Blood Pressure and Heart Rate Responses following Dietary Protein Intake in Older Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Protocol
2.3. Measurements
2.4. Data and Statistical Analysis
3. Results
3.1. Systolic Blood Pressure (SBP)
3.2. Diastolic Blood Pressure (DBP)
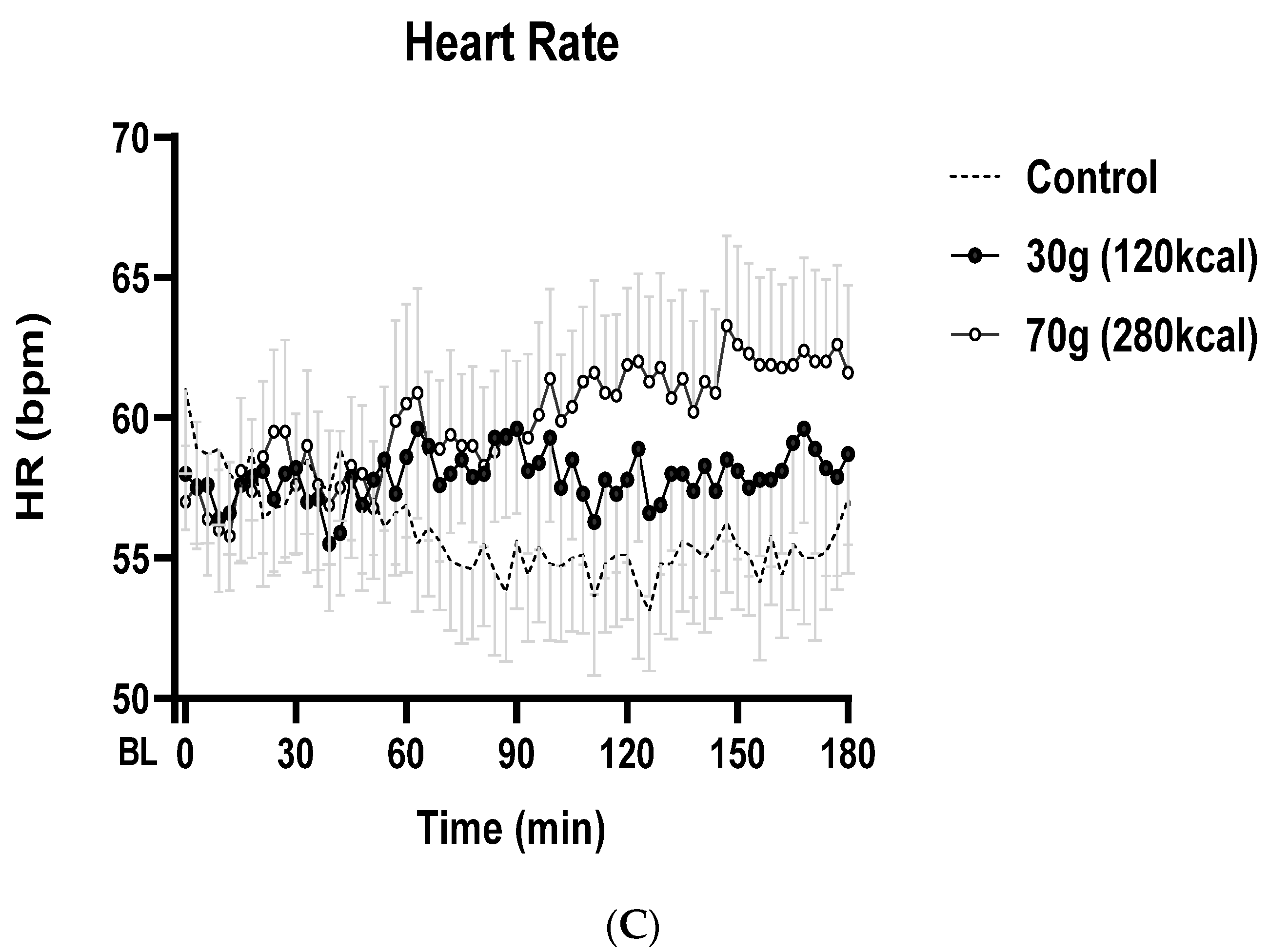
3.3. Heart Rate (HR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batsis, J.A. Obesity in the Older Adult: Special Issue. J. Nutr. Gerontol. Geriatr. 2019, 38, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Heber, D. Sarcopenic obesity in the elderly and strategies for weight management. Nutr. Rev. 2012, 70, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaum, C.S.; Xue, Q.L.; Michelon, E.; Semba, R.D.; Fried, L.P. The association between obesity and the frailty syndrome in older women: The women’s health and aging studies. J. Am. Geriatr. Soc. 2005, 53, 927–934. [Google Scholar] [CrossRef]
- Lapane, K.L.; Resnik, L. Obesity in nursing homes: An escalating problem. J. Am. Geriatr. Soc. 2005, 53, 1386–1391. [Google Scholar] [CrossRef]
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 2004, 12, 913–920. [Google Scholar] [CrossRef]
- Zizza, C.A.; Herring, A.; Stevens, J.; Popkin, B.M. Obesity affects nursing-care facility admission among whites but not blacks. Obes. Res. 2002, 10, 816–823. [Google Scholar] [CrossRef]
- Elkins, J.S.; Whitmer, R.A.; Sidney, S.; Sorel, M.; Yaffe, K.; Johnston, S.C. Midlife obesity and long-term risk of nursing home admission. Obesity 2006, 14, 1472–1478. [Google Scholar] [CrossRef]
- Fagius, J.; Ellerfelt, K.; Lithell, H.; Berne, C. Increase in muscle nerve sympathetic activity after glucose intake is blunted in the elderly. Clin. Auton. Res. 1996, 6, 195–203. [Google Scholar] [CrossRef]
- Aronow, W.S.; Ahn, C. Association of postprandial hypotension with incidence of falls, syncope, coronary events, stroke, and total mortality at 29-month follow-up in 499 older nursing home residents. J. Am. Geriatr. Soc. 1997, 45, 1051–1053. [Google Scholar] [CrossRef]
- Jansen, R.M.; Lipsitz, L.A. Postprandial hypotension: Epidemiology, pathophysiology, and clinical management. Ann. Intern. Med. 1995, 122, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Trahair, L.G.; Rigda, R.; Hutchison, A.T.; Feinle-Bisset, C.; Luscombe-Marsh, N.; Hausken, T.; Jones, K.; Horowitz, M.; Chapman, I.M.; et al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R845–R854. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vloet, L.C.M.; Smits, R.; Jansen, R.W.M.M. The effect of meals at different mealtimes on blood pressure and symptoms in geriatric patients with postprandial hypotension. J. Gerontol. Ser. A 2003, 58, 1031–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentilcore, D.; Hausken, T.; Meyer, J.H.; Chapman, I.M.; Horowitz, M.; Jones, K.L. Effects of intraduodenal glucose, fat, and protein on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am. J. Clin. Nutr. 2008, 87, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giezenaar, C.; Oberoi, A.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of age on blood pressure and heart rate responses to whey protein in younger and older men. J. Am. Geriatr. Soc. 2021, 69, 1291–1299. [Google Scholar] [CrossRef]
- Chapman, I.; Oberoi, A.; Giezenaar, C.; Soenen, S. Rational Use of Protein Supplements in the elderly–relevance of gastrointestinal mechanisms. Nutrients 2021, 13, 1227. [Google Scholar] [CrossRef]
- Pham, H.; Holen, I.S.; Phillips, L.K.; Hatzinikolas, S.; Huynh, L.Q.; Wu, T.; Hausken, T.; Rayner, C.K.; Horowitz, M.; Jones, K.L. The Effects of a Whey Protein and Guar Gum-Containing Preload on Gastric Emptying, Glycaemia, Small Intestinal Absorption and Blood Pressure in Healthy Older Subjects. Nutrients 2019, 11, 2666. [Google Scholar] [CrossRef] [Green Version]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigoz, P.Y.; Vellas, M.B.; Garry, P.P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 2009, 54, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Grandi, E.; Dinelli, G.; Hrelia, S.; Borghi, C. Short-Term Hemodynamic Effects of Modern Wheat Products Substitution in Diet with Ancient Wheat Products: A Cross-Over, Randomized Clinical Trial. Nutrients 2018, 10, 1666. [Google Scholar] [CrossRef] [Green Version]
- Peitzman, S.J.; Berger, S.R. Postprandial Blood Pressure Decrease in Well Elderly Persons. Arch. Intern. Med. 1989, 149, 286–288. [Google Scholar] [CrossRef]

| Control | WP30 | WP70 | p-Value | |
|---|---|---|---|---|
| SBP (mmHg) | ||||
| Mean 0–60 min | 132 ± 6 | 127 ± 4 | 134 ± 5 | 0.123 |
| Mean 60–120 min | 129 ± 6 | 124 ± 5 | 131 ± 6 | 0.092 |
| Mean 120–180 min | 133 ± 7 | 122 ± 6 | 125 ± 5 | 0.016 |
| Max. change from baseline | −15 ± 2 | −24 ± 2 | −25 ± 4 | 0.020 |
| Time to nadir (min) | 90 ± 8 | 111 ± 22 | 136 ± 11 | 0.084 |
| DBP (mmHg) | ||||
| Mean 0–60 min | 77 ± 7 | 73 ± 2 | 75 ± 3 | 0.235 |
| Mean 60–120 min | 75 ± 3 | 71 ± 3 | 72 ± 3 | 0.414 |
| Mean 120–180 min | 75 ± 3 | 70 ± 23 | 72 ± 3 | 0.202 |
| Max. change from baseline | −13 ± 2 | 15 ± 1 | −17 ± 2 | 0.130 |
| Time to nadir (min) | 89 ± 19 | 98 ± 19 | 108 ± 11 | 0.740 |
| HR (bpm) | ||||
| Mean 0–60 min | 55 ± 3 | 58 ± 3 | 59 ± 3 | 0.057 |
| Mean 60–120 min | 53 ± 2 | 60 ± 3 | 60 ± 3 | 0.007 |
| Mean 120–180 min | 53 ± 2 | 58 ± 3 | 62 ± 3 | 0.005 |
| Max. change from baseline Time to nadir (min) | 2 ± 1 71 ± 25 | 6 ± 1 112 ± 17 | 10 ± 2 120 ± 17 | 0.002 0.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberoi, A.; Giezenaar, C.; Lange, K.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Blood Pressure and Heart Rate Responses following Dietary Protein Intake in Older Men. Nutrients 2022, 14, 1913. https://doi.org/10.3390/nu14091913
Oberoi A, Giezenaar C, Lange K, Jones KL, Horowitz M, Chapman I, Soenen S. Blood Pressure and Heart Rate Responses following Dietary Protein Intake in Older Men. Nutrients. 2022; 14(9):1913. https://doi.org/10.3390/nu14091913
Chicago/Turabian StyleOberoi, Avneet, Caroline Giezenaar, Kylie Lange, Karen L. Jones, Michael Horowitz, Ian Chapman, and Stijn Soenen. 2022. "Blood Pressure and Heart Rate Responses following Dietary Protein Intake in Older Men" Nutrients 14, no. 9: 1913. https://doi.org/10.3390/nu14091913
APA StyleOberoi, A., Giezenaar, C., Lange, K., Jones, K. L., Horowitz, M., Chapman, I., & Soenen, S. (2022). Blood Pressure and Heart Rate Responses following Dietary Protein Intake in Older Men. Nutrients, 14(9), 1913. https://doi.org/10.3390/nu14091913








