Magnesium—A Potential Key Player in Inflammatory Bowel Diseases?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Hair Sample Collection and SEM and EDX Measurement
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. Patients Included
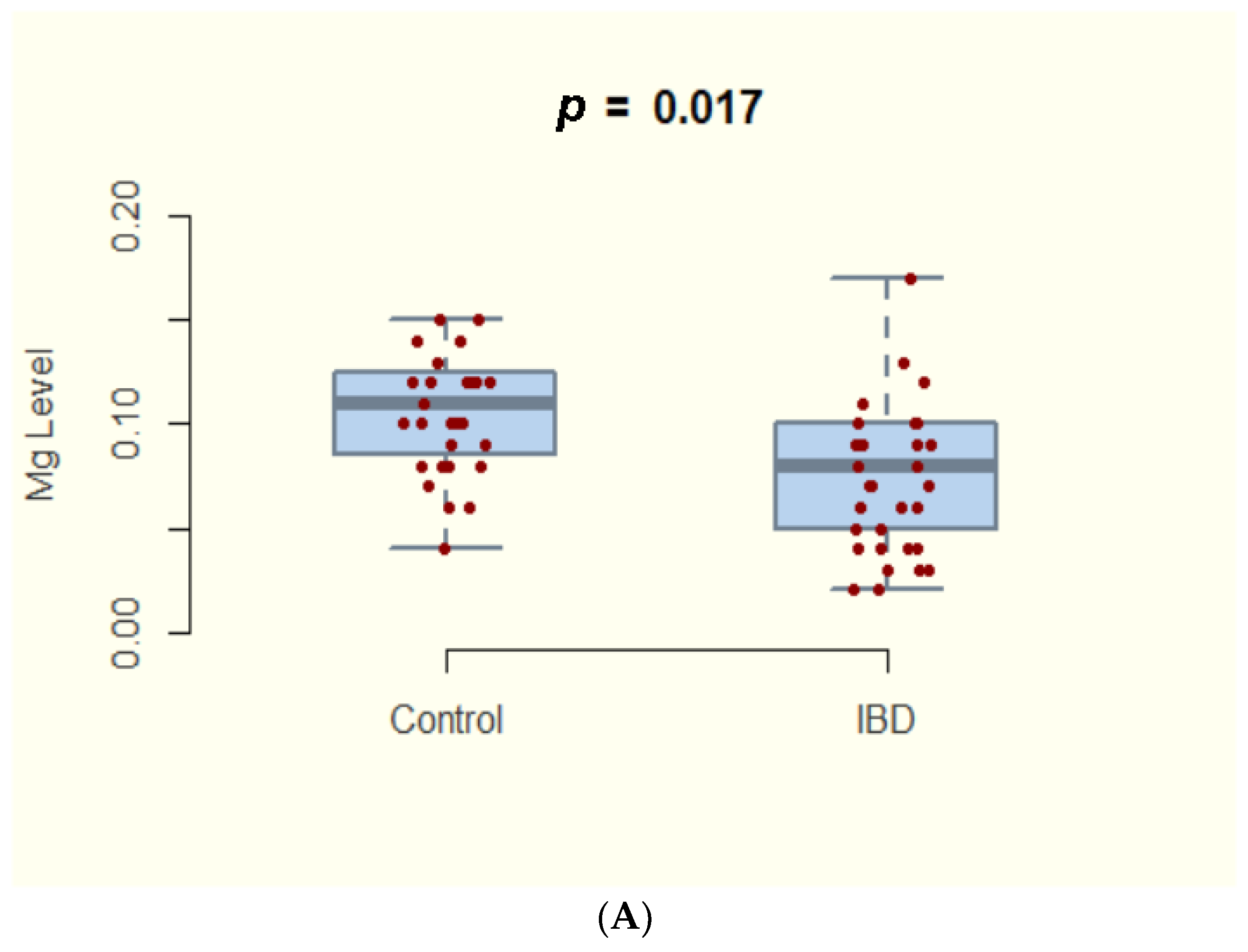
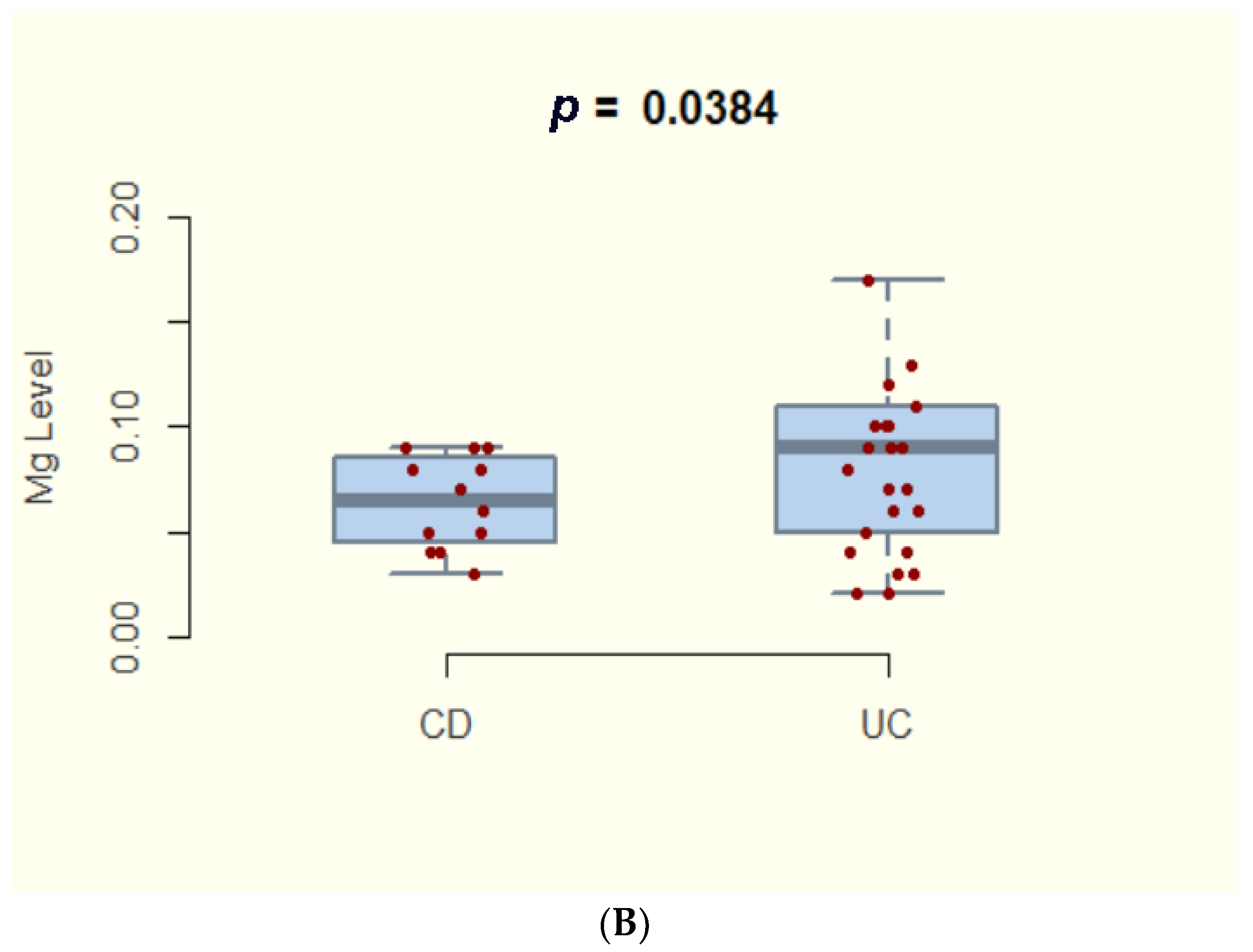
3.2. Magnesium Deficiency in IBD and Subtypes
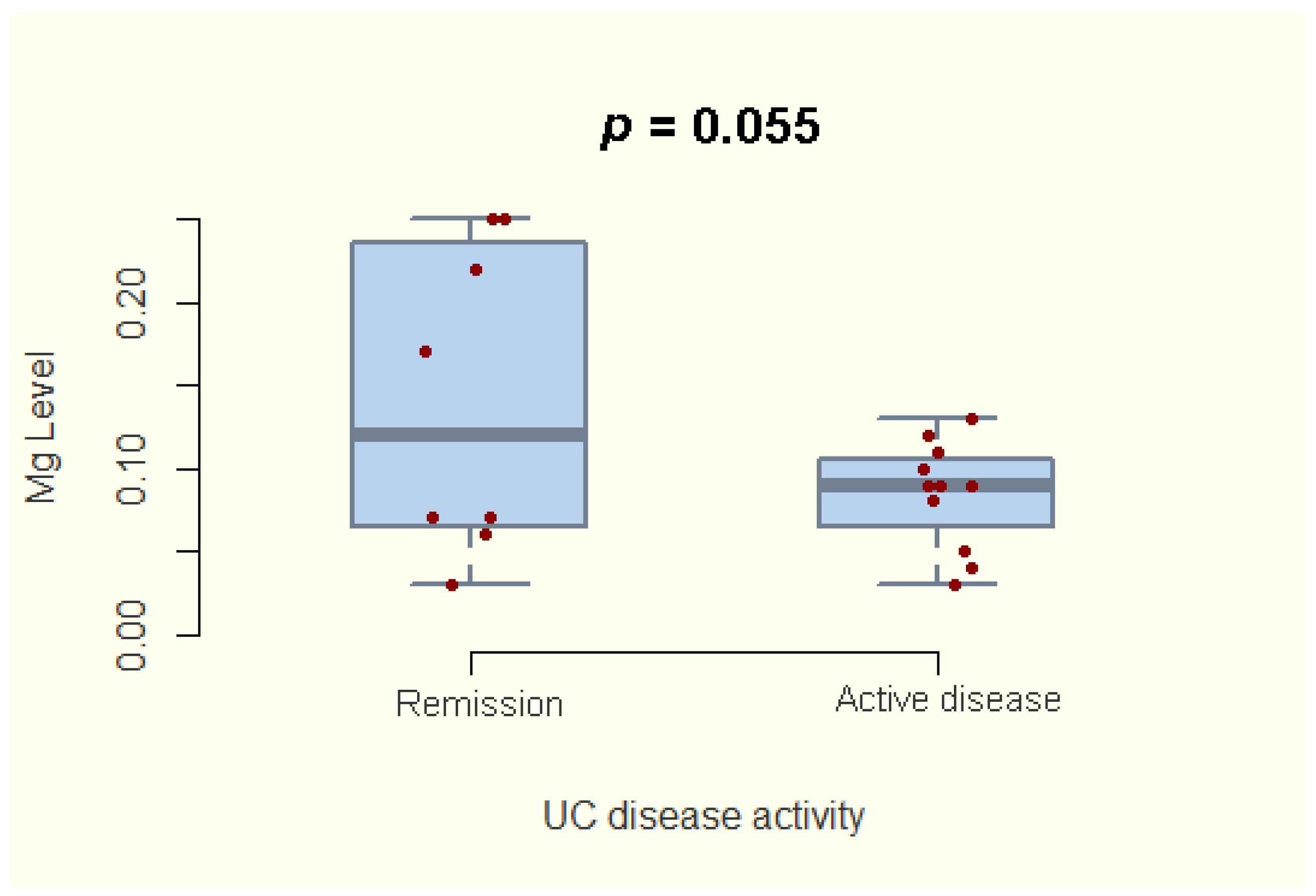
3.3. Magnesium Deficiency and Disease Activity
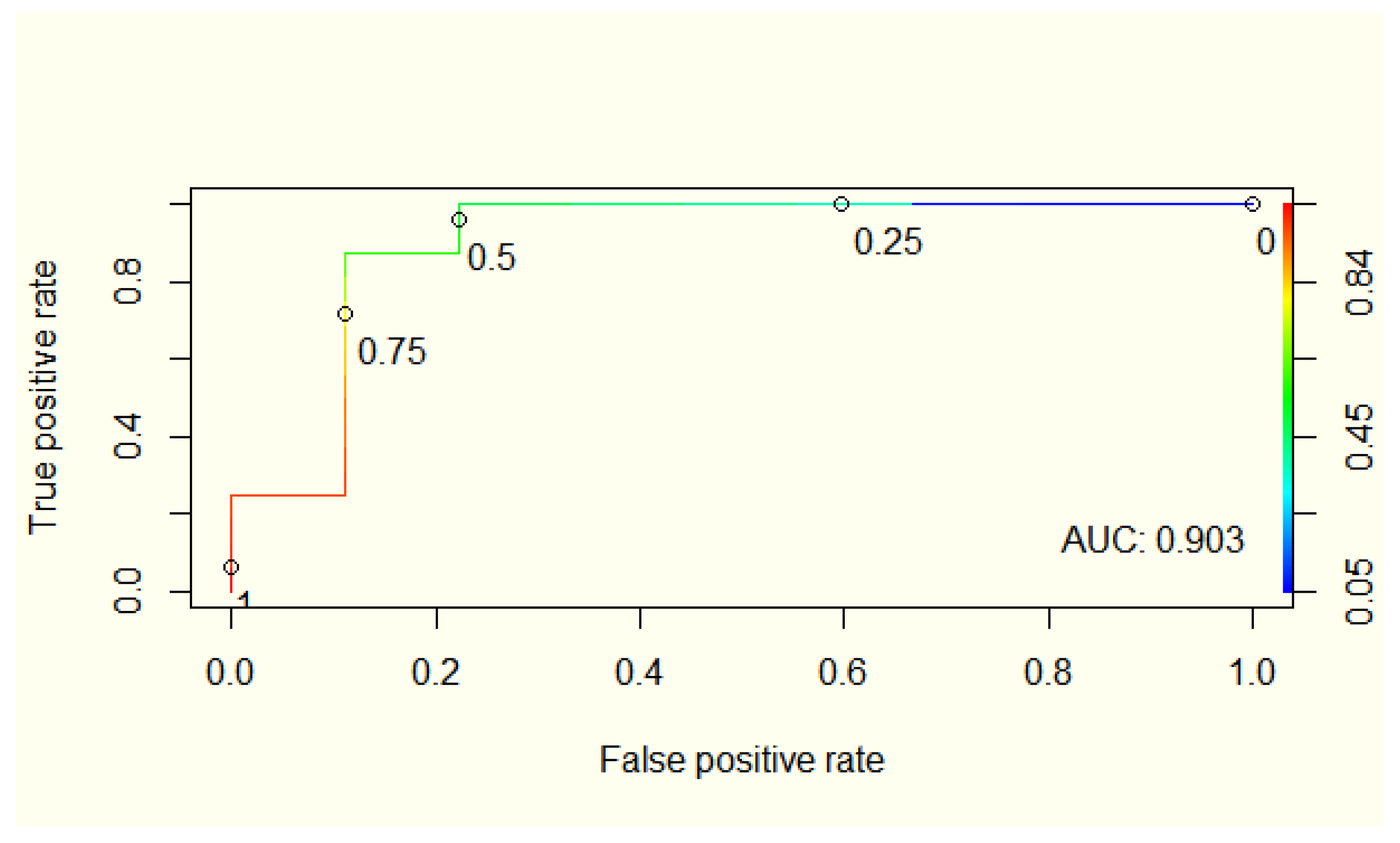
3.4. Integrating Magnesium Concentration in a Predictive Model for UC Disease Activity
3.5. Nutritional Status and Magnesium Hair Concentration
3.6. Psychological Status and Magnesium Hair Concentration
3.7. Sleep Quality and Magnesium Hair Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Valentini, L.; Schaper, L.; Buning, C.; Hengstermann, S.; Koernicke, T.; Tillinger, W.; Guglielmi, F.W.; Norman, K.; Buhner, S.; Ockenga, J.; et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008, 24, 694–702. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Parekh, N.; Bechtold, M.L.; Jamal, M.M. National Trends and In-Hospital Outcomes of Adult Patients With Inflammatory Bowel Disease Receiving Parenteral Nutrition Support. JPEN J. Parenter. Enter. Nutr. 2016, 40, 412–416. [Google Scholar] [CrossRef]
- Song, S.M.; Kim, Y.; Oh, S.H.; Kim, K.M. Nutritional status and growth in Korean children with Crohn’s disease: A single-center study. Gut Liver 2014, 8, 500–507. [Google Scholar] [CrossRef][Green Version]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Cao, Y.; Zhen, S.; Taylor, A.W.; Appleton, S.; Atlantis, E.; Shi, Z. Magnesium Intake and Sleep Disorder Symptoms: Findings from the Jiangsu Nutrition Study of Chinese Adults at Five-Year Follow-Up. Nutrients 2018, 10, 1354. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B. Magnesium intake and depression in adults. J. Am. Board Fam. Med. JABFM 2015, 28, 249–256. [Google Scholar] [CrossRef]
- Cho, J.M.; Yang, H.R. Hair Mineral and Trace Element Contents as Reliable Markers of Nutritional Status Compared to Serum Levels of These Elements in Children Newly Diagnosed with Inflammatory Bowel Disease. Biol. Trace Elem. Res. 2018, 185, 20–29. [Google Scholar] [CrossRef]
- Gîlcă-Blanariu, G.E.; Coroabă, A.; Ciocoiu, M.; Trifan, A.; Dimofte, G.; Diaconescu, S.; Afrăsânie, V.A.; Balan, G.G.; Pinteală, T.; Ștefănescu, G. Hair EDX Analysis-A Promising Tool for Micronutrient Status Evaluation of Patients with IBD? Nutrients 2021, 13, 2572. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- De Simone, B.; Davies, J.; Chouillard, E.; Di Saverio, S.; Hoentjen, F.; Tarasconi, A.; Sartelli, M.; Biffl, W.L.; Ansaloni, L.; Coccolini, F.; et al. WSES-AAST guidelines: Management of inflammatory bowel disease in the emergency setting. World J. Emerg. Surg. 2021, 16, 23. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Shah, Y.; Trivedi, C.; Yang, Y.X. Albumin as a prognostic marker for ulcerative colitis. World J. Gastroenterol. 2017, 23, 8008–8016. [Google Scholar] [CrossRef]
- Lee, S.-H.; Walshe, M.; Oh, E.H.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Byeon, J.-S.; Myung, S.-J.; Yang, S.-K.; Greener, T.; et al. Early Changes in Serum Albumin Predict Clinical and Endoscopic Outcomes in Patients With Ulcerative Colitis Starting Anti-TNF Treatment. Inflamm. Bowel Dis. 2020, 27, 1452–1461. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mathôt, R.A.; van der Kleij, D.; Rispens, T.; Ashruf, Y.; Jansen, J.M.; Rietdijk, S.; Löwenberg, M.; Ponsioen, C.Y.; Singh, S.; et al. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate With Response to Infliximab Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 251–258.e2. [Google Scholar] [CrossRef]
- Lees, C.W.; Heys, D.; Ho, G.T.; Noble, C.L.; Shand, A.G.; Mowat, C.; Boulton-Jones, R.; Williams, A.; Church, N.; Satsangi, J.; et al. A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2007, 26, 411–419. [Google Scholar] [CrossRef]
- Ali, T.; Orr, W.C. Sleep disturbances and inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1986–1995. [Google Scholar] [CrossRef]
- Ali, T.; Madhoun, M.F.; Orr, W.C.; Rubin, D.T. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 2440–2443. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef]
- Hess, W.M.; Seegmiller, R.E.; Gardner, J.S.; Allen, J.V.; Barendregt, S. Human hair morphology: A scanning electron microscopy study on a male Caucasoid and a computerized classification of regional differences. Scanning Microsc. 1990, 4, 375–386. [Google Scholar] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Stritt, S.; Nurden, P.; Favier, R.; Favier, M.; Ferioli, S.; Gotru, S.K.; van Eeuwijk, J.M.; Schulze, H.; Nurden, A.T.; Lambert, M.P.; et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg2+ homeostasis and cytoskeletal architecture. Nat. Commun. 2016, 7, 11097. [Google Scholar] [CrossRef]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef]
- Yakut, M.; Ustün, Y.; Kabaçam, G.; Soykan, I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur. J. Intern. Med. 2010, 21, 320–323. [Google Scholar] [CrossRef]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef]
- Delves, H.T. Effect of inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2001, 38, 289–291. [Google Scholar]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef]
- Wołowiec, P.; Michalak, I.; Chojnacka, K.; Mikulewicz, M. Hair analysis in health assessment. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 419, 139–171. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Fujino, R.; Kimura, O.; Endo, T. Essential and Non-essential Elements in Scalp Hair of Diabetics: Correlations with Glycated Hemoglobin (HbA1c). Biol. Pharm. Bull. 2018, 41, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Seneczko, M. Selenium balance in patients suffering from psoriasis vulgaris in different development phases Part 1. Concentration of selenium in selected morphotic components and excreta and activity of glutation peroxidase in red blood cells. Adv. Dermatol. Allergol. 2004, 21, 36–46. [Google Scholar]
- Coroaba, A.; Chiriac, A.E.; Sacarescu, L.; Pinteala, T.; Minea, B.; Ibanescu, S.A.; Pertea, M.; Moraru, A.; Esanu, I.; Maier, S.S.; et al. New insights into human hair: SAXS, SEM, TEM and EDX for Alopecia Areata investigations. PeerJ 2020, 8, e8376. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef]
- Kruis, W.; Phuong Nguyen, G. Iron Deficiency, Zinc, Magnesium, Vitamin Deficiencies in Crohn’s Disease: Substitute or Not? Dig. Dis. 2016, 34, 105–111. [Google Scholar] [CrossRef]
- Mah, J.; Pitre, T. Oral magnesium supplementation for insomnia in older adults: A Systematic Review & Meta-Analysis. BMC Complementary Med. Ther. 2021, 21, 125. [Google Scholar] [CrossRef]
| Patient Characteristics | Study Group n = 37 | Control Group n = 31 | p-Value | |
|---|---|---|---|---|
| UC (n = 25) | CD (n = 12) | |||
| Age, median (Q25; Q75) | 43.5 (30; 59.5) | 32 (29; 42) | 0.05 § | |
| 46 (32.5; 65.5) | 33 (27.5; 44.5) | |||
| Sex (M/F), n (%) | 19/18 (51.4/48.6) | 16/15 (51.6/48.4) | 0.981 Ϯ | |
| 13/12 (52/48) | 6/6 (50/50) | |||
| BMI (average ± SD) | 21.97 ± 1.5 | 23.08 ± 2.2 | 0.259 # | |
| 22.61 ± 1.95 | 21.97 ± 1.5 | |||
| Disease activity score | Mayo score 3 (1; 7) | CDAI score 146.5 (52.5; 276.5) | ||
| Active disease, n (%) | 22 (59.5%) | NA | NA | |
| 16 (64% *) | 6 (50% *) | NA | 0.65 Ϯ | |
| PSQI Component | t | df | p | CI |
|---|---|---|---|---|
| Global | −1.05 | 35 | 0.3 | −0.472, 0.158 |
| Component 1—subjective sleep quality | −1.212 | 35 | 0.234 | −0.493, 0.132 |
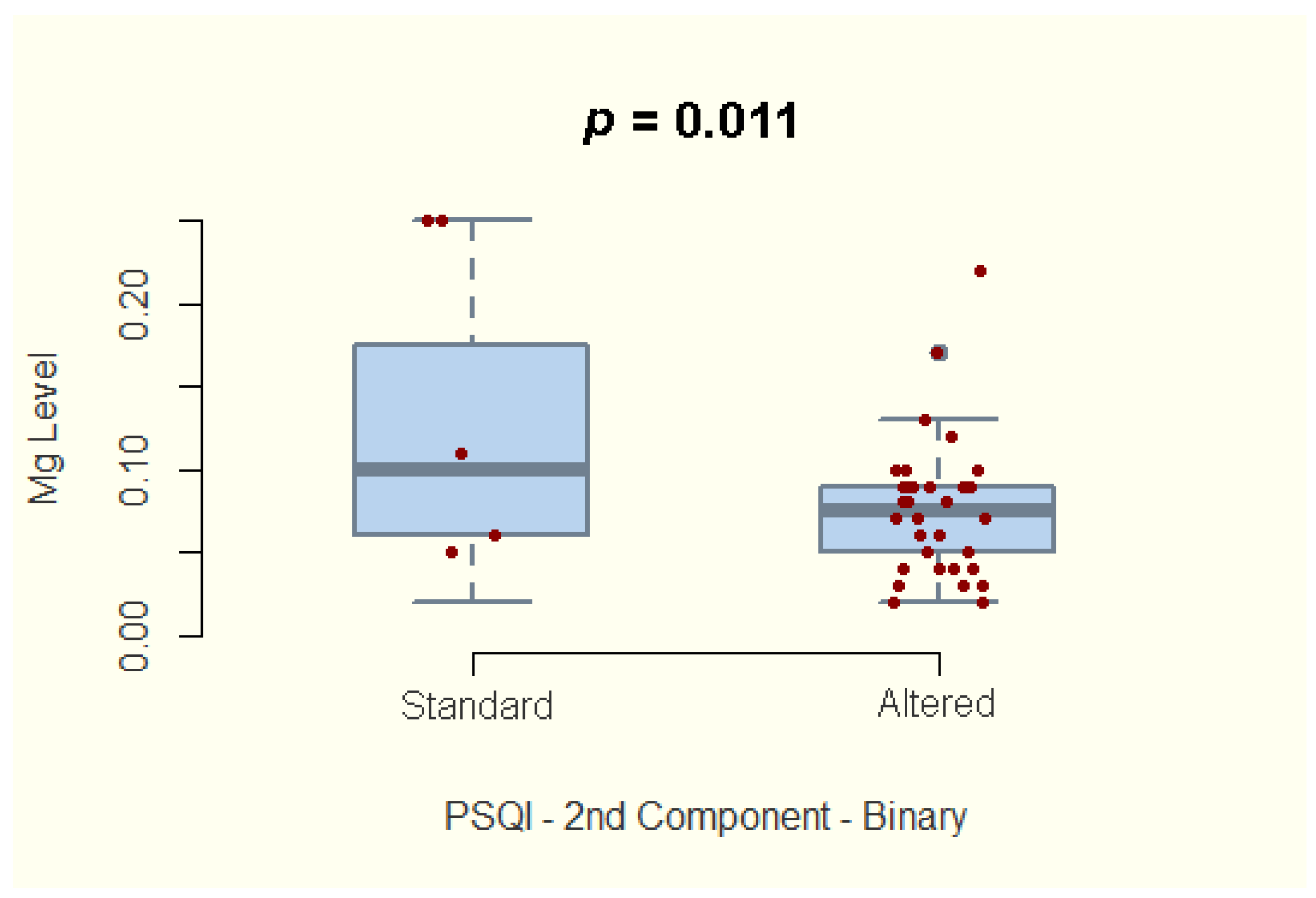
| Component 2—sleep latency | −2.681 | 35 | 0.011 | −0.65, −0.102 |
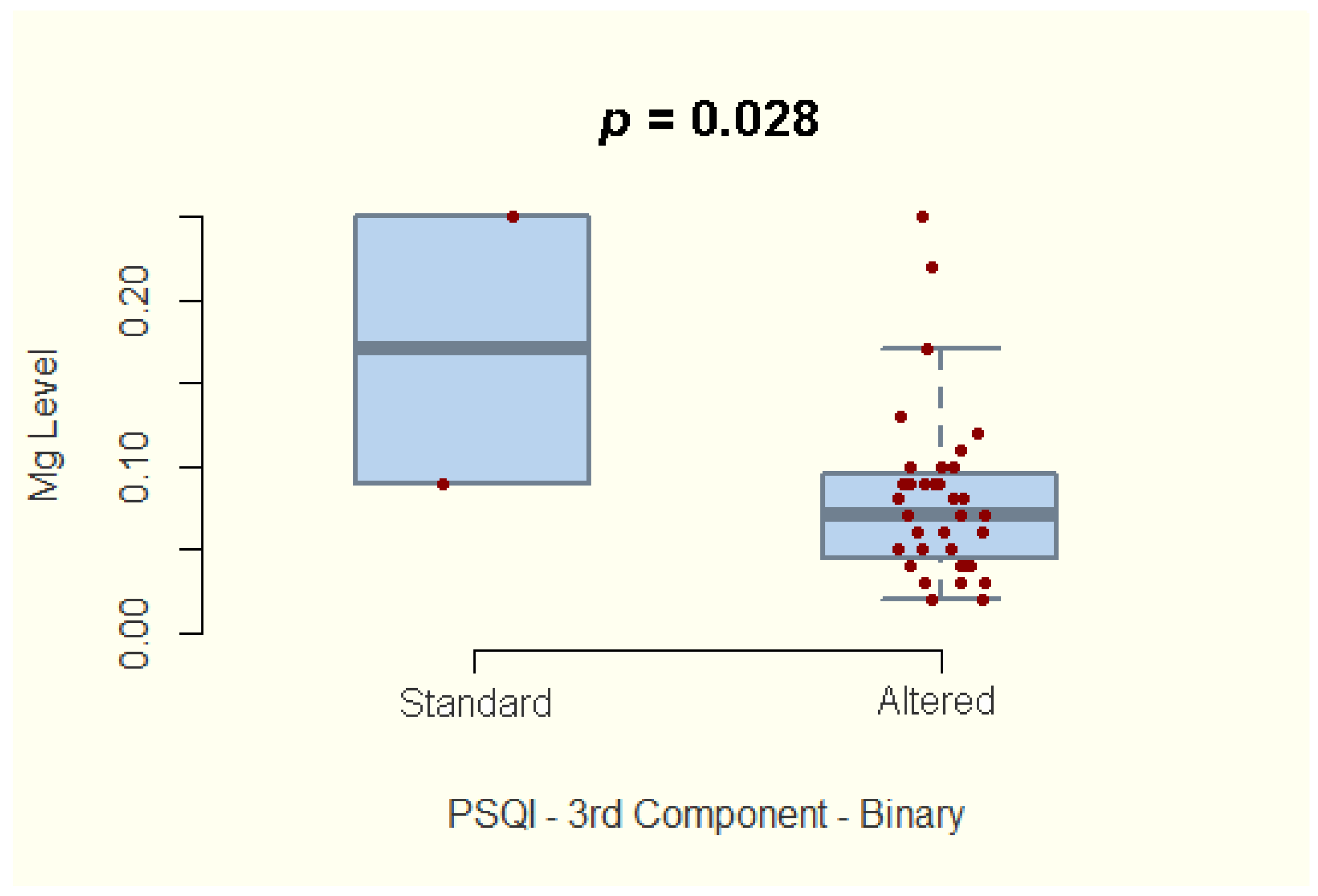
| Component 3—Sleep duration | −2.285 | 35 | 0.028 | −0.613, −0.041 |
| Component 4—Habitual sleep efficiency | −0.706 | 35 | 0.485 | −0.426, 0.214 |
| Component 5—sleep disturbances | 0.644 | 35 | 0.524 | −0.224, 0.418 |
| Component 6—Use of sleeping medication | −0.942 | 35 | 0.353 | −0.224, 0.418 |
| Component 7—Daytime dysfunction | −0.725 | 35 | 0.474 | −0.429, 0.211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilca-Blanariu, G.-E.; Trifan, A.; Ciocoiu, M.; Popa, I.V.; Burlacu, A.; Balan, G.G.; Olteanu, A.V.; Stefanescu, G. Magnesium—A Potential Key Player in Inflammatory Bowel Diseases? Nutrients 2022, 14, 1914. https://doi.org/10.3390/nu14091914
Gilca-Blanariu G-E, Trifan A, Ciocoiu M, Popa IV, Burlacu A, Balan GG, Olteanu AV, Stefanescu G. Magnesium—A Potential Key Player in Inflammatory Bowel Diseases? Nutrients. 2022; 14(9):1914. https://doi.org/10.3390/nu14091914
Chicago/Turabian StyleGilca-Blanariu, Georgiana-Emmanuela, Anca Trifan, Manuela Ciocoiu, Iolanda Valentina Popa, Alexandru Burlacu, Gheorghe G. Balan, Andrei Vasile Olteanu, and Gabriela Stefanescu. 2022. "Magnesium—A Potential Key Player in Inflammatory Bowel Diseases?" Nutrients 14, no. 9: 1914. https://doi.org/10.3390/nu14091914
APA StyleGilca-Blanariu, G.-E., Trifan, A., Ciocoiu, M., Popa, I. V., Burlacu, A., Balan, G. G., Olteanu, A. V., & Stefanescu, G. (2022). Magnesium—A Potential Key Player in Inflammatory Bowel Diseases? Nutrients, 14(9), 1914. https://doi.org/10.3390/nu14091914










