam, A.; et al. Risk of Infection Associated with Administration of Intravenous Iron: A Systematic Review and Meta-analysis. JAMA 2021, 4, e2133935. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Freburger, J.K.; Ellis, A.R.; Wang, L.; Winkelmayer, W.C.; Kshirsagar, A.V. Infection Risk with Bolus versus Maintenance Iron Supplementation in Hemodialysis Patients. J. Am. Soc. Nephrol. 2013, 24, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Lenga, I.; Lok, C.; Marticorena, R.; Hunter, J.; Dacouris, N.; Goldstein, M. Role of oral iron in the management of long-term hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2007, 2, 688–693. [Google Scholar] [CrossRef]
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral iron supplementation in iron-deficient women: How much and how often? Mol. Aspects Med. 2020, 75, 100865. [Google Scholar] [CrossRef]
- Buttarello, M.; Pajola, R.; Novello, E.; Rebeschini, M.; Cantaro, S.; Oliosi, F.; Naso, A.; Plebani, M. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am. J. Clin. Pathol. 2010, 133, 949–954. [Google Scholar] [CrossRef]
- Mitsuiki, K.; Harada, A.; Miyata, Y. Assessment of iron deficiency in chronic hemodialysis patients: Investigation of cutoff values for reticulocyte hemoglobin content. Clin. Exp. Nephrol. 2003, 7, 52–57. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef]
- Bullock, G.C.; Delehanty, L.L.; Talbot, A.L.; Gonias, S.L.; Tong, W.H.; Rouault, T.A.; Dewar, B.; Macdonald, J.M.; Chruma, J.J.; Goldfarb, A.N. Iron control of erythroid development by a novel aconitase-associated regulatory pathway. Blood 2010, 116, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, J.W.; Cook, J.D.; Scribner, B.H.; Finch, C.A. Iron balance in hemodialysis patients. Ann. Intern Med. 1977, 87, 710–713. [Google Scholar] [CrossRef]
- Kuragano, T.; Kitamura, K.; Matsumura, O.; Matsuda, A.; Hara, T.; Kiyomoto, H.; Murata, T.; Fujimoto, S.; Hase, H.; Joki, N.; et al. ESA Hyporesponsiveness Is Associated with Adverse Events in Maintenance Hemodialysis (MHD) Patients, But Not with Iron Storage. PLoS ONE 2016, 11, e0147328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, C.; Cheng, J.; Zhang, P.; Jiang, H.; Chen, J. Haemoglobin variability and all-cause mortality in haemodialysis patients: A systematic review and meta-analysis. Nephrology 2019, 24, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Kimata, N.; Tsuchiya, K.; Akiba, T.; Nitta, K. Differences in the Characteristics of Dialysis Patients in Japan Compared with Those in Other Countries Differences in the Characteristics of Dialysis Patients in Japan Compared with Those in Other Countries. Blood Purif. 2015, 40, 275–279. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Magzoub, D.; Aldehami, M.A.; Behiry, A.A.; Bhagavathula, A.S.; Hajji, R. The Effect of Iron Supplementation on FGF23 in Chronic Kidney Disease Patients: A Systematic Review and Time-Response Meta-Analysis. Biol. Trace Elem. Res. 2021, 199, 4516–4524. [Google Scholar] [CrossRef]
- Iwasaki, T.; Fujimori, A.; Nakanishi, T.; Okada, S.; Hanawa, N.; Hasuike, Y.; Kuragano, T. Saccharated ferric oxide attenuates haematopoietic response induced by epoetin beta pegol in patients undergoing haemodialysis. BMC Nephrol. 2021, 22, 124. [Google Scholar] [CrossRef]
- Karaboyas, A.; Morgenstern, H.; Fleischer, N.L.; Vanholder, R.C.; Dhalwani, N.N.; Schaeffner, E.; Schaubel, D.E.; Akizawa, T.; James, G.; Sinsakul, M.V.; et al. Inflammation and Erythropoiesis-Stimulating Agent Response in Hemodialysis Patients: A Self-matched Longitudinal Study of Anemia Management in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Med. 2020, 2, 286–296. [Google Scholar] [CrossRef]
| Variables | All | Effective | No Effect | p-Value |
|---|---|---|---|---|
| N (courses) | 108 | 84 | 24 | |
| Age (years) | 68.7 ± 12.8 | 69.0 ± 13.0 | 67.8 ± 12.9 | 0.67 |
| Sex | ||||
| Men | 78 | 60 | 18 | 0.80 |
| Women | 30 | 24 | 6 | |
| Duration of dialysis (years) † | 7.3 (2.8–17.7) | 6.7 (2.9–17.5) | 8.0 (3.0–18.5) | 0.89 |
| Primary diagnosis | ||||
| Chronic glomerulonephritis | 36 | 28 | 8 | 0.42 |
| Diabetes nephropathy | 40 | 30 | 10 | |
| Renal sclerosis | 24 | 18 | 6 | |
| Other | 8 | 8 | 0 | |
| Hb (g/dL) | 10.5 ± 0.7 | 10.5 ± 0.7 | 10.4 ± 0.7 | 0.28 |
| RBC (×104/μL) | 345.9 ± 35.6 | 346.0 ± 36.7 | 345.5 ± 33.0 | 0.95 |
| MCH (pg) | 30.5 ± 2.1 | 30.6 ± 2.2 | 30.1 ± 1.3 | 0.41 |
| MCV (fL) | 96.3 ± 5.5 | 96.6 ± 5.8 | 95.3 ± 4.4 | 0.29 |
| s-Fe (μg/dL) | 51.4 ± 14.6 | 51.6 ± 14.8 | 50.4 ± 14.5 | 0.72 |
| TIBC (μg/dL) | 285.5 ± 41.2 | 284.4 ± 41.7 | 289.4 ± 41.0 | 0.60 |
| TSAT (%) | 18.2 ± 5.4 | 18.4 ± 5.6 | 17.7 ± 4.7 | 0.57 |
| Serum ferritin (ng/mL) | 25.8 ± 11.3 | 24.9 ± 11.8 | 28.9 ± 9.4 | 0.13 |
| Albumin (g/dL) | 3.4 ± 0.3 | 3.4 ± 0.3 | 3.5 ± 0.3 | 0.07 |
| C-reactive protein (mg/dL) † | 0.10 (0.05–0.25) | 0.09 (0.04–0.17) | 0.19 (0.10–0.62) | <0.01 |
| nPCR | 0.92 ± 0.17 | 0.91 ± 0.14 | 0.94 ± 0.24 | 0.44 |
| Kt/V | 1.51 ± 0.25 | 1.52 ± 0.26 | 1.49 ± 0.23 | 0.64 |
| Darbepoetin α (μg/week) | 20 (10–30) | 20 (10–32.5) | 15 (10–22.5) | 0.09 |
| PPI/H2 blocker | 64 | 52 | 12 | 0.35 |
| Oral iron preparation | ||||
| Sodium ferrous citrate | 68 | 55 | 13 | 0.34 |
| Ferric citrate hydrate | 40 | 29 | 11 |
| Variables | Courses, n | Effective, n | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| CRP (mg/dL) | |||||
| ≤0.1 | 60 | 53 | 1.00 | ||
| >0.1 | 48 | 31 | 0.24 | (0.09–0.659) | 0.005 |
| Variables | Courses, n | Effective, n | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| Hb (g/dL) | |||||
| <10.7 | 52 | 33 | 1.00 | ||
| ≥10.7 | 32 | 29 | 7.99 | (3.29–19.4) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, C.; Tsuchiya, K.; Kanemitsu, M.; Maeda, K. Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study. Nutrients 2023, 15, 125. https://doi.org/10.3390/nu15010125
Ogawa C, Tsuchiya K, Kanemitsu M, Maeda K. Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study. Nutrients. 2023; 15(1):125. https://doi.org/10.3390/nu15010125
Chicago/Turabian StyleOgawa, Chie, Ken Tsuchiya, Mineko Kanemitsu, and Kunimi Maeda. 2023. "Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study" Nutrients 15, no. 1: 125. https://doi.org/10.3390/nu15010125
APA StyleOgawa, C., Tsuchiya, K., Kanemitsu, M., & Maeda, K. (2023). Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study. Nutrients, 15(1), 125. https://doi.org/10.3390/nu15010125






