Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection Criteria and Data Extraction
2.3. Methodological Quality Assessment
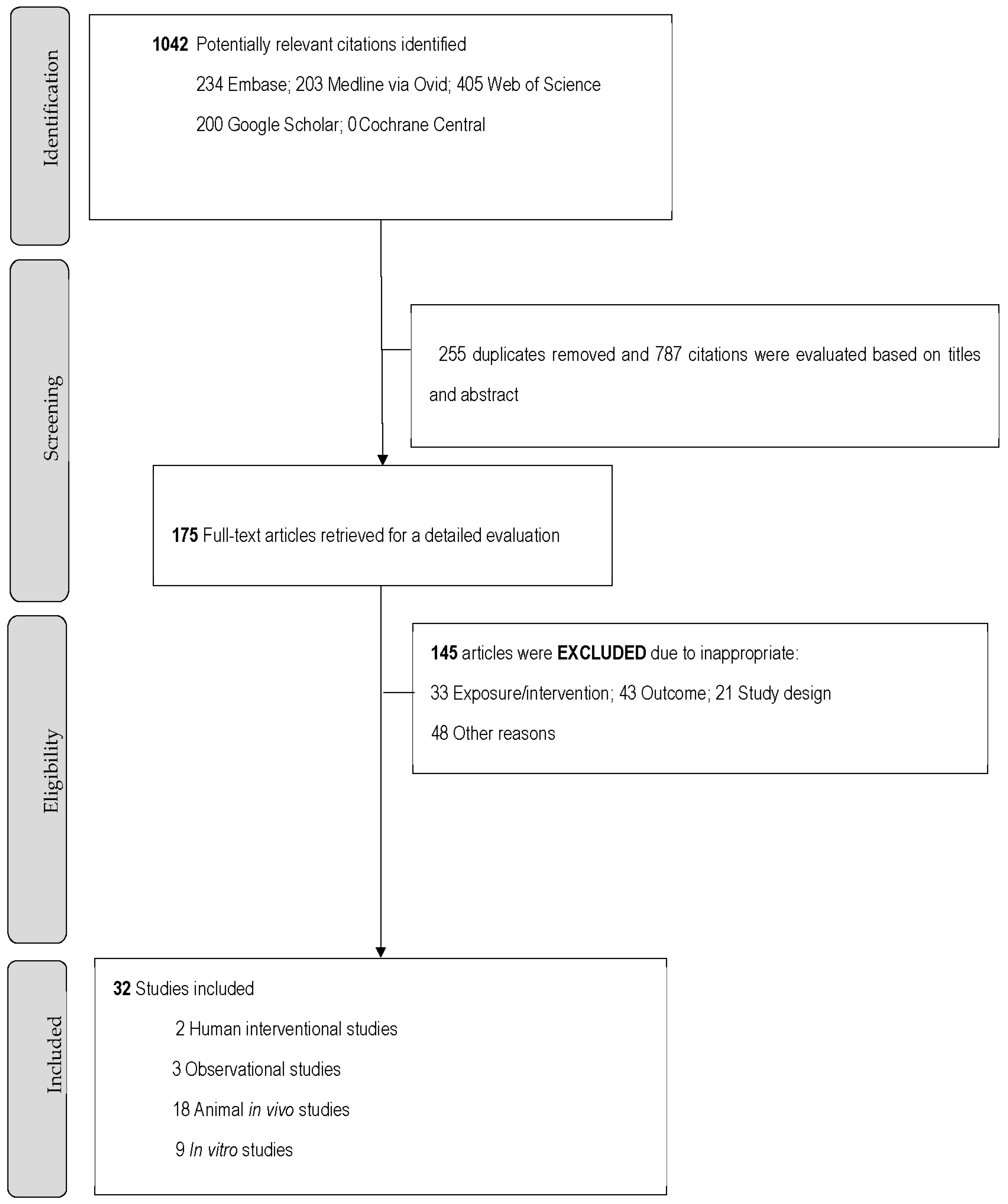
3. Results
3.1. Literature Search and Study Characteristics
3.2. Buckwheat Consumption and the Development of GI Mucosal Inflammation and Symptoms
3.3. Buckwheat and Its GI Anti-Cancer Cell Line Activity
3.4. Effects of Buckwheat on the GI Microbiome
3.5. Study Quality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Han, L.; Liu, F.; Yin, S.; Peng, Q.; Wang, M. A mini-review of isolation, chemical properties and bioactivities of polysaccharides from buckwheat (Fagopyrum Mill). Int. J. Biol. Macromol. 2019, 127, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Noreen, S.; Rizwan, B.; Khan, M.; Farooq, S. Health Benefits of Buckwheat (Fagopyrum Esculentum), Potential Remedy for Diseases, Rare to Cancer: A Mini Review. Infect. Disord. Drug Targets 2021, 21, e170721189478. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef]
- Ajamian, M.; Rosella, G.; Newnham, E.D.; Biesiekierski, J.R.; Muir, J.G.; Gibson, P.R. Effect of Gluten Ingestion and FODMAP Restriction on Intestinal Epithelial Integrity in Patients with Irritable Bowel Syndrome and Self-Reported Non-Coeliac Gluten Sensitivity. Mol. Nutr. Food Res. 2021, 65, e1901275. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef]
- Valido, E.; Stoyanov, J.; Bertolo, A.; Hertig-Godeschalk, A.; Zeh, R.M.; Flueck, J.L.; Minder, B.; Stojic, S.; Metzger, B.; Bussler, W.; et al. Systematic Review of the Effects of Oat Intake on Gastrointestinal Health. J. Nutr. 2021, 151, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lietz, G.; Seal, C. Buckwheat and CVD Risk Markers: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbäck, D.; Wieslander, G. A Review on Epidemiological and Clinical Studies on Buckwheat Allergy. Plants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Harzing, A.W. Publish or Perish. 2007. Available online: https://harzing.com/resources/publish-or-perish (accessed on 17 January 2022).
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- NIH National Heart Lung and Blood Institute. Quality Assessment Tool for before-after (Pre-Post) Studies with no Control Group. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 27 February 2022).
- NIH National Heart Lung and Blood Institute. Quality Assessment of Controlled Intervention Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 27 February 2022).
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Dinu, M.; Macchia, D.; Pagliai, G.; Gori, A.M.; Cesari, F.; Marcucci, R.; Sofi, F.; Casini, A. Symptomatic efficacy of buckwheat products in Non-Celiac Gluten Sensitivity (NCGS). Asia Pac. J. Clin. Nutr. 2017, 26, 630–636. [Google Scholar]
- De Francischi, M.L.P.; Salgado, J.; Da Costa, C.P. Immunological analysis of serum for buckwheat fed celiac patients. Mater. Veg. 1994, 46, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, C.; Guo, Y.; Niu, K.; Momma, H.; Kobayashi, Y.; Fukudo, S.; Nagatomi, R. Staple Foods Consumption and Irritable Bowel Syndrome in Japanese Adults: A Cross-Sectional Study. PLoS ONE 2015, 10, e0119097. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, V.I.; Isakov, V.A.; Morozov, S.V.; Vlasova, A.V.; Naydenova, M.A. Association of food patterns with different forms of small intestinal bacterial overgroth syndrome and treatment efficacy. Ter. arkhiv 2019, 91, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, V.; Isakov, V.A.; Vlasova, A.V.; Naidenova, M.A. Features of nutrition pattern of patients with small intestinal bacterial overgrowth resistant to therapy. Vopr Pitan 2019, 88, 31–38. [Google Scholar]
- Giménez-Bastida, J.A.; Laparra-Llopis, J.M.; Baczek, N.; Zielinski, H. Buckwheat and buckwheat enriched products exert an anti-inflammatory effect on the myofibroblasts of colon CCD-18Co. Food Funct. 2018, 9, 3387–3397. [Google Scholar] [CrossRef]
- Ishii, S.; Katsumura, T.; Shiozuka, C.; Ooyauchi, K.; Kawasaki, K.; Takigawa, S.; Fukushima, T.; Tokuji, Y.; Kinoshita, M.; Ohnishi, M.; et al. Anti-Inflammatory Effect of Buckwheat Sprouts in Lipopolysaccharide-Activated Human Colon Cancer Cells and Mice. Biosci. Biotechnol. Biochem. 2008, 72, 3148–3157. [Google Scholar] [CrossRef] [Green Version]
- Afroz, S.; Ikoma, T.; Yagi, A.; Kogure, K.; Tokumura, A.; Tanaka, T. Concentrated Phosphatidic Acid in Cereal Brans as Potential Protective Agents against Indomethacin-Induced Stomach Ulcer. J. Agric. Food Chem. 2016, 64, 6950–6957. [Google Scholar] [CrossRef]
- Gāliņa, D.; Ansonska, L.; Valdovska, A. Effect of Probiotics and Herbal Products on Intestinal Histomorphological and Immunological Development in Piglets. Veter Med. Int. 2020, 2020, 3461768. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Wang, X.; Ding, C.; Liu, J.; Li, W.; Sun, Y. High-Salt Diet-Induced Gastritis in C57BL/6 Mice is Associated with Microbial Dysbiosis and Alleviated by a Buckwheat Diet. Mol. Nutr. Food Res. 2020, 64, e1900965. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Wei, C.; Luo, T.; Deng, Z.; Fan, Y.; Zheng, L. A polysaccharide from Fagopyrum esculentum Moench bee pollen alleviates microbiota dysbiosis to improve intestinal barrier function in antibiotic-treated mice. Food Funct. 2020, 11, 10519–10533. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Q.; Zhao, S.; Yan, B.; Zhou, X. Impact of Buckwheat Fermented Milk Combined with High-Fat Diet on Rats’ Gut Microbiota and Short-Chain Fatty Acids. J. Food Sci. 2019, 84, 3833–3842. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Olejnik, A.; Rychlik, J.; Kreft, I.; Drożdżyńska, A.; Walkowiak, J. The cytotoxic effect of artificially digested buckwheat products on HT-29 colon cancer cells. J. Cereal Sci. 2018, 83, 68–73. [Google Scholar] [CrossRef]
- Świątecka, D.; Markiewicz, L.H.; Wroblewska, B. In vitro evaluation of the effect of the buckwheat protein hydrolysate on bacterial adhesion, physiology and cytokine secretion of Caco-2 cells. Central Eur. J. Immunol. 2013, 38, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Cui, C.-B.; Kang, I.-J.; Kim, S.Y.; Ham, S.-S. Cytotoxic Effect of Buckwheat (Fagopyrum esculentum Moench) Hull Against Cancer Cells. J. Med. Food 2007, 10, 232–238. [Google Scholar] [CrossRef]
- Zhou, X.L.; Meng, X.X.; Wang, Q.; Zhou, Y.M.; Li, Z.J. Comparative Anti-Tumor Activity Study of Tartary Buckwheat Flavonoids and Amphibian Peptides. Adv. Mater. Res. 2013, 781–784, 1270–1274. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, Z.-D.; Zhou, Y.-M.; Shi, R.-H.; Li, Z.-J. The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules 2019, 24, 3092. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Duan, S.; Jia, H.; Bai, C.; Zhang, L.; Wang, Z. Flavonoids from tartary buckwheat induce G2/M cell cycle arrest and apoptosis in human hepatoma HepG2 cells. Acta Biochim. Biophys. Sin. 2014, 46, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ishikawa, W.; Huang, X.; Tomotake, H.; Kayashita, J.; Watanabe, H.; Kato, N. A buckwheat protein product suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by reducing cell proliferation. J. Nutr. 2001, 131, 1850–1853. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and Carbohydrates in Buckwheat Honey Regulate the Human Intestinal Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef]
- Amelchanka, S.; Kreuzer, M.; Leiber, F. Utility of buckwheat (Fagopyrum esculentum Moench) as feed: Effects of forage and grain on in vitro ruminal fermentation and performance of dairy cows. Anim. Feed Sci. Technol. 2010, 155, 111–121. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Yan, B.-B.; Xiao, Y.; Zhou, Y.-M.; Liu, T.-Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory Function of Buckwheat-Resistant Starch Supplementation on Lipid Profile and Gut Microbiota in Mice Fed with a High-Fat Diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.; Yan, B.; Zhao, S.; Zhou, X. Regulation of tartary buckwheat-resistant starch on intestinal microflora in mice fed with high-fat diet. Food Sci. Nutr. 2020, 8, 3243–3251. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Deng, J.-C.; Li, Q.-Y.; Cao, Y.-J.; Lin, Y.-C.; Bai, W.-D.; Liu, B.; Rao, P.-F.; Ni, L.; Lv, X.-C. Protective Mechanism of Common Buckwheat (Fagopyrum esculentum Moench.) against Nonalcoholic Fatty Liver Disease Associated with Dyslipidemia in Mice Fed a High-Fat and High-Cholesterol Diet. J. Agric. Food Chem. 2020, 68, 6530–6543. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Qin, F.; Qiu, J. Anti-diabetic effects of the soluble dietary fiber from tartary buckwheat bran in diabetic mice and their potential mechanisms. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, S.; Xia, Y.; Huang, J.; Ye, J.; Xuan, Z.; Li, P.; Du, B. Probiotic-fermented black tartary buckwheat alleviates hyperlipidemia and gut microbiota dysbiosis in rats fed with a high-fat diet. Food Funct. 2021, 12, 6045–6057. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2019, 8, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Petersen, I.L.; Llopis, M.L. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef]
- Mu, C.; Ding, N.; Hao, X.; Zhao, Y.; Wang, P.; Zhao, J.; Ren, Y.; Zhang, C.; Zhang, W.; Xiang, B. Effects of different proportion of buckwheat straw and corn straw on performance, rumen fermentation and rumen microbiota composition of fattening lambs. Small Rumin. Res. 2019, 181, 21–28. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Q.; Wang, S.; Diao, Q.; Zhang, N. The Facilitating Effect of Tartary Buckwheat Flavonoids and Lactobacillus plantarum on the Growth Performance, Nutrient Digestibility, Antioxidant Capacity, and Fecal Microbiota of Weaned Piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef] [Green Version]
- Giménez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Shin, Y.; Jung, S.; Kim, S.-Y.; Jo, Y.-H.; Kim, C.-T.; Yun, M.-K.; Lee, S.-J.; Sohn, J.; Yu, H.-J.; et al. The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response. Molecules 2017, 22, 1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Shen, S.-R.; Lai, Y.-J.; Wu, S.-C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Lim, T.-G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.-O. Comparison of Anti-Inflammatory Effects of Flavonoid-Rich Common and Tartary Buckwheat Sprout Extracts in Lipopolysaccharide-Stimulated RAW 264.7 and Peritoneal Macrophages. Oxidative Med. Cell. Longev. 2017, 2017, 9658030. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Park, C.-H.; Kim, D.-W. Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264.7). J. Integr. Med. 2013, 11, 246–252. [Google Scholar] [CrossRef]
- Choi, S.Y.; Choi, J.Y.; Lee, J.M.; Lee, S.; Cho, E.J. Tartary buckwheat on nitric oxide-induced inflammation in RAW264.7 macrophage cells. Food Funct. 2015, 6, 2664–2670. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Li, Y.; Lu, K.; Yin, R.; Ming, J. Phenolics extracted from tartary (Fagopyrum tartaricum L. Gaerth) buckwheat bran exhibit antioxidant activity, and an antiproliferative effect on human breast cancer MDA-MB-231 cells through the p38/MAP kinase pathway. Food Funct. 2017, 8, 177–188. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Carbonaro, M.; Grant, G. Absorption of Quercetin and Rutin in Rat Small Intestine. Ann. Nutr. Metab. 2005, 49, 178–182. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of beta diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Beards, E.; Tuohy, K.; Gibson, G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe 2010, 16, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ichikawa, S.; Aihara, Y.; Yokota, S. Buckwheat allergy in 90,000 school children in Yokohama. Arerugi 1998, 47, 26–33. [Google Scholar]
- Fok, J.S.; Kette, F.; Smith, W.B.; Smith, A.; Ahmadie, A.; Heddle, R.; Hissaria, P. Buckwheat allergy in Australia. Intern. Med. J. 2019, 49, 1552–1553. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Lead Author, Year; Country | Study Type | Population Characteristic | Sample Size; Follow-Up (mos) | GI Outcome Finding | Risk of Bias |
|---|---|---|---|---|---|
| Dinu, 2017; Italy | RCT Crossover | Adults with non-CeD gluten sensitivity | 19 3 (2 periods) | ↓ abdominal pain and bloating vs. gluten-free diet | Moderate [23] |
| De Francischi, 1994; Brazil | non-RCT | Children with CeD | 4 1 | ⇄ toxic prolamines to children CeD | High [24] |
| Zheng, 2015; Japan | Observational | Healthy adults and adults with IBS | 1082 36 | ↑ prevalence of IBS in moderate to high buckwheat consumption regardless of socio-demographic profile, anthropometric, and lifestyle-related factors | Moderate [25] |
| Pilipenko, 2019; Pilipenko, 2018; Russia | Observational | Individuals with SIBO | 458 2 | ↑ resistance to SIBO (0.41 ± 0.47 high buckwheat consumption vs. 0.14 ± 0.35 relative to the rate of consumption of cereals, p < 0.001) compared to those with a resolution of SIBO | Moderate [26,27] |
| Giménez-Bastida, 2018; USA | In vitro with human myofibroblasts of colon CCD-18Co cell line | - | - | ↓ TNFα induced colonic myofibroblast migration vs. control | Reliable w/o restrictions [28] |
| Lead Author, Year; Country | Study Type | Population Characteristic | Sample Size;Follow-Up (mos) | GI Outcome Finding | Risk of Bias |
|---|---|---|---|---|---|
| Ishii, 2008; Japan | Animal In vivo | Mice | 10 2 | ↓ IL6 and TNFα in the spleen and liver vs. oral LPS | Reliable w/o restrictions [29] |
| Afroz 2016; Japan | Animal In vivo | Mice | 35 1.25 | ↓ gastric mucosal lesions | Reliable with restrictions [30] |
| Gāliņa, 2020; Latvia | Animal In vivo | Pig | 44 1.5 | ⇄ histomorphology and immune system of the intestinal mucosa | Reliable w/o restrictions [31] |
| Li, 2020; China | Animal In vivo | Mice | 40 2 | ↓ gastric mucosa inflammation vs. high salt diet ↓ lymphocyte infiltration vs. high salt diet | Reliable w/o restrictions [32] |
| Zhu, 2020; China | Animal In vivo | Mice | 60 2 | ↓ intestinal mucosal and recess destruction vs. natural resolution ↓ intestinal inflammation vs. natural resolution | Reliable w/o restrictions [33] |
| Zhou, 2019, China | Animal In vivo | Rats | 32 1 | ↓ colon IL6, TNFa, and LPS with a high-fat diet with buckwheat vs. a high-fat diet | Reliable w/o Restrictions [34] |
| Lead Author, Year; Country | Sample Source; Study Type | Buckwheat Preparation; Control | GI Derived Cancer Cell Line Tested | Significant Finding | Risk of Bias |
|---|---|---|---|---|---|
| Dziedzic, 2018; Poland | Human In vitro | Buckwheat digestate;Blank cell culture | Human colon adenocarcinoma cell line HT-29 | + cytotoxicity capacity of buckwheat bran, groats, and raw grain | Reliable with restrictions [35] |
| Ishii, 2008; Japan | Human In vitro | Buckwheat ethanol extract; LPS | Human colon cancer cell line (CoLotC) | ↓ IL8 expression ⇄ cytotoxicity | Reliable w/o restrictions [29] |
| Swiatecka, 2013; Poland | Human In vitro | Buckwheat protein hydrolysate; Blank cell culture | CaCo-2 cell line | + cytotoxicity ↑ IL8 expression | Reliable with restrictions [36] |
| Kim, 2007; Korea | Human In vitro | Buckwheat ethanol extract None | Gastric carcinoma cell line, Hepatocellular carcinoma | + cytotoxicity in a dose-response manner | Reliable with restrictions [37] |
| Zhou, 2013; China | Human In vitro | Buckwheat flavonoids extract; Blank cell culture | Human gastric cancer MGC80-3 | + cytotoxicity | Reliable w/o restrictions [38] |
| Zhou, 2019; China | Human In vitro | Buckwheat flavonoids extract; Blank cell culture | Human gastric cancer MGC80-3 | + cytotoxicity | Reliable w/o restrictions [39] |
| Li, 2014; China | Human In vitro | Buckwheat flavonoids extract; Blank cell culture | Human hepatoma HepG2 cells | + cytotoxicity in a dose-response and time-dependent manner + anti-oxidant capacity | Reliable w/o restrictions [40] |
| Liu, 2001; Japan | Rat In vivo | Buckwheat proteins Casein | 1,2-dimethyl hydrazine-induced colonic tumors | ↓ incidence of bloody stools ⇄ incidence of colonic tumors ↓ proliferation of colonic epithelium | Reliable with restrictions [41] |
| Lead Author, Year; Country | Population Study Type Type of Buckwheat Diet Comparison | Microbial Diversity Findings | SCFAs | Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|
| α Diversity | β Diversity | Acetate | Propionate | Butyrate | Total | |||
| Jiang, 2020; China | Healthy adult In vitro Common buckwheat Negative control | ↓ Chao1 and Shannon indices ↑ Simpson index | + β diversity among buckwheat groups vs. negative control | - | - | - | - | Reliable w/o restrictions [42] |
| Amelchanka, 2010; Switzerland | Cows In vitro Common buckwheat Basal diet/grass clover hay | - | - | ⇄ | ⇄ | ⇄ | ⇄ | Reliable w/ restrictions [43] |
| Li, 2020; China | Mice In vivo Common buckwheat High salt diet | ↑ α diversity | Influenced the return to control microbiome profile after a high salt diet | - | - | - | - | Reliable w/o restrictions [32] |
| Zhou, 2018; China | Mice In vivo Tartary buckwheat High-fat diet | - | - | ↑ | ↑ | ↑ | - | Reliable w/o restrictions [44] |
| Zhou, 2019; China | Mice In vivo Tartary buckwheat High-fat diet | - | - | ↑ | ↑ | ↑ | - | Reliable w/o restrictions [45] |
| Zhou, 2020; China | Mice In vivo Tartary buckwheat High-fat diet | ⇄ Shannon, Chao, and Ace indices | - | ⇄ | ↑ | ↑ | ↑ | Reliable w/o restrictions [46] |
| Huang, 2020; China | Mice In vivo Common buckwheat High-fat diet | - | High-dose buckwheat consumption has significantly different β-diversity | ↑ | ⇄ | ↑ | ↑ | Reliable w/o restrictions [47] |
| Wu, 2021; China | Mice In vivo Tartary buckwheat None | - | - | ↑ | ↑ | ↑ | - | Reliable w/o restrictions [48] |
| Zhu, 2020; China | Mice In vivo Common buckwheat Natural resolution after ceftriaxone exposure | ↑ Shannon, Chao, and Ace indices | - | - | - | - | - | Reliable w/o restrictions [33] |
| Liu, 2021; China | Rats In vivo Tartary buckwheat High-fat diet | ↑ α diversity | - | ↑ | ↑ | ⇄ | ↑ | Reliable w/o restrictions [7] |
| Zhou, 2019; China | Rats In vivo Tartary buckwheat High-fat diet | ⇄ Shannon and Simpson indices ↑ Chao and Ace indices | - | ⇄ | ⇄ | ⇄ | ⇄ | Reliable w/o restrictions [34] |
| Ren, 2021; China | Rats In vivo Tartary buckwheat High-fat diet | ↑ Shannon and Simpson indices | BTB and GTB have significantly different β-diversity with a high-fat diet | - | - | - | - | Reliable w/o restrictions [49] |
| Peng, 2019; China | Rats In vivo Tartary buckwheat High-fat diet | ↑ Chao index | - | ⇄ | ⇄ | ⇄ | - | Reliable w/o restrictions [50] |
| Fotschki, 2020; Poland | Rats In vivo Common buckwheat Normal diet | - | - | ↑ | ⇄ | ↑ | ↑ | Reliable w/o restrictions [51] |
| Mu, 2019; China | Lambs In vivo Common buckwheat Normal diet | ↓ Chao1, Ace, Shannon, and Simpson indices with increasing buckwheat concentration | - | ↓ | ↑ | ↑ | ↑ | Reliable w/o restrictions [52] |
| Cui, 2019; China | Pig In vivo Tartary buckwheat Basal diet | ⇄ Observed species and Chao, Shannon, and Simpson indices | - | - | - | - | - | Reliable w/o restrictions [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valido, E.; Stoyanov, J.; Gorreja, F.; Stojic, S.; Niehot, C.; Kiefte-de Jong, J.; Llanaj, E.; Muka, T.; Glisic, M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients 2023, 15, 1. https://doi.org/10.3390/nu15010001
Valido E, Stoyanov J, Gorreja F, Stojic S, Niehot C, Kiefte-de Jong J, Llanaj E, Muka T, Glisic M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients. 2023; 15(1):1. https://doi.org/10.3390/nu15010001
Chicago/Turabian StyleValido, Ezra, Jivko Stoyanov, Frida Gorreja, Stevan Stojic, Christa Niehot, Jessica Kiefte-de Jong, Erand Llanaj, Taulant Muka, and Marija Glisic. 2023. "Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health" Nutrients 15, no. 1: 1. https://doi.org/10.3390/nu15010001
APA StyleValido, E., Stoyanov, J., Gorreja, F., Stojic, S., Niehot, C., Kiefte-de Jong, J., Llanaj, E., Muka, T., & Glisic, M. (2023). Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients, 15(1), 1. https://doi.org/10.3390/nu15010001









