Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA
Abstract
:1. Introduction
2. Materials and Methods
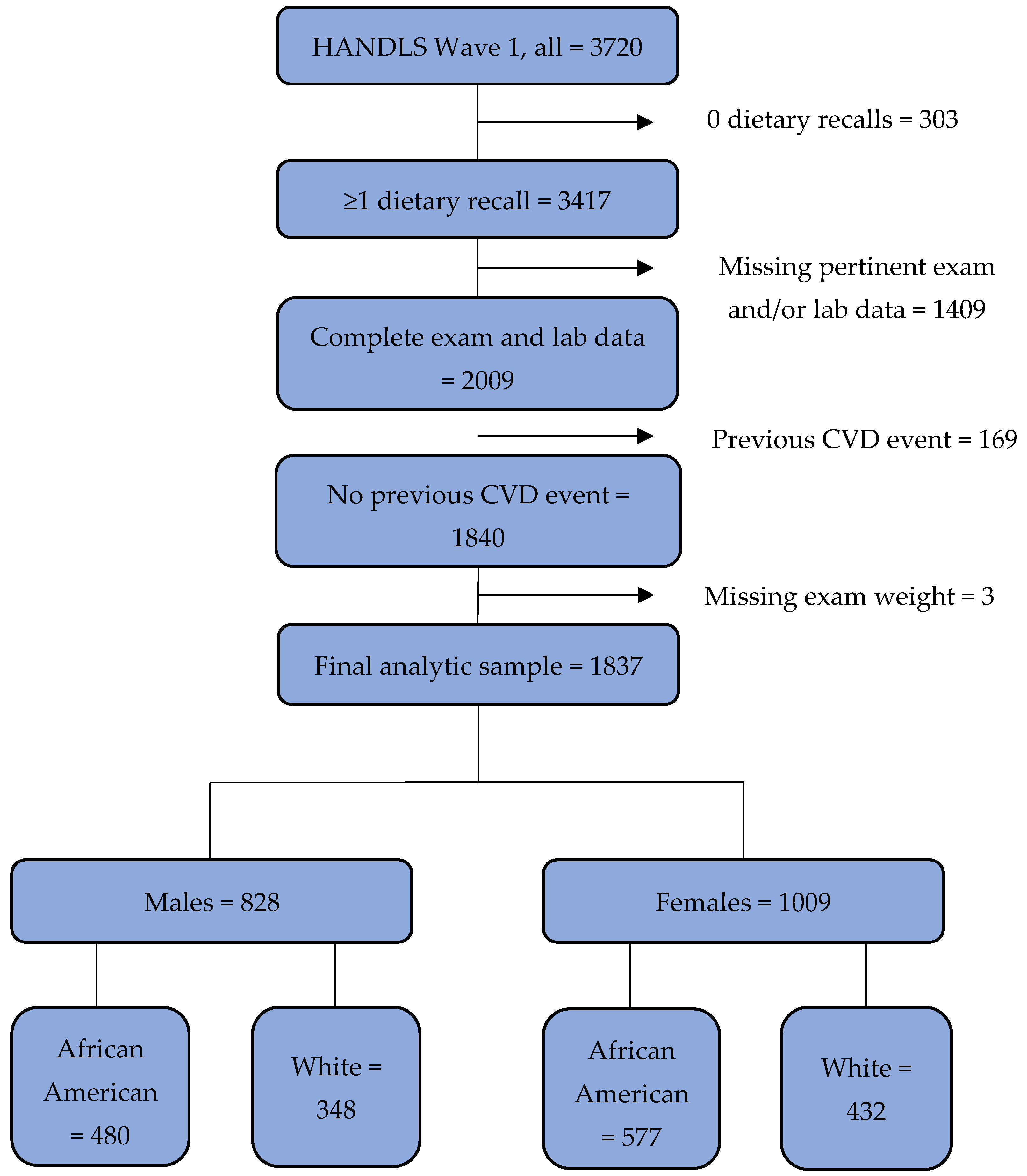
2.1. Study Sample
2.2. Flavonoid Intake Assessment
2.3. Metabolic Syndrome and Its Risk Factors
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Usual Total Flavonoid Intake Distributions
3.3. Flavonoid Intake—MetS Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirode, G.; Wong, R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH dietary pattern and cardiometabolic outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food. Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.; Rocha, S.; de Freitas, V. Going “green” in the prevention and management of atherothrombotic diseases: The role of dietary polyphenols. J. Clin. Med. 2021, 10, 1490. [Google Scholar] [CrossRef]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and insulin-resistance: From molecular evidences to clinical trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [Green Version]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012, 1259, 87–94. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, Y.; Cheng, G.; Zhang, Y.; Cai, S. Comparative study of dietary flavonoids with different structures as α-glucosidase inhibitors and insulin sensitizers. J. Agric. Food. Chem. 2019, 67, 10521–10533. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health, National Cancer Institute. Dietary Assessment Primer: Learn More about Usual Dietary Intake. Available online: https://dietassessmentprimer.cancer.gov/learn/usual.html (accessed on 21 March 2022).
- National Institutes of Health, National Cancer Institute, Division of Cancer Control and Population Sciences, Epidemiology and Genomics Research Program. Usual Dietary Intakes: The NCI Method. Available online: https://epi.grants.cancer.gov/diet/usualintakes/method.html (accessed on 2 December 2021).
- National Institutes of Health, National Cancer Institute, Division of Cancer Control and Population Sciences, Epidemiology and Genomics Research Program. Usual Dietary Intakes: SAS macros for Analysis of a Single Dietary Component. Available online: https://epi.grants.cancer.gov/diet/usualintakes/macros_single.html (accessed on 2 December 2021).
- Fanelli Kuczmarski, M.; Sebastian, R.S.; Goldman, J.D.; Murayi, T.; Steinfeldt, L.C.; Eosso, J.R.; Moshfegh, A.J.; Zonderman, A.B.; Evans, M.K. Dietary flavonoid intakes are associated with race but not income in an urban population. Nutrients 2018, 10, 1749. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.K.; Lepkowski, J.M.; Powe, N.R.; LaVeist, T.; Kuczmarski, M.F.; Zonderman, A.B. Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn. Dis. 2010, 20, 267–275. Available online: https://www.ethndis.org/priorarchives/ethn-20-03-267.pdf (accessed on 30 November 2021). [PubMed]
- National Institutes of Health, National Institute on Aging, Intramural Research Program. Healthy Aging in Neighborhoods of Diversity across the Life Span: Study Protocols. Available online: https://handls.nih.gov/02Protocol.htm (accessed on 2 February 2021).
- US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. 2004 HHS Poverty Guidelines. Available online: https://aspe.hhs.gov/2004-hhs-poverty-guidelines (accessed on 2 February 2021).
- National Institutes of Health, National Institute on Aging, Intramural Research Program. Healthy Aging in Neighborhoods of Diversity across the Life Span. Available online: https://handls.nih.gov/ (accessed on 2 February 2021).
- Wilkinson, G.S. The Wide Range Achievement Test (WRAT-3), 3rd ed.; Wide Range, Inc.: Wilmington, DE, USA, 1993. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The U.S. Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Steinfeldt, L.; Anand, J.; Murayi, T. Food reporting patterns in the USDA Automated Multiple-Pass Method. Procedia Food Sci. 2013, 2, 145–156. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture, Food Surveys Research Group. FNDDS Documentation and Databases. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 2 December 2021).
- U.S. Department of Agriculture, Food Surveys Research Group. Flavonoid Database. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-flavonoid-database/ (accessed on 21 March 2022).
- Sebastian, R.S.; Wilkinson Enns, C.; Goldman, J.D.; Steinfeldt, L.C.; Martin, C.L.; Moshfegh, A.J. Flavonoid Values for USDA Survey Foods and Beverages 2007–2010 [Documentation]. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fndds/FlavonoidDB_documentation_0710.pdf (accessed on 21 March 2022).
- Sebastian, R.S.; Wilkinson Enns, C.; Goldman, J.D.; Steinfeldt, L.C.; Martin, C.L.; Clemens, J.C.; Murayi, T.; Moshfegh, A.J. New, publicly available flavonoid data products: Valuable resources for emerging science. J. Food Compos. Anal. 2017, 64, 68–72. [Google Scholar] [CrossRef]
- Sebastian, R.S.; Wilkinson Enns, C.; Goldman, J.D.; Martin, C.L.; Steinfeldt, L.C.; Murayi, T.; Moshfegh, A.J. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among U.S. adults. J. Nutr. 2015, 145, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Kuczmarski, M.T.; Wang, Y.; Mason, M.A.; Evans, M.K.; Zonderman, A.B. Receiver-operating characteristics of adiposity for metabolic syndrome: The Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Public Health Nutr. 2011, 14, 77–92. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health, National Cancer Institute. Dietary Assessment Primer: Learn More about Regression Calibration. Available online: https://dietassessmentprimer.cancer.gov/learn/regression.html (accessed on 2 December 2021).
- Sohrab, G.; Hosseinpour-Niazi, S.; Hejazi, J.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int. J. Food Sci. Nutr. 2013, 64, 661–667. [Google Scholar] [CrossRef]
- Qu, R.; Jia, Y.; Liu, J.; Jin, S.; Han, T.; Na, L. Dietary flavonoids, copper intake, and risk of metabolic syndrome in Chinese adults. Nutrients 2018, 10, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary polyphenol intake is associated with HDL-cholesterol and a better profile of other components of the metabolic syndrome: A PREDIMED-Plus sub-study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, Y.J.; Yang, Y.K.; Kim, J.Y.; Kwon, O. Dietary flavan-3-ols intake and metabolic syndrome risk in Korean adults. Nutr. Res. Pract. 2012, 6, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet. 2019, 32, 578–590. [Google Scholar] [CrossRef]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between polyphenol intake and hypertension in adults and older adults: A population-based study in Brazil. PLoS ONE 2016, 11, e0165791. [Google Scholar] [CrossRef]
- The Multiple Source Method (MSM). Available online: https://msm.dife.de (accessed on 15 January 2022).
- Harttig, U.; Haubrock, J.; Knüppel, S.; Boeing, H.; EFCOVAL Consortium. The MSM program: Web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur. J. Clin. Nutr. 2011, 65, S87–S91. [Google Scholar] [CrossRef] [Green Version]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Soltani, S.; Boozari, M.; Cicero, A.F.G.; Jamialahmadi, T.; Sahebkar, A. Effects of phytochemicals on macrophage cholesterol efflux capacity: Impact on atherosclerosis. Phytother. Res. 2021, 35, 2854–2878. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Waśkiewicz, A.; Drygas, W.; Cicha-Mikołajczyk, A.; Zujko, K.; Szcześniewska, D.; Kozakiewicz, K.; Witkowska, A.M. Dietary habits and dietary antioxidant intake are related to socioeconomic status in Polish adults: A nationwide study. Nutrients 2020, 12, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Determinants of Health. Available online: https://www.healthypeople.gov/2020/about/foundation-health-measures/Determinants-of-Health (accessed on 15 January 2022).
- Ramires, E.K.N.M.; Menezes, R.C.E.; Longo-Silva, G.; Santos, T.G.D.; Marinho, P.M.; Silveira, J.A.C.D. Prevalence and factors associated with metabolic syndrome among Brazilian adult population: National Health Survey-2013. Arq. Bras. Cardiol. 2018, 110, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.Y.; Jung, C.H.; Park, J.S.; Rhee, E.J.; Kim, S.W. Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res. Clin. Pract. 2005, 67, 70–77. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Obhi, H.K.; Weiss, J.; Canas, J.A.; Beydoun, H.A.; Evans, M.K.; Zonderman, A.B. Systematic inflammation is associated with depressive symptoms differentially by sex and race: A longitudinal study of urban adults. Mol. Psychiatry 2020, 25, 1286–1300. [Google Scholar] [CrossRef] [Green Version]
- Fanelli Kuczmarski, M.; Mason, M.A.; Allegro, D.; Zonderman, A.B.; Evans, M.K. Diet quality inversely associated with C-reactive protein levels in urban low-income African American and White adults. J. Acad. Nutr. Diet. 2013, 113, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.A.; Dwyer, J.T.; Erdman, J.W.; King, J.C.; Lyle, B.J.; Schneeman, B.O.; Weaver, C.M.; serving as an ad hoc Working Group on a Framework for Developing Recommended Intakes for Dietary Bioactives. Perspective: Framework for developing recommended intakes of bioactive dietary substances. Adv. Nutr. 2021, 12, 1087–1099. [Google Scholar] [CrossRef]
- Sebastian, R.S.; Fanelli Kuczmarski, M.; Wilkinson Enns, C.; Goldman, J.D.; Murayi, T.; Moshfegh, A.J.; Zonderman, A.B.; Evans, M.K. Application of the Database of Flavonoid Values for USDA Food Codes 2007-2010 in assessing intake differences between the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study and What We Eat in America (WWEIA), NHANES. J. Food Comp. Anal. 2021, 104, 104124. [Google Scholar] [CrossRef]
- National Institutes of Health, National Cancer Institute, Division of Cancer Control and Population Sciences, Epidemiology and Genomics Research Program. Difficulties Posed by Intra-Individual Variation. Available online: https://epi.grants.cancer.gov/diet/usualintakes/difficulties.html (accessed on 2 December 2021).
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Characteristic | All | African American | White | All | African American | White |
| Demographic: | ||||||
| Age 3, y, % (SE) | ||||||
| 30–49 | 60.3 (1.7) | 59.3 (1.8) | 62.0 (1.4) | 60.3 (3.7) | 60.3 (5.3) | 60.2 (2.3) |
| 50–64 | 39.7 (1.7) | 40.7 (1.8) | 38.0 (1.4) | 39.7 (3.7) | 39.7 (5.3) | 39.8 (2.3) |
| Poverty status 3,4, % (SE) | ||||||
| <125% poverty | 16.7 (3.0) | 19.5 (4.4) | 11.6 (1.7) | 22.8 (1.7) | 28.1 (2.6) | 13.4 (2.3) |
| >125% poverty | 83.3 (3.0) | 80.5 (4.4) | 88.4 (1.7) | 77.2 (1.7) | 71.9 (2.6) | 86.6 (2.3) |
| Education, % (SE) | ||||||
| <High school diploma/GED | 24.3 (3.6) | 28.3 (3.2) | 17.0 (7.3) | 22.4 (2.5) | 25.1 (3.5) | 17.6 (6.2) |
| High school diploma/GED | 31.9 (2.1) | 37.0 (2.8) | 22.5 (10.0) | 32.2 (1.8) | 35.5 (0.8) | 26.4 (6.9) |
| Post-secondary Education | 40.2 (8.4) | 32.9 (3.0) | 53.6 (22.7) | 41.4 (6.3) | 37.3 (3.4) | 48.6 (18.4) |
| Not reported | 3.5 (3.6) | 1.7 (2.0) | 6.8 (5.6) | 4.0 (3.8) | 2.1 (2.1) | 7.3 (5.8) |
| Literacy level 5, % (SE) | ||||||
| ≤8th grade | 36.6 (0.9) | 47.7 (1.6) | 16.1 (6.3) | 29.1 (4.7) | 37.1 (6.0) | 15.1 (4.6) |
| >8th grade | 62.3 (1.2) | 51.6 (1.9) | 82.0 (7.0) | 70.1 (5.1) | 62.0 (6.6) | 84.3 (4.5) |
| Not reported | 1.1 (0.5) | 0.7 (0.4) | 1.9 (0.7) | 0.8 (0.5) | 0.9 (0.7) | 0.6 (0.5) |
| Lifestyle: | ||||||
| Smoking status 6, % (SE) | ||||||
| Currently smoking | 49.8 (4.3) | 56.4 (4.7) | 37.7 (9.9) | 35.8 (3.9) | 38.4 (3.9) | 31.3 (8.1) |
| Not currently smoking | 47.9 (4.0) | 40.8 (5.2) | 61.0 (9.1) | 61.3 (4.4) | 58.3 (4.8) | 66.6 (9.6) |
| Not reported | 2.3 (1.0) | 2.8 (1.1) | 1.3 (1.0) | 2.8 (1.1) | 3.2 (1.7) | 2.2 (1.6) |
| Self-reported health measures: | ||||||
| Health status, % (SE) | ||||||
| Excellent/very good | 41.8 (3.0) | 36.2 (1.6) | 51.9 (10.6) | 40.6 (3.8) | 36.0 (2.6) | 48.7 (9.1) |
| Good | 40.4 (0.8) | 45.8 (1.4) | 30.6 (3.3) | 40.1 (3.1) | 43.1 (3.9) | 35.0 (4.6) |
| Fair/poor | 17.8 (2.4) | 18.0 (1.7) | 17.4 (7.4) | 19.2 (2.7) | 20.9 (2.7) | 16.3 (4.8) |
| Menopause status, % (SE) | ||||||
| Premenopausal | - | - | - | 54.8 (1.5) | 55.2 (3.1) | 54.1 (2.8) |
| Postmenopausal | - | - | - | 42.4 (2.9) | 40.6 (5.3) | 45.4 (2.4) |
| Not reported | - | - | - | 2.8 (1.8) | 4.1 (2.9) | 0.5 (0.4) |
| Diagnostic health measures: | ||||||
| Body mass index 7, mean (SE) | 27.6 (0.2) | 27.0 (0.2) | 28.6 (0.2) | 30.9 (0.6) | 32.0 (0.7) | 28.9 (1.7) |
| Metabolic syndrome 8, % (SE) | 28.2 (2.2) | 23.1 (4.7) | 37.8 (5.2) | 37.9 (5.6) | 39.8 (3.5) | 34.7 (11.2) |
| MetS risk factors: 9 | ||||||
| Elevated triglycerides 10, % (SE) | 30.3 (4.9) | 24.1 (5.2) | 41.6 (7.2) | 24.1 (2.7) | 20.2 (2.8) | 30.8 (5.2) |
| Low HDL-C 11, % (SE) | 35.8 (1.8) | 31.2 (3.6 | 44.3 (1.4) | 48.7 (4.0) | 50.9 (1.4) | 44.9 (10.8) |
| Elevated blood glucose 12, % (SE) | 30.2 (2.1) | 28.5 (1.3) | 33.5 (6.0) | 26.2 (4.0) | 26.7 (3.6) | 25.2 (5.1) |
| Elevated blood pressure 13, % (SE) | 40.6 (3.0) | 40.2 (2.2) | 41.4 (4.7) | 44.2 (1.8) | 51.9 (4.6) | 30.7 (8.3) |
| Elevated waist circumference 14, % (SE) | 34.1 (1.7) | 29.4 (2.1) | 42.7 (4.5) | 71.0 (4.8) | 77.3 (3.9) | 60.0 (12.1) |
| Percentiles of Usual Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 5th | 10th | 25th | 50th | 75th | 90th | 95th | ||
| N | ------------------------------------------------------- mg (SE) ------------------------------------------------- | ||||||||
| Males: | |||||||||
| All | 828 | 187.4 (10.6) | 22.8 (3.6) | 33.9 (4.3) | 64.6 (6.0) | 126.3 (8.9) | 239.3 (13.5) | 409.2 (26.4) | 558.6 (43.4) |
| African American | 480 | 172.7 (15.3) | 20.8 (3.9) | 31.5 (4.6) | 60.4 (6.9) | 117.1 (11.4) | 219.6 (18.7) | 377.4 (36.1) | 510.5 (55.0) |
| White | 348 | 214.5 (17.4) | 27.1 (4.4) | 39.5 (6.3) | 75.8 (9.7) | 147.2 (14.2) | 276.9 (22.9) | 467.5 (38.4) | 632.5 (53.6) |
| Females: | |||||||||
| All | 1009 | 255.2 (34.6) | 19.5 (3.9) | 31.9 (5.7) | 68.5 (9.6) | 149.8 (19.3) | 315.6 (39.7) | 588.2 (82.2) | 837.8 (129.2) |
| African American | 577 | 212.4 (37.4) | 16.4 (3.0) | 26.4 (4.4) | 56.8 (8.0) | 125.4 (18.7) | 261.4 (44.4) | 486.6 (87.4) | 702.7 (139.3) |
| White | 432 | 329.9 (35.9) | 31.4 (8.5) | 48.7 (11.0) | 98.6 (16.5) | 204.5 (24.5) | 413.3 (42.7) | 742.1 (83.1) | 1053.7 (136.5) |
| Metabolic Syndrome/Risk Factor 7 | Males | Females | ||||
|---|---|---|---|---|---|---|
| All | African American | White | All | African American | White | |
| -------------------------------------------------Odds ratio (95% CI)------------------------------------------------- | ||||||
| Metabolic syndrome | 0.62 (0.53, 0.71) | 0.72 (0.53, 0.98) | 0.72 (0.52, 0.98) | 1.22 (0.92, 1.61) | 1.24 (0.93, 1.65) | 1.23 (0.93, 1.63) |
| Elevated triglycerides | 0.86 (0.71, 1.04) | 0.96 (0.74, 1.25) | 0.96 (0.73, 1.26) | 1.00 (0.81, 1.24) | 1.15 (0.90, 1.48) | 1.15 (0.90, 1.47) |
| Low HDL-C | 0.52 (0.45, 0.61) | 0.58 (0.42, 0.81) | 0.57 (0.41, 0.81) | 1.34 (1.07, 1.69) | 1.36 (1.02, 1.82) | 1.35 (1.02, 1.79) |
| Elevated blood glucose | 0.79 (0.69, 0.89) | 0.79 (0.72, 0.87) | 0.79 (0.71, 0.87) | 1.61 (1.18, 2.18) | 1.57 (1.20, 2.06) | 1.56 (1.19, 2.04) |
| Elevated blood pressure | 0.78 (0.49, 1.24) | 0.80 (0.51, 1.24) | 0.79 (0.50, 1.25) | 0.90 (0.68, 1.18) | 0.79 (0.47, 1.33) | 0.79 (0.47, 1.32) |
| Elevated waist circumference | 1.85 (0.55, 6.19) | 1.94 (0.69, 5.48) | 1.98 (0.68, 5.74) | 0.84 (0.50, 1.41) | 0.81 (0.46 1.43) | 0.81 (0.47, 1.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian, R.S.; Fanelli Kuczmarski, M.T.; Goldman, J.D.; Moshfegh, A.J.; Zonderman, A.B.; Evans, M.K. Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA. Nutrients 2022, 14, 1924. https://doi.org/10.3390/nu14091924
Sebastian RS, Fanelli Kuczmarski MT, Goldman JD, Moshfegh AJ, Zonderman AB, Evans MK. Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA. Nutrients. 2022; 14(9):1924. https://doi.org/10.3390/nu14091924
Chicago/Turabian StyleSebastian, Rhonda S., Marie T. Fanelli Kuczmarski, Joseph D. Goldman, Alanna J. Moshfegh, Alan B. Zonderman, and Michele K. Evans. 2022. "Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA" Nutrients 14, no. 9: 1924. https://doi.org/10.3390/nu14091924
APA StyleSebastian, R. S., Fanelli Kuczmarski, M. T., Goldman, J. D., Moshfegh, A. J., Zonderman, A. B., & Evans, M. K. (2022). Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA. Nutrients, 14(9), 1924. https://doi.org/10.3390/nu14091924






