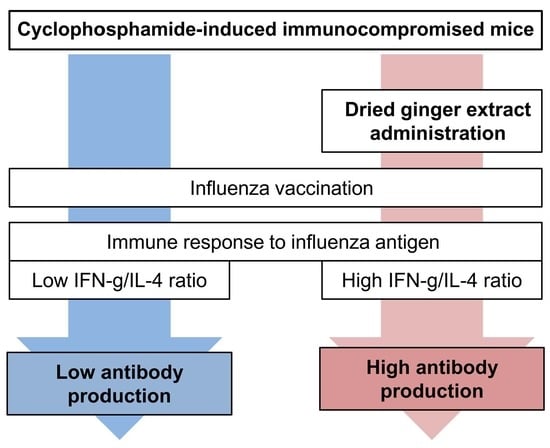
Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
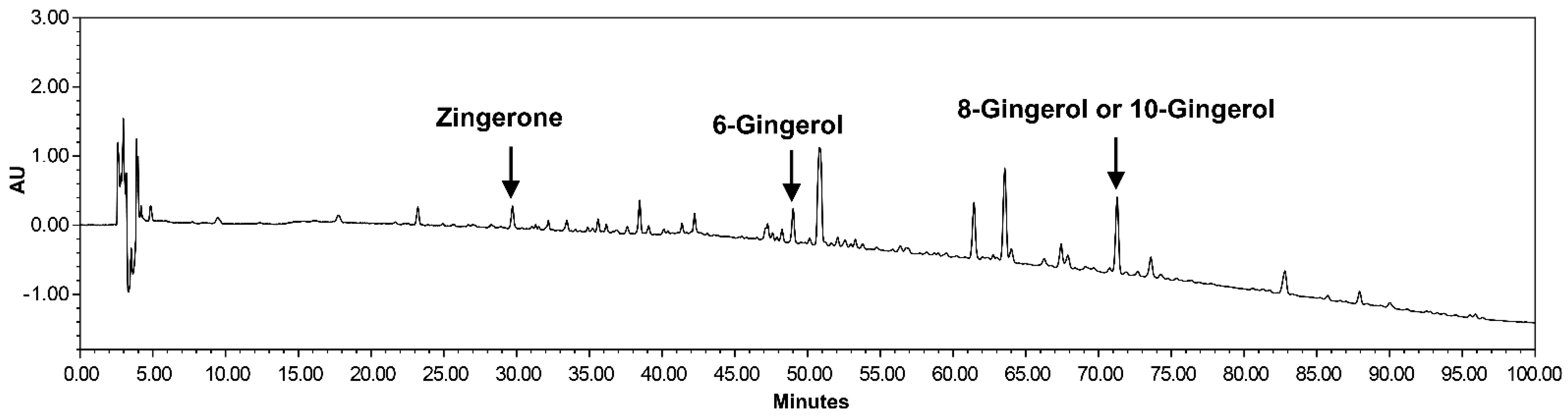
2.2. Preparation of Dried Ginger Extracts
2.3. Immunization
2.4. Measurement of Influenza-Specific Antibody Subclasses by Enzyme-Linked Immunosorbent Assay
2.5. Isolation and Culture of Splenocytes for Flu Antigen Stimulation
2.6. Flow Cytometric Analyses of Isolated Splenic Cells
2.7. Cytometric Bead Array of Splenocyte Supernatants after Influenza Antigen Stimulation
2.8. Determination of Antibody Titers by Hemagglutination Inhibition Assay
2.9. Statistical Analyses
3. Results
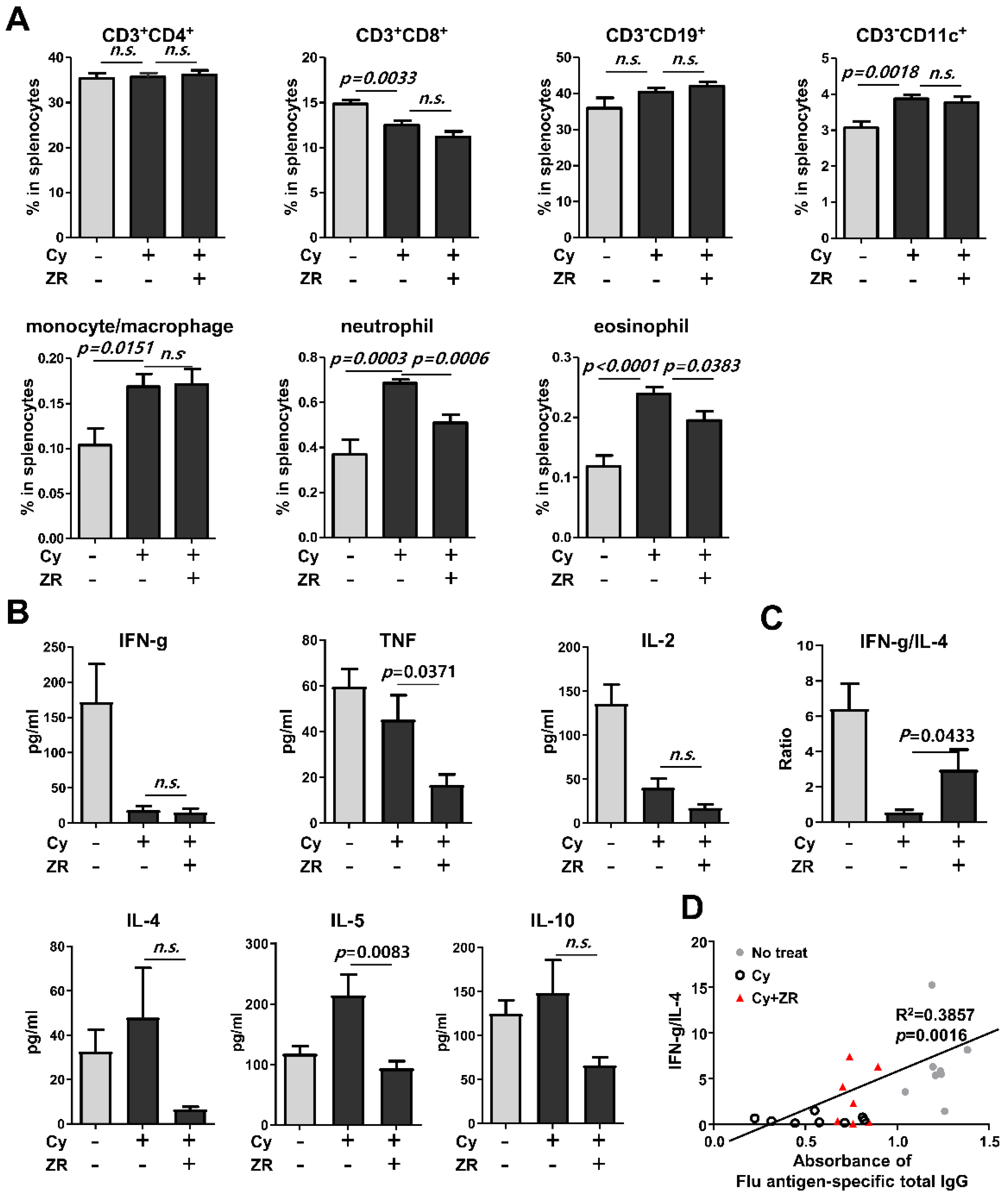
3.1. Zingiberis Processum Rhizoma Enhances Flu Antigen-Specific Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice
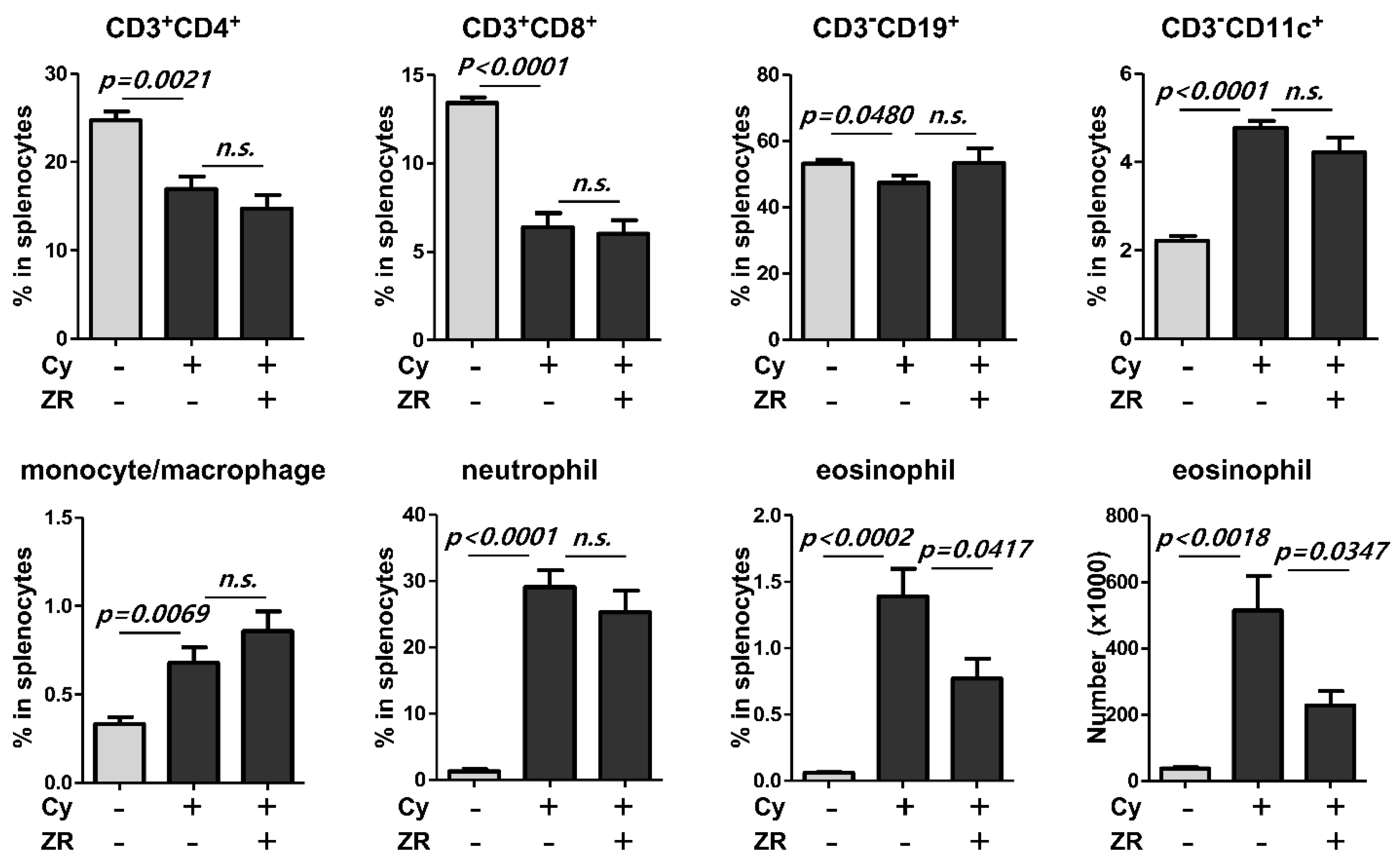
3.2. Zingiberis Processum Rhizoma Restores T Helper Type 1/T Helper Type 2 Balance to a Normal State in Cy-Induced Immunocompromised Mice
3.3. Zingiberis Processum Rhizoma Suppresses the Increase of Eosinophil in the Spleen before Vaccination after Cyclophosphamide Injection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Influenza Strategy 2019–2030; World Health Organization (WHO): Geneva, Switzerland, 2019. [Google Scholar]
- Bosaeed, M.; Kumar, D. Seasonal influenza vaccine in immunocompromised persons. Hum. Vaccin. Immunother. 2018, 14, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Halasa, N.B.; Savani, B.N.; Asokan, I.; Kassim, A.; Simons, R.; Summers, C.; Bourgeois, J.; Clifton, C.; Vaughan, L.A.; Lucid, C.; et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol. Blood Marrow Transplant. 2016, 22, 528–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Campbell, P.; Hoschler, K.; Hidalgo, L.; Al-Dabbagh, M.; Wilson, L.; Humar, A. Randomized controlled trial of adjuvanted versus nonadjuvanted influenza vaccine in kidney transplant recipients. Transplantation 2016, 100, 662–669. [Google Scholar] [CrossRef]
- Noh, J.Y.; Song, J.Y.; Choi, W.S.; Lee, J.; Seo, Y.B.; Kwon, Y.J.; Ko, G.J.; Cha, D.R.; Kang, Y.S.; Lee, Y.K.; et al. Immunogenicity of trivalent influenza vaccines in patients with chronic kidney disease undergoing hemodialysis: MF59-adjuvanted versus non-adjuvanted vaccines. Hum. Vaccin. Immunother. 2016, 12, 2902–2908. [Google Scholar] [CrossRef] [Green Version]
- Natori, Y.; Shiotsuka, M.; Slomovic, J.; Hoschler, K.; Ferreira, V.; Ashton, P.; Rotstein, C.; Lilly, L.; Schiff, J.; Singer, L.; et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin. Infect. Dis. 2018, 66, 1698–1704. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, M.; Jena, L.; Rath, S.N.; Kumar, S. Identification of suitable natural inhibitor against influenza A (H1N1) neuraminidase protein by molecular docking. Genom. Inform. 2016, 14, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, A.; Jafarzadeh, S.; Nemati, M. Therapeutic potential of ginger against COVID-19: Is there enough evidence? J. Tradit. Chin. Med. Sci. 2021, 8, 267–279. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Fazelian, S.; Rahimlou, M.; Kern, F.G.; Heshmati, S.; Omidi, A.; Persad, E.; Heshmati, J. Effect of ginger (Zingiber officinale) supplementation on oxidative stress parameters: A systematic review and meta-analysis. J. Food Biochem. 2021, 45, e13612. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef]
- Rasool, A.; Khan, M.U.; Ali, M.A.; Anjum, A.A.; Ahmed, I.; Aslam, A.; Mustafa, G.; Masood, S.; Ali, M.A.; Nawaz, M. Anti-avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (Ginger) and Allium sativum (Garlic) in chick embryos. Pak. J. Pharm. Sci. 2017, 30, 1341–1344. [Google Scholar]
- Chang, J.S.; Wang, K.C.; Yeh, C.F.; Shieh, D.E.; Chiang, L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 145, 146–151. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; Filho, J.W.G.O.; Ferreira, J.R.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-Oxidative and Anti-Inflammatory Effects of Ginger in Health and Physical Activity: Review of Current Evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [Google Scholar]
- Ahui, M.L.; Champy, P.; Ramadan, A.; Pham Van, L.; Araujo, L.; Brou André, K.; Diem, S.; Damotte, D.; Kati-Coulibaly, S.; Offoumou, M.A.; et al. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int. Immunopharmacol. 2008, 8, 1626–1632. [Google Scholar] [CrossRef]
- Skibinski, D.A.G.; Jones, L.A.; Zhu, Y.O.; Xue, L.W.; Au, B.; Lee, B.; Naim, A.N.M.; Lee, A.; Kaliaperumal, N.; Low, J.G.H.; et al. Induction of human t-cell and cytokine responses following vaccination with a novel influenza vaccine. Sci. Rep. 2018, 8, 18007. [Google Scholar] [CrossRef]
- Miyauchi, K.; Sugimoto-Ishige, A.; Harada, Y.; Adachi, Y.; Usami, Y.; Kaji, T.; Inoue, K.; Hasegawa, H.; Watanabe, T.; Hijikata, A.; et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 2016, 17, 1447–1458. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Ginger and its health claims: Molecular aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell. Dev. Biol. Anim. 2004, 40, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar] [CrossRef]
- Rose, S.; Misharin, A.; Perlman, H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytom. A 2012, 81, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, O.; Akan, H.; Koç, H.; Beksaç, M.; Ilhan, O.; Ozcan, M.; Yalçín, S.; Gürman, G.; Konuk, N.; Uysal, A. Eosinophilia after allogeneic bone marrow transplantation using busulfan and cyclophosphamide conditioning regimen. Bone Marrow Transpl. 1996, 18, 261. [Google Scholar]
- Thomson, A.W.; Mathie, I.H.; Sewell, H.F. Cyclophosphamide-induced eosinophilia in the rat: Concomitant changes in T-cell subsets, B cells and large granular lymphocytes within lymphoid tissues. Immunology 1987, 60, 383–388. [Google Scholar] [PubMed]
- Mathie, I.H.; Sewell, H.F.; Thomson, A.W. Generation of large granular lymphocytes and lymphocyte subset changes linked with cyclophosphamide-induced eosinophilia in rats--and the effects of ciclosporin. Scand. J. Immunol. 1987, 26, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009, 85, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Kont, I.; Fürst, R. Benefits of ginger and its constituent 6-Shogaol in inhibiting inflammatory processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Afrida, I.R.; Fatchiyah, F.; Widodo, N.; Amin, M.; Djati, M.S. Shogaol, Bisdemethoxycurcumin, and Curcuminoid: Potential zingiber compounds against COVID-19. Biointerface Res. Appl. Chem. 2021, 11, 12869–12876. [Google Scholar] [CrossRef]
- Haridas, M.; Sasidhar, V.; Nath, P.; Abhithaj, J.; Sabu, A.; Rammanohar, P. Compounds of citrus medica and zingiber officinale for COVID-19 inhibition: In silico evidence for cues from ayurveda. Future J. Pharm. Sci. 2021, 7, 13. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, H.; You, S. Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination. Nutrients 2022, 14, 1984. https://doi.org/10.3390/nu14091984
Kim J, Lee H, You S. Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination. Nutrients. 2022; 14(9):1984. https://doi.org/10.3390/nu14091984
Chicago/Turabian StyleKim, Jihyun, Hoyoung Lee, and Sooseong You. 2022. "Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination" Nutrients 14, no. 9: 1984. https://doi.org/10.3390/nu14091984
APA StyleKim, J., Lee, H., & You, S. (2022). Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination. Nutrients, 14(9), 1984. https://doi.org/10.3390/nu14091984