Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cow’s Milk-Allergic Patients, Sera, PBMC Samples
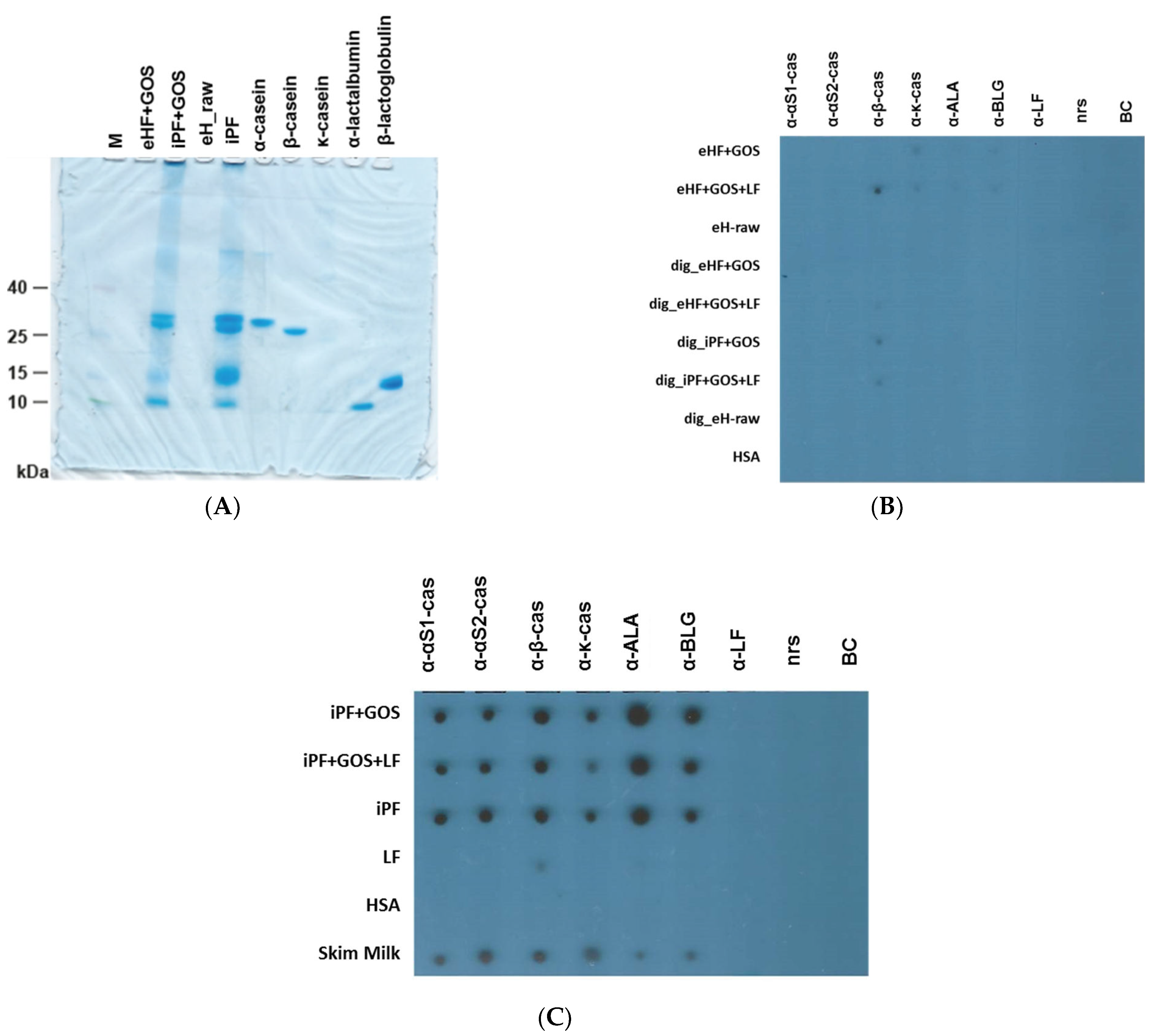
2.2. Allergens, Materials, Infant Formulas, Antibodies, SDS-PAGE
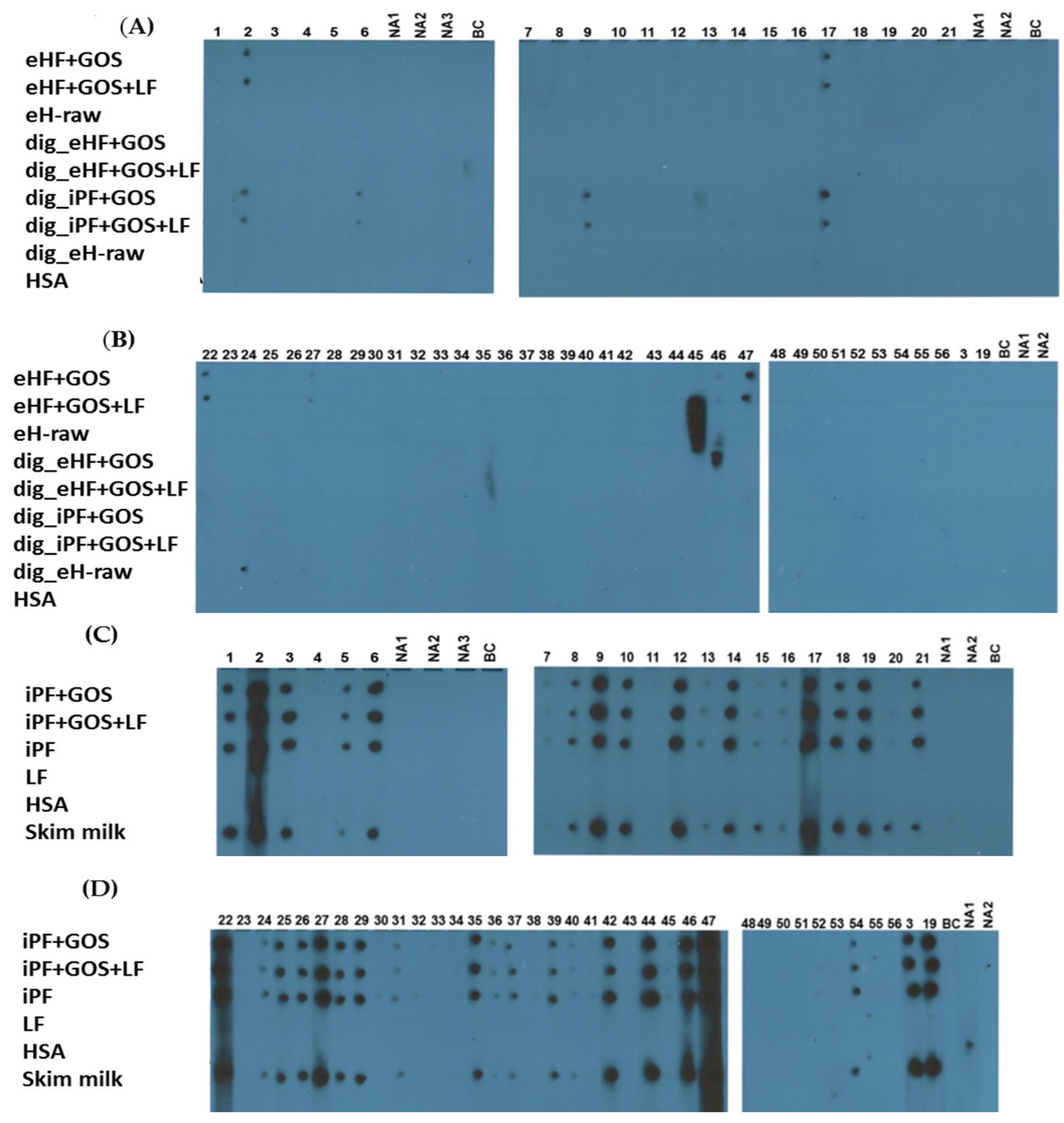
2.3. Immune Dot Blot
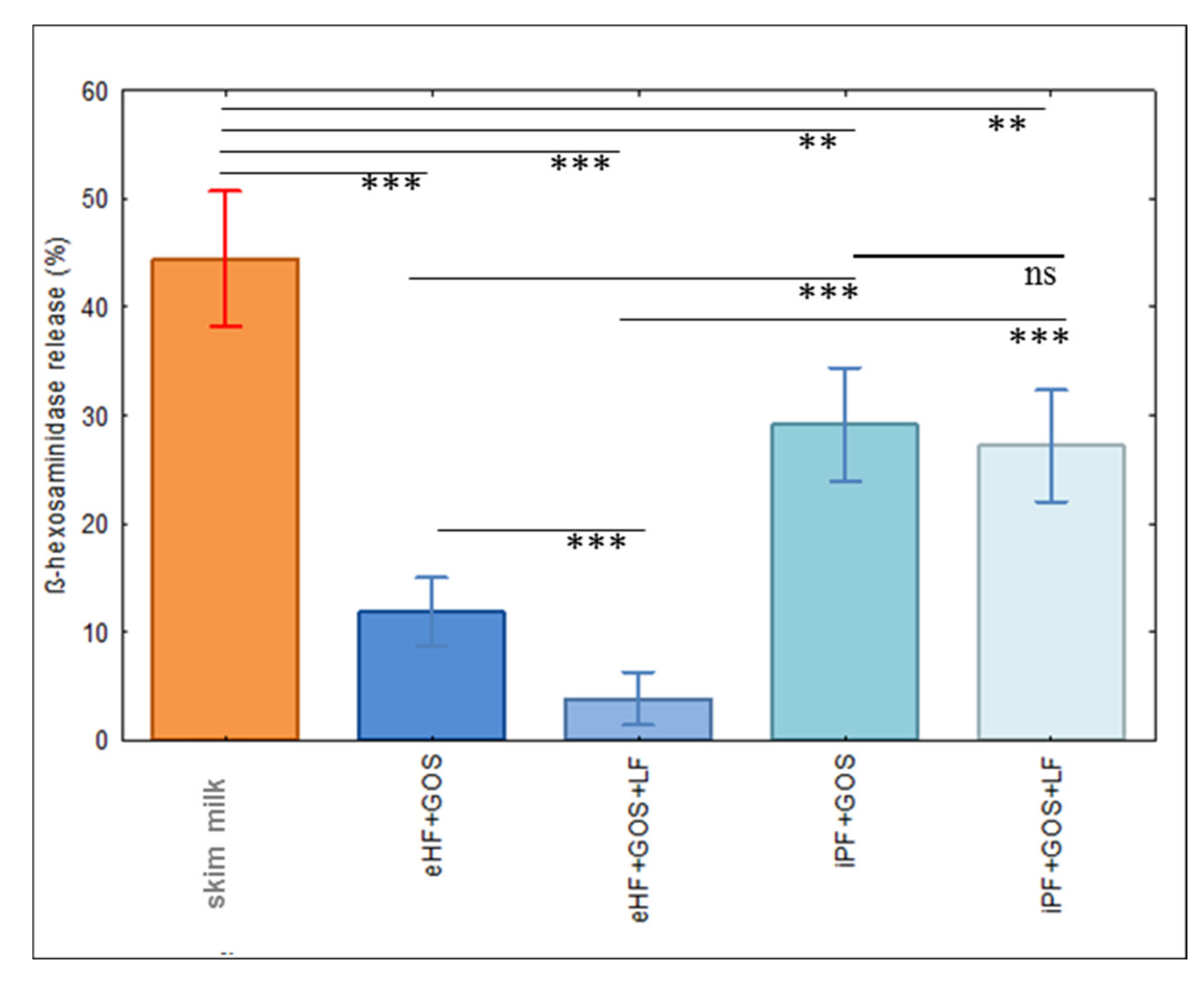
2.4. Basophil Degranulation Assays
2.5. FACS-Based Analysis of the Proliferation of CD4+ and CD8+ T Cells in Response to Antigens by CFSE Dilution
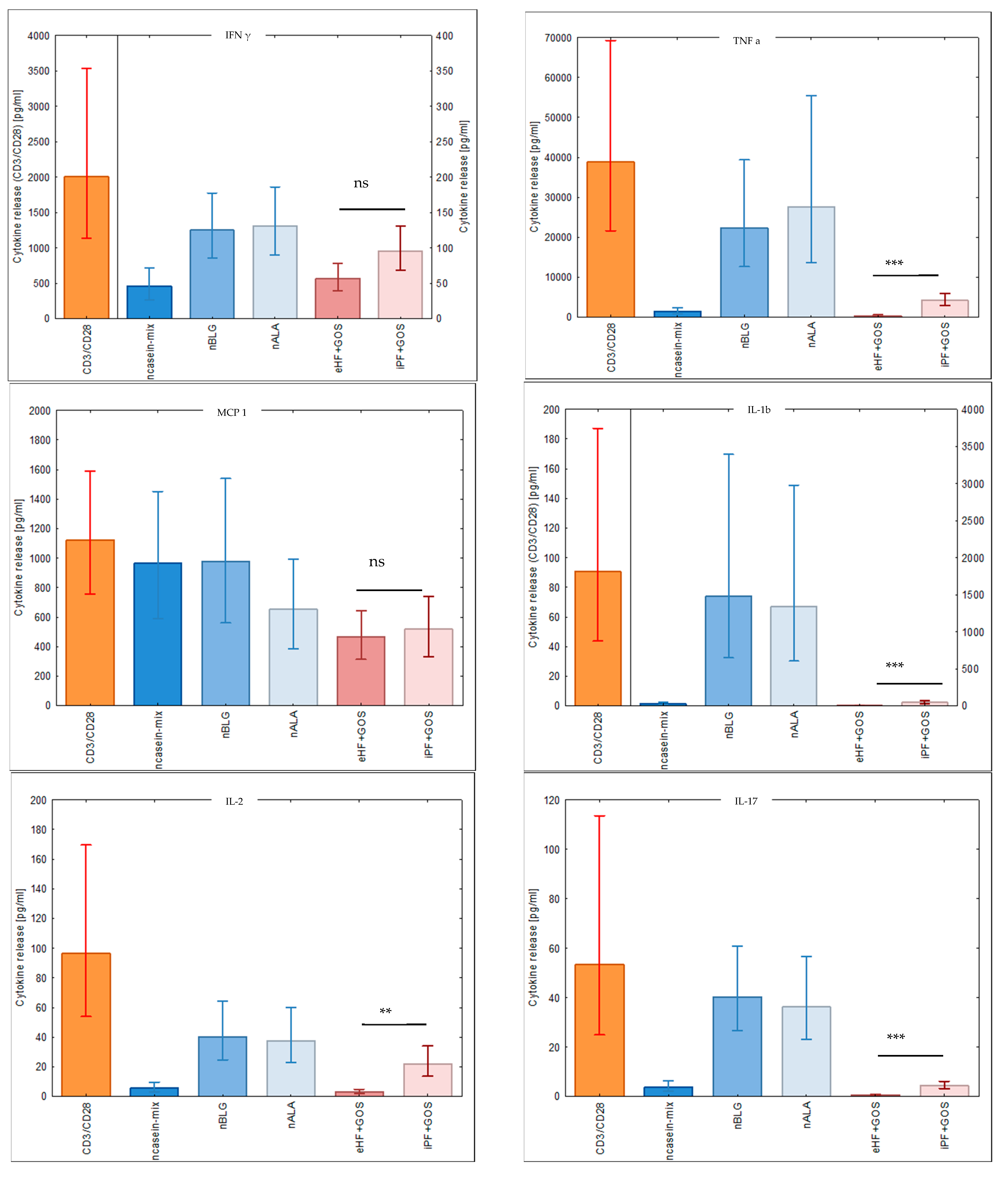
2.6. Measurement of Cytokine Levels
2.7. Statistical Analysis
3. Results
3.1. Extensively Hydrolyzed Infant Formulas Lack Intact Cow’s Milk Allergens
3.2. Extensively Hydrolyzed Formulas and In Vitro Digested Intact Protein Formulas Show Strongly Reduced IgE Reactivity
3.3. Limosilactobacillus fermentum CECT5716 (LF)-Containing Extensively Hydrolyzed Formula Shows the Strongest Reduction of Allergenic Activity
3.4. Extensively Hydrolyzed GOS-Containing Infant Formula Induces T Cell Proliferation Similarly to GOS-Containing Intact Protein Formula
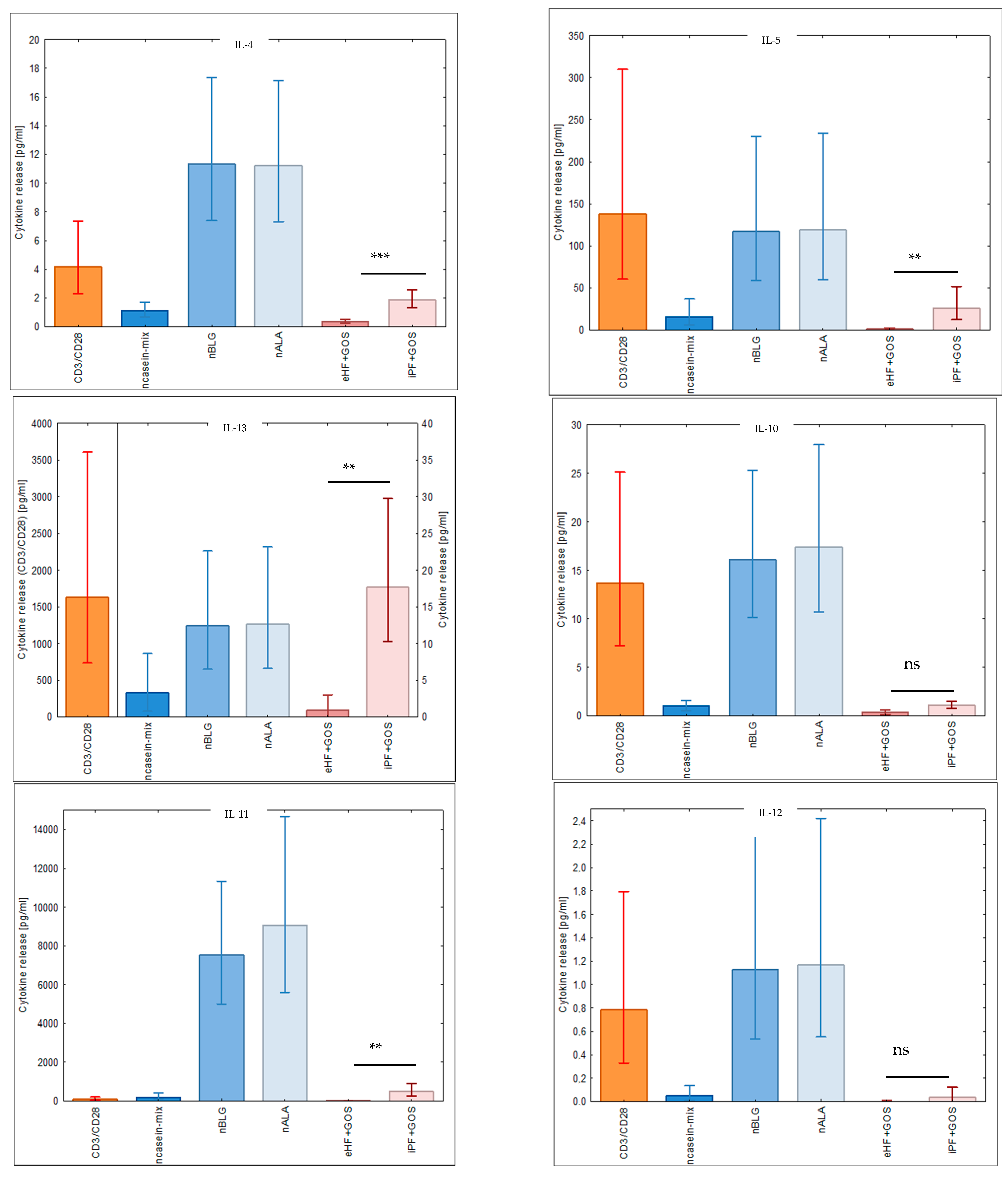
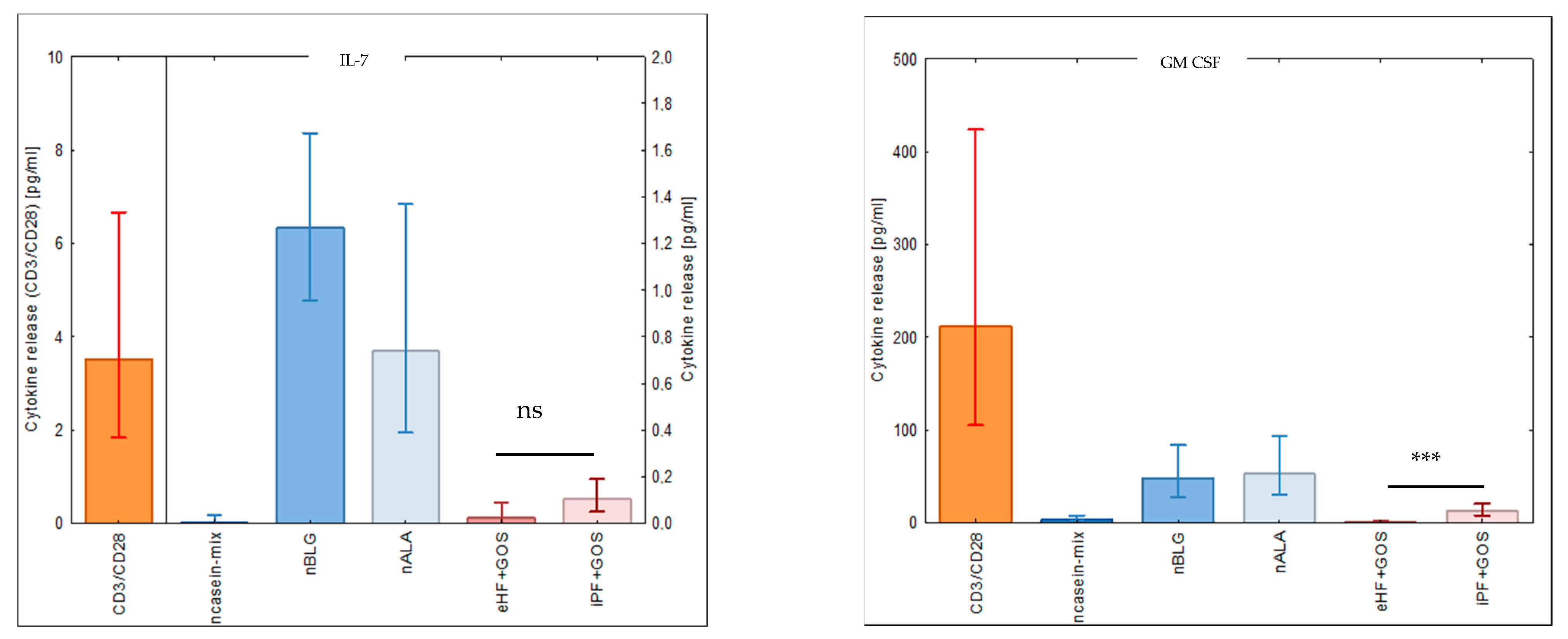
3.5. Extensively Hydrolyzed GOS-Containing Infant Formula Induces Lower Secretion of Inflammatory Cytokines than GOS-Containing Intact Protein Formula
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arasi, S.; Cafarotti, A.; Fiocchi, A. Cow’s milk allergy. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ostblom, E.; Lilja, G.; Pershagen, G.; van Hage, M.; Wickman, M. Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin. Exp. Allergy 2008, 38, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food allergies: The basics. Gastroenterology 2015, 148, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Karsonova, A.V.; Riabova, K.A.; Khaitov, M.R.; Elisyutina, O.G.; Ilina, N.; Fedenko, E.S.; Fomina, D.S.; Beltyukov, E.; Bondarenko, N.L.; Evsegneeva, I.V.; et al. Milk-Specific IgE Reactivity without Symptoms in Albumin-Sensitized Cat Allergic Patients. Allergy Asthma Immunol. Res. 2021, 13, 668–670. [Google Scholar] [CrossRef]
- Sampson, H.A.; Ho, D.G. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J. Allergy Clin. Immunol. 1997, 100, 444–451. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Balic, N.; Geller, B.; Nystrand, M.; Härlin, A.; Thalhamer, J.; Scheiblhofer, S.; Niggemann, B.; et al. Microarray and allergenic activity assessment of milk allergens. Clin. Exp. Allergy 2010, 40, 1809–1818. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27, 1–250. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Gros, M.; van Neerven, R.J.J.; Faas, M.M.; de Vos, P. Immunomodulating properties of protein hydrolysates for application in cow’s milk allergy. Pediatr. Allergy Immunol. 2015, 26, 206–217. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef]
- Linhart, B.; Freidl, R.; Elisyutina, O.; Khaitov, M.; Karaulov, A.; Valenta, R. Molecular Approaches for Diagnosis, Therapy and Prevention of Cows Milk Allergy. Nutrients 2019, 11, 1492. [Google Scholar] [CrossRef]
- Zepeda-Ortega, B.; Goh, A.; Xepapadaki, P.; Sprikkelman, A.; Nicolaou, N.; Hernandez, R.E.H.; Latiff, A.H.A.; Yat, M.T.; Diab, M.; Hussaini, B.A.; et al. Strategies and Future Opportunities for the Prevention, Diagnosis, and Management of Cow Milk Allergy. Front. Immunol. 2021, 10, 608372. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen immunotherapy for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy 2017, 72, 1133. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Focke-Tejkl, M.; Reininger, R.; Civaj, V.; Campana, R.; Thalhamer, J.; Scheiblhofer, S.; Balic, N.; et al. Infant milk formulas differ regarding their allergenic activity and induction of T-cell and cytokine responses. Allergy 2017, 72, 416–424. [Google Scholar] [CrossRef]
- Meulenbroek, L.A.; Oliveira, S.; den Hartog Jager, C.F.; Klemans, R.J.; Lebens, A.F.; van Baalen, T.; Knulst, A.C.; Bruijnzeel-Koomen, C.A.; Garssen, J.; Knippels, L.M.; et al. The degree of whey hydrolysis does not uniformly affect in vitro basophil and T cell responses of cow’s milk-allergic patients. Clin. Exp. Allergy 2014, 44, 529–539. [Google Scholar] [CrossRef]
- Fritsché, R. Animal models in food allergy: Assessment of allergenicity and preventive activity of infant formulas. Toxicol. Lett. 2003, 140–141, 303–309. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Guinot, M.; Guillon, B.; Bernard, H.; Chikhi, A.; Hazebrouck, S.; Junot, C. Administration of extensive hydrolysates from caseins and Lactobacillus rhamnosus GG probiotic does not prevent cow’s milk proteins allergy in a mouse model. Front. Immunol. 2020, 11, 1700. [Google Scholar] [CrossRef]
- Graversen, K.B.; Larsen, J.M.; Pedersen, S.S.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Halken, S.; Licht, T.R.; Bahl, M.I.; Bøgh, K.L. Partially Hydrolysed Whey Has Superior Allergy Preventive Capacity Compared to Intact Whey Regardless of Amoxicillin Administration in Brown Norway Rats. Front. Immunol. 2021, 12, 705543. [Google Scholar] [CrossRef]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and 15 management of ana-phylaxis: Summary report--Second National Institute of Allergy and 16 Infectious Dis-ease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Sakwinska, O.; Moine, D.; Delley, M.; Combremont, S.; Rezzonico, E.; Descombes, P.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Microbiota in Breast Milk of Chinese Lactating Mothers. PLoS ONE 2016, 11, e0160856. [Google Scholar] [CrossRef]
- Garib, V.; Ben-Ali, M.; Kundi, M.; Curin, M.; Yaakoubi, R.; Ben-Mustapha, I.; Mekki, N.; Froeschl, R.; Perkmann, T.; Valenta, R.; et al. Profound differences in IgE and IgG recognition of micro-arrayed allergens in hyper-IgE syndromes. Allergy 2022, 77, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Eckl-Dorna, J.; Campana, R.; Valenta, R.; Niederberger, V. Poor association of allergen-specific antibody, T- and B-cell responses revealed with recombinant allergens and a CFSE dilution-based assay. Allergy 2015, 70, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Curin, M.; Banerjee, S.; Chen, K.W.; Garmatiuk, T.; Resch-Marat, Y.; Carvalho-Queiroz, C.; Blatt, K.; Gafvelin, G.; Grönlund, H.; et al. A hypoallergenic peptide mix containing T cell epitopes of the clinically relevant house dust mite allergens. Allergy 2019, 74, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol. Rev. 2011, 241, 241–259. [Google Scholar] [CrossRef]
- Strobel, S. Immunity induced after a feed of antigen during early life: Oral tolerance v. sensitisation. Proc. Nutr. Soc. 2001, 60, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Han, G.; Li, A.; Kong, X. Lactobacillus fermentum CECT5716 Alleviates the Inflammatory Response in Asthma by Regulating TLR2/TLR4 Expression. Front. Nutr. 2022, 9, 931427. [Google Scholar] [CrossRef]
- Stapel, S.O.; Asero, R.; Ballmer-Weber, B.K.; Knol, E.F.; Strobel, S.; Vieths, S.; Kleine-Tebbe, J. EAACI Task Force, Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy 2008, 63, 793–796. [Google Scholar] [CrossRef]

| Number | Age | Sex | CMP-Related Clinical | SPT CMP | Allergy to Other Food Allergen |
|---|---|---|---|---|---|
| Years | F/M | Symptoms | Sources | ||
| 1 | 0.9 | M | A, AD | pos | no |
| 2 | 12.0 | M | A, AD | pos | lentil |
| 3 | 5.0 | M | A, AD | pos | egg, wheat, walnut |
| 4 | 2.4 | F | A, AD | pos | egg |
| 5 | 8.0 | M | A | pos | egg |
| 6 | 0.5 | M | AD | pos | wheat, fish |
| 7 | 4.0 | M | AD | n.d. | peach, apricot |
| 8 | 8.0 | F | A, AD | n.d. | beef, egg |
| 9 | 4.1 | M | A, AD | n.d. | beef, pork, egg, wheat |
| 10 | 1.9 | F | AD | pos | egg, potato, wheat |
| 11 | 3.4 | M | AD | n.d. | egg |
| 12 | 3.0 | F | AD | n.d. | beef, egg |
| 13 | 1.3 | M | AD | n.d. | egg |
| 14 | 6.1 | M | A, AD | n.d. | egg |
| 15 | 4.5 | F | AD | pos | egg |
| 16 | 1.6 | M | AD | n.d. | no |
| 17 | 8.0 | F | A, AD | n.d. | no |
| 18 | 10 | F | AD | n.d. | egg, fruits, vegetables, cereals, fish, meat |
| 19 | 6.0 | M | AD | n.d. | fish, egg, meat, potato |
| 20 | 1.1 | M | A, AD | pos | wheat, fish |
| 21 | 8.0 | M | A, AD | pos | egg, walnut |
| 22 | 2.9 | F | A, GI | pos | no |
| 23 | 2.9 | F | A, GI | n.d. | egg |
| 24 | 7.2 | M | AD, GI | pos | peanut |
| 25 | 3.7 | M | A, AD, GI | n.d. | egg |
| 26 | 5.0 | F | A, AD, GI | pos | egg, nuts, caviar |
| 27 | 2.9 | F | A, AD, GI | n.d. | no |
| 28 | 2.5 | F | A, AD, GI | n.d. | no |
| 29 | 4.1 | M | AD, GI | n.d. | no |
| 30 | 11.2 | M | A, GI | pos | no |
| 31 | 6.0 | M | AD, GI | n.d. | egg |
| 32 | 6.1 | F | A, GI | pos | egg |
| 33 | 4.2 | M | A, AD, GI | n.d. | no |
| 34 | 1.3 | M | AD, GI | pos | egg, cereals, fish |
| 35 | 10.6 | M | A, AD, GI | pos | egg |
| 36 | 7.3 | F | AD, GI | n.d. | egg, oat |
| 37 | 3.4 | M | A, GI | n.d. | egg |
| 38 | 1.4 | M | A, AD, GI | n.d. | egg, cereals |
| 39 | 5.6 | M | AD, GI | n.d. | egg, wheat, caviar |
| 40 | 6.6 | M | AD, GI | n.d. | egg, wheat, caviar |
| 41 | 1.8 | M | A, AD, GI | n.d. | egg, wheat, walnut |
| 42 | 4.8 | M | A, AD, GI | n.d. | egg, walnut, peanut, soy |
| 43 | 10.9 | M | A, AD, GI | pos | no |
| 44 | 3.2 | M | A, AD, GI | n.d. | egg, wheat, nuts, soy |
| 45 | 2.5 | M | A, AD, GI | n.d. | egg |
| 46 | 2.3 | M | A, AD, GI | n.d. | egg |
| 47 | 10.8 | M | A, AD, GI | n.d. | peanut, soy, walnut |
| 52 | 1.7 | M | AD, GI | n.d. | egg, salmon, codfish |
| 54 | 2.5 | M | A | n.d. | egg, walnut |
| NCMA 48 | 1.7 | M | no | n.d. | peanut |
| NCMA 49 | 11.3 | M | no | n.d. | hazelnut, codfish |
| NCMA 50 | 11.1 | M | no | n.d. | no |
| NCMA 51 | 8.2 | M | no | n.d. | no |
| NCMA 53 | 1.5 | M | no | n.d. | egg |
| NCMA 55 | 5.3 | M | no | n.d. | peanut |
| NCMA 56 | 9.4 | M | no | n.d. | peanut |
| NA1 | 3.0 | F | no | neg | no |
| NA2 | 2.1 | M | no | neg | no |
| NA3 | 0.7 | F | no | neg | no |
| No. | Abbreviation | Description | BCA mg/mL | BCA Peptide mg/mL |
|---|---|---|---|---|
| 1 | eH_raw | Raw material: extensively hydrolyzed whey protein | n.a. | 93.29 |
| 2 | iPF | HiPP standard cow’s milk infant formula (HiPP Pre BIO, powder) w/o synbiotics | 25.45 | n.a. |
| 3 | LF | Limosilactobacillus fermentum CECT5716 (originally obtained from human milk) | 2.26 | n.a. |
| 4 | eHF + GOS | HiPP HA infant formula (HiPP Pre HA Combiotik®, liquid) | n.a. | 20.92 |
| 5 | eHF + GOS + LF | HiPP HA infant formula (HiPP Pre HA Combiotik®, liquid) + L. ferm. CECT5716 | n.d. | n.d. |
| 6 | iPF + GOS | HiPP standard cow’s milk infant formula (HiPP Pre Bio Combiotik®, liquid) | 22.64 | n.a. |
| 7 | iPF + GOS + LF | HiPP standard cow’s milk infant formula (HiPP Pre Bio Combiotik®, liquid) + L. ferm. CECT5716 | n.d. | n.d. |
| 8 | dig_eH_raw | In vitro-digested raw material: extensively hydrolyzed whey protein | n.a. | 5.27 |
| 9 | dig_eHF + GOS | In vitro-digested HiPP HA infant formula (HiPP Pre HA Combiotik®, liquid) | n.a. | 6.30 |
| 10 | dig_eHF + GOS + LF | In vitro-digested HiPP HA infant formula (HiPP Pre HA Combiotik®, liquid) + L. ferm. CECT5716 | n.a. | 8.94 |
| 11 | dig_iPF + GOS | In vitro-digested HiPP standard cow’s milk infant formula (HiPP Pre Bio Combiotik®, liquid) | 8.66 | n.a. |
| 12 | dig_iPF + GOS + LF | In vitro-digested HiPP standard cow’s milk infant formula (HiPP Pre Bio Combiotik®, liquid) + L. ferm. CECT5716 | 8.97 | n.a. |
| 13 | HSA | Human serum albumin (neg. ctl.) | 1 | n.a. |
| 14 | Skim milk powder | Commercial cow’s milk powder, Sigma Aldrich (pos. ctl.) | 1 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freidl, R.; Garib, V.; Linhart, B.; Haberl, E.M.; Mader, I.; Szépfalusi, Z.; Schmidthaler, K.; Douladiris, N.; Pampura, A.; Varlamov, E.; et al. Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity. Nutrients 2023, 15, 111. https://doi.org/10.3390/nu15010111
Freidl R, Garib V, Linhart B, Haberl EM, Mader I, Szépfalusi Z, Schmidthaler K, Douladiris N, Pampura A, Varlamov E, et al. Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity. Nutrients. 2023; 15(1):111. https://doi.org/10.3390/nu15010111
Chicago/Turabian StyleFreidl, Raphaela, Victoria Garib, Birgit Linhart, Elisabeth M. Haberl, Isabelle Mader, Zsolt Szépfalusi, Klara Schmidthaler, Nikos Douladiris, Alexander Pampura, Evgeniy Varlamov, and et al. 2023. "Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity" Nutrients 15, no. 1: 111. https://doi.org/10.3390/nu15010111
APA StyleFreidl, R., Garib, V., Linhart, B., Haberl, E. M., Mader, I., Szépfalusi, Z., Schmidthaler, K., Douladiris, N., Pampura, A., Varlamov, E., Lepeshkova, T., Beltyukov, E., Naumova, V., Taka, S., Nosova, D., Guliashko, O., Kundi, M., Kiyamova, A., Katsamaki, S., & Valenta, R. (2023). Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity. Nutrients, 15(1), 111. https://doi.org/10.3390/nu15010111








