Biomarkers of Redox Balance Adjusted to Exercise Intensity as a Useful Tool to Identify Patients at Risk of Muscle Disease through Exercise Test
Abstract
:1. Introduction
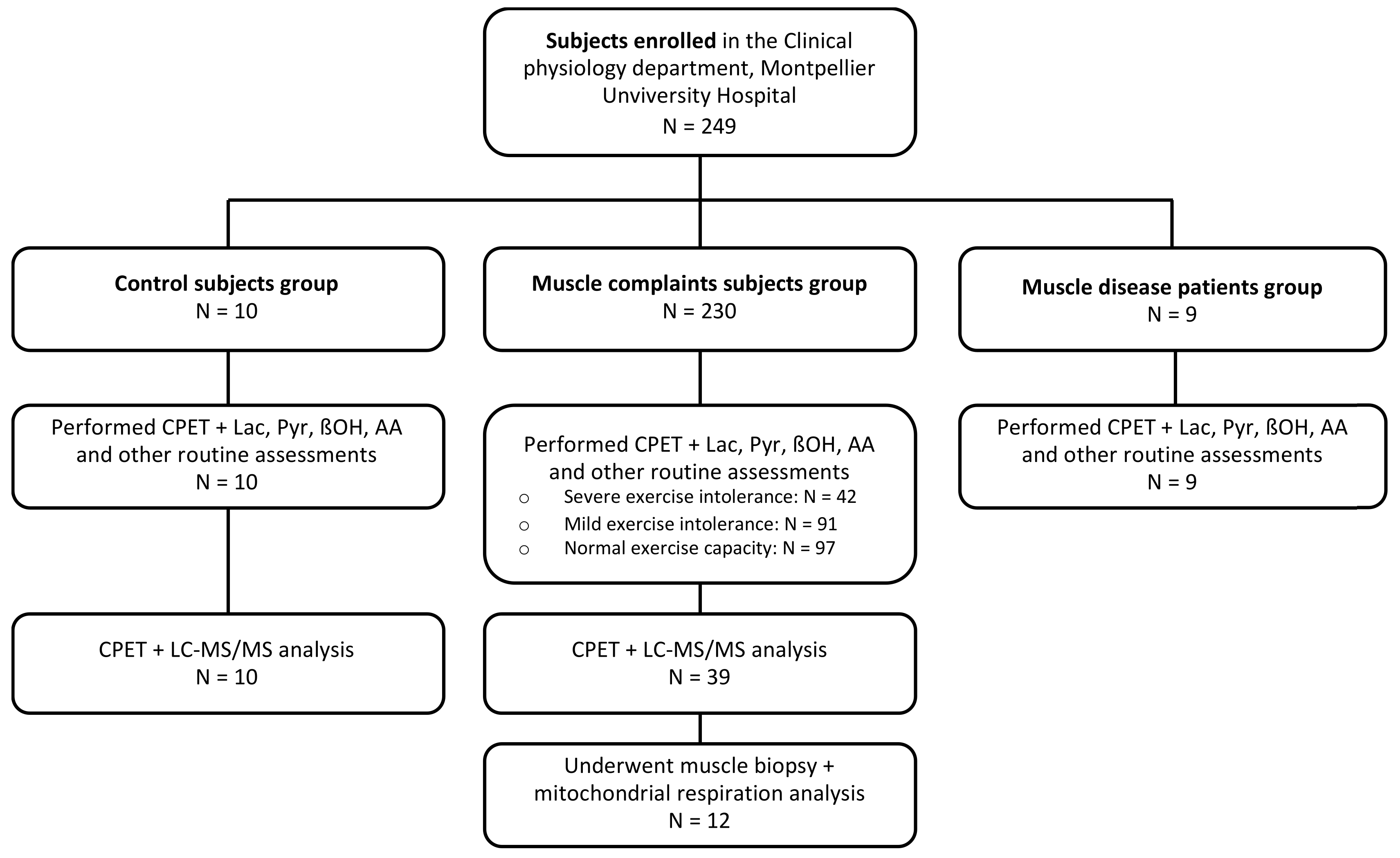
2. Materials and Methods
2.1. Population
2.2. Assessments
2.3. Cardiopulmonary Exercise Test
2.4. Samples
2.5. Energy and Redox Biomarkers
2.6. TCA Cycle-Related Metabolites
2.7. Skeletal Muscle Biopsy
2.8. Mitochondrial Respiration
2.9. Statistical Analysis
3. Results
3.1. Energy Substrate and Redox Markers Assessed during a Standardized CPET Are Associated with the Aerobic Exercise Capacity
3.2. Energy and Redox Markers Assessed during a Standardized CPET Are Associated with Muscle Fiber Mitochondrial Respiration
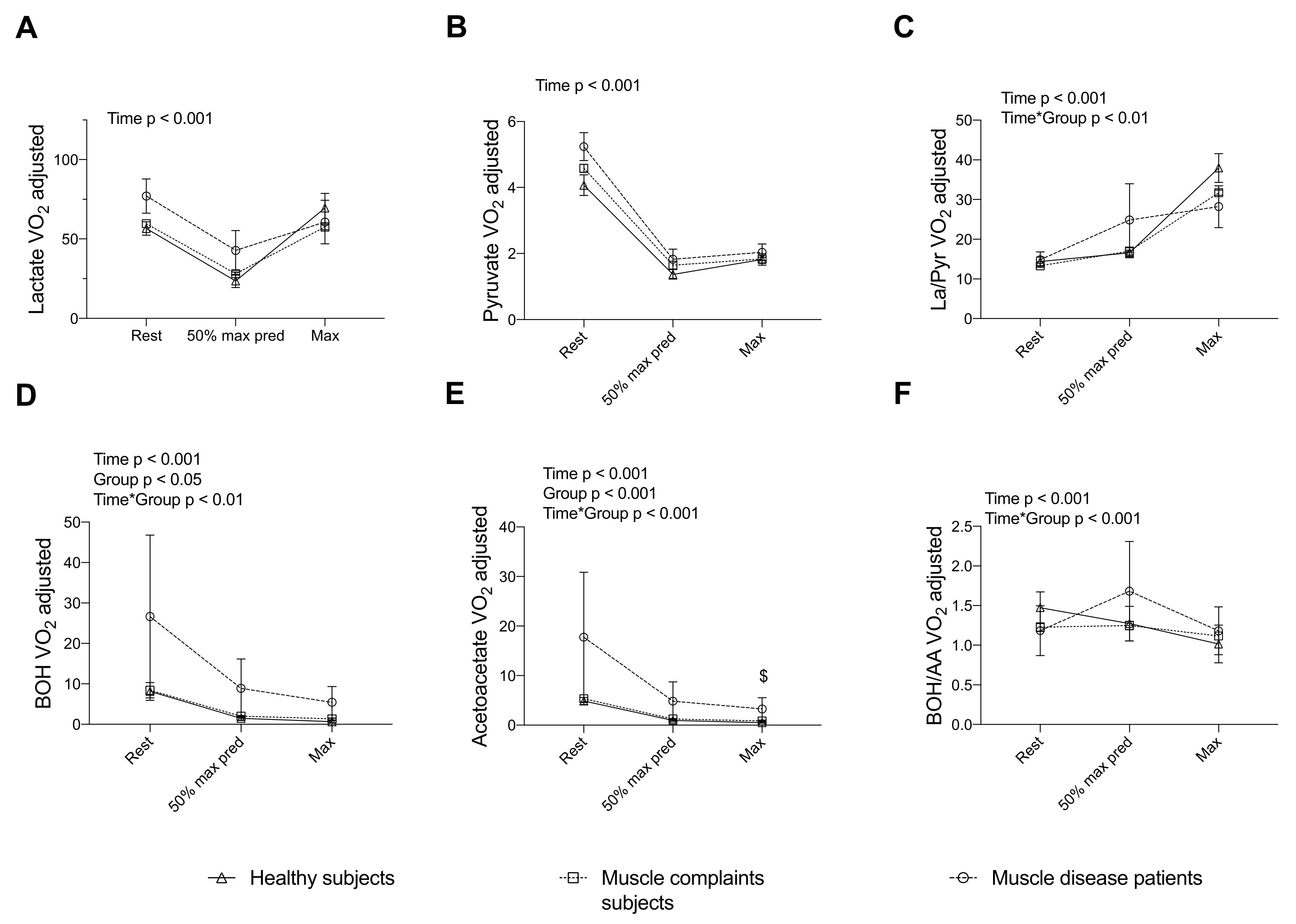
3.3. Energy and Redox Markers Assessed during a Standardized CPET Can Discriminate Subjects with Muscle Complaints and Patients with Muscle Disease from Healthy Subjects
3.4. Completing the Assessment of the TCA Cycle Intermediates during a CPET Reveals a Blunted Exercise-Induced Adaptation of Other Intermediates in Muscle Complaint Subjects with Severe Exercise Intolerance
4. Discussion
4.1. Validity of Exercise Values of V’O2-Adjusted Energy Substrates and Muscle Function/Respiration
4.2. Discriminative Value of Exercise-Induced Increase in Lactate, Pyruvate and Ratios in Muscle Diseases
4.3. Discriminative Value of Exercise-Induced Increase in β-Hydroxybutyrate, Acetoacetate and Ratio in Muscle Diseases
4.4. Screening for “At-Risk” Patients for Muscle Oxidative Metabolism Diseases
4.5. Study Limitations
4.6. Exercise Metabolomics: Potential Issues for Nutritional Interventions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domingo-Horne, R.M.; Salajegheh, M.K. An Approach to Myopathy for the Primary Care Clinician. Am. J. Med. 2018, 131, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterer, J.; Löscher, W.N.; Wanschitz, J.; Quasthoff, S.; Grisold, W. Secondary myopathy due to systemic diseases. Acta Neurol. Scand. 2016, 134, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Update review about metabolic myopathies. Life 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowtell, J.L.; Marwood, S.; Bruce, M.; Constantin-Teodosiu, D.; Greenhaff, P.L. Tricarboxylic acid cycle intermediate pool size: Functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med. 2007, 37, 1071–1088. [Google Scholar] [CrossRef]
- Kujoth, C.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Medicine: Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Haas, R.H.; Parikh, S.; Falk, M.J.; Saneto, R.P.; Wolf, N.I.; Darin, N.; Wong, L.J.; Cohen, B.H.; Naviaux, R.K. The in-depth evaluation of suspected mitochondrial disease. Mol. Genet. Metab. 2008, 94, 16–37. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef]
- Murayama, K.; Shimura, M.; Liu, Z.; Okazaki, Y.; Ohtake, A. Recent topics: The diagnosis, molecular genesis, and treatment of mitochondrial diseases. J. Hum. Genet. 2019, 64, 113–125. [Google Scholar] [CrossRef]
- Finsterer, J.; Zarrouk-Mahjoub, S. Biomarkers for detecting mitochondrial disorders. J. Clin. Med. 2018, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, K.R.; Wald, J.; Weisman, I.M.; Zeballos, R.J.; Schork, M.A.; Blaivas, M.; Rubenfire, M.; Martinez, F.J. Unexplained exertional limitation: Characterization of patients with a mitochondrial myopathy. Am. J. Respir. Crit. Care Med. 2001, 164, 425–432. [Google Scholar] [CrossRef]
- Riley, M.S.; Nicholls, D.P.; Cooper, C.B. Cardiopulmonary exercise testing and metabolic myopathies. Ann. Am. Thorac. Soc. 2017, 14, S129–S139. [Google Scholar] [CrossRef]
- Elliot, D.L.; Buist, N.R.M.; Goldberg, L.; Kennaway, N.G.; Phil, D.; Powell, B.R.; Kuehl, K.S. Metabolic myopathies: Evaluation by graded exercise testing. Medicine 1989, 68, 163–172. [Google Scholar] [CrossRef]
- Blomstrand, E.; Rådegran, G.; Saltin, B. Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J. Physiol. 1997, 501 Pt 2, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Zoll, J.; Sanchez, H.; N’Guessan, B.; Ribera, F.; Lampert, E.; Bigard, X.; Serrurier, B.; Fortin, D.; Geny, B.; Veksler, V.; et al. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 2002, 543, 191–200. [Google Scholar] [CrossRef]
- Dysgaard Jeppesen, T.; Olsen, D.; Vissing, J. Cycle ergometry is not a sensitive diagnostic test for mitochondrial myopathy. J. Neurol. 2003, 250, 293–299. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Sirvent, P.; Perrey, S.; Raynaud, E.; Mercier, J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J. Appl. Physiol. 2004, 97, 2132–2138. [Google Scholar] [CrossRef]
- Delaney, N.F.; Sharma, R.; Tadvalkar, L.; Clish, C.B.; Haller, R.G.; Mootha, V.K. Metabolic profiles of exercise in patients with McArdle disease or mitochondrial myopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 8402–8407. [Google Scholar] [CrossRef] [Green Version]
- Fernández Guerra, J.; Montemayor, T.; Bautista, J.; Márquez, R.; Jiménez, L.; Arenas, J.; Campos, Y.; Castillo, J. Role of cardiopulmonary exercise test in patients with mitochondrial myopathies. Med. Clin. 2000, 114, 121–127. [Google Scholar] [CrossRef]
- Yan, B.; Jiye, A.; Wang, G.; Lu, H.; Huang, X.; Liu, Y.; Zha, W.; Hao, H.; Zhang, Y.; Liu, L.; et al. Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J. Appl. Physiol. 2009, 106, 531–538. [Google Scholar] [CrossRef]
- Corkey, B.E.; Deeney, J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020, 11, 567796. [Google Scholar] [CrossRef]
- Davies, A.O.; Samuelson, W.M. Nonequilibrium of two redox couplets in human plasma: Lactate-pyruvate and beta-hydroxybutyrate-acetoacetate. Crit. Care Med. 1986, 14, 936–941. [Google Scholar] [CrossRef]
- Chan, A.; Gold, R.; Arp, S.; Pflughaupt, K.W.; Toyka, K.V.; Reichmann, H. A standardized bicycle ergometer test in the diagnosis and monitoring of mitochondrial myopathies. Nervenarzt 1998, 69, 472–484. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med.-Open 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Radtke, T.; Crook, S.; Kaltsakas, G.; Louvaris, Z.; Berton, D.; Urquhart, D.S.; Kampouras, A.; Rabinovich, R.A.; Verges, S.; Kontopidis, D.; et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur. Respir. Rev. 2019, 28, 180101. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.H.Y.; de Craen, A.J.M.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.M.; Westendorp, R.G.J.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Jaffrin, M.Y.; Morel, H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med. Eng. Phys. 2008, 30, 1257–1269. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Froelicher, V.F. Principles of Exercise Testing and Interpretation. J. Cardiopulm. Rehabil. Prev. 2005, 50, 160–182. [Google Scholar] [CrossRef]
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [CrossRef]
- Saks, V.A.; Veksler, V.I.; Kuznetsov, A.V.; Kay, L.; Sikk, P.; Tiivel, T.; Tranqui, L.; Olivares, J.; Winkler, K.; Wiedemann, F.; et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol. Cell. Biochem. 1998, 184, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Mallows, C.L. Some comments on Cp. Technometrics 2000, 42, 87–94. [Google Scholar] [CrossRef]
- Gibala, M.J.; Tarnopolsky, M.A.; Graham, T.E. Tricarboxylic acid cycle intermediates in human muscle at rest and during prolonged cycling. Am. J. Physiol.-Endocrinol. Metab. 1997, 272, E239–E244. [Google Scholar] [CrossRef] [PubMed]
- Hammarén, E.; Rafsten, L.; Kreuter, M.; Lindberg, C. Modified exercise test in screening for mitochondrial myopathies-Adjustment of workload in relation to muscle strength. Eur. Neurol. 2004, 51, 38–41. [Google Scholar] [CrossRef]
- Mettauer, B.; Zoll, J.; Sanchez, H.; Lampert, E.; Ribera, F.; Veksler, V.; Bigard, X.; Mateo, P.; Epailly, E.; Lonsdorfer, J.; et al. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J. Am. Coll. Cardiol. 2001, 38, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Sharma, G.; Jiang, W.; Maptue, N.R.; Malloy, C.R.; Sherry, A.D.; Khemtong, C. Metabolism of hyperpolarized 13C-acetoacetate to β-hydroxybutyrate detects real-time mitochondrial redox state and dysfunction in heart tissue. NMR Biomed. 2019, 32, e4091. [Google Scholar] [CrossRef]
- Sharma, G.; Wen, X.; Maptue, N.R.; Hever, T.; Malloy, C.R.; Sherry, A.D.; Khemtong, C. Co-Polarized [1-13C]Pyruvate and [1,3-13C2]Acetoacetate Provide a Simultaneous View of Cytosolic and Mitochondrial Redox in a Single Experiment. ACS Sens. 2021, 6, 3967–3977. [Google Scholar] [CrossRef]
- Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. β-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int. J. Mol. Sci. 2018, 19, 2247. [Google Scholar] [CrossRef] [Green Version]
- Mey, J.T.; Erickson, M.L.; Axelrod, C.L.; King, W.T.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. β-Hydroxybutyrate is reduced in humans with obesity-related NAFLD and displays a dose-dependent effect on skeletal muscle mitochondrial respiration in vitro. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E184–E195. [Google Scholar] [CrossRef]
- Monsalves-Alvarez, M.; Morales, P.E.; Castro-Sepulveda, M.; Sepulveda, C.; Rodriguez, J.M.; Chiong, M.; Eisner, V.; Lavandero, S.; Troncoso, R. β-Hydroxybutyrate Increases Exercise Capacity Associated with Changes in Mitochondrial Function in Skeletal Muscle. Nutrients 2020, 12, 1930. [Google Scholar] [CrossRef]
- Huang, T.Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E1053–E1067. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; David Arnold, W.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef]
- Sahlin, K.; Jorfeldt, L.; Henriksson, K.G.; Lewis, S.F.; Haller, R.G. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle’s disease. Clin. Sci. 1995, 88, 687–693. [Google Scholar] [CrossRef]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Korpela, M.; Isohanni, P.; Marjavaara, S.K.; Tyni, T.; Kiuru-Enari, S.; et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef]
- Kurihara, M.; Sugiyama, Y.; Tanaka, M.; Sato, K.; Mitsutake, A.; Ishiura, H.; Kubota, A.; Sakuishi, K.; Hayashi, T.; Iwata, A.; et al. Diagnostic Values of Venous Peak Lactate, Lactate-to-pyruvate Ratio, and fold Increase in Lactate from Baseline in Aerobic Exercise Tests in Patients with Mitochondrial Diseases. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Zierz, S.; Meessen, S.; Jerusalem, F. Lactat- und pyruvatblutspiegel in der diagnostik mitochondrialer myopathien. Nervenarzt 1989, 60, 545–548. [Google Scholar]
- Debray, F.G.; Mitchell, G.A.; Allard, P.; Robinson, B.H.; Hanley, J.A.; Lambert, M. Diagnostic accuracy of blood lactate-to-pyruvate molar ratio in the differential diagnosis of congenital lactic acidosis. Clin. Chem. 2007, 53, 916–921. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2015, 17, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, 105906. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Karaa, A.; Koenig, M.K.; Anselm, I.; Brunel-Guitton, C.; Christodoulou, J.; Cohen, B.H.; Dimmock, D.; Enns, G.M.; et al. Patient care standards for primary mitochondrial disease: A consensus statement from the mitochondrial medicine society. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, J.P. Uncatalyzed and amine catalyzed decarboxylation of acetoacetic acid: An examination in terms of No Barrier Theory. Bioorg. Chem. 2002, 30, 32–52. [Google Scholar] [CrossRef]
- Hagenfeldt, L.; Wahren, J. Human forearm muscle metabolism during exercise III uptake, release and oxidation of β-hydroxybutyrate and observations on the β-hydroxybutyrate/acetoacetate ratio. Scand. J. Clin. Lab. Invest. 1968, 21, 314–320. [Google Scholar] [CrossRef]
- Bashir, B.; Fahmy, A.A.; Raza, F.; Banerjee, M. Non-diabetic ketoacidosis: A case series and literature review. Postgrad. Med. J. 2021, 97, 667–671. [Google Scholar] [CrossRef]
- Kim, M.J.; Hong, S.H.; Cho, W.; Park, D.H.; Lee, E.B.; Song, Y.; Choe, Y.S.; Lee, J.H.; Jang, Y.; Lee, W.; et al. Breath acetone measurement-based prediction of exercise-induced energy and substrate expenditure. Sensors 2020, 20, 6878. [Google Scholar] [CrossRef]
- Munnich, A.; Rötig, A.; Chretien, D.; Saudubray, J.M.; Cormier, V.; Rustin, P. Clinical presentations and laboratory investigations in respiratory chain deficiency. Eur. J. Pediatr. 1996, 155, 262–274. [Google Scholar] [CrossRef]
- Turki, A.; Hayot, M.; Carnac, G.; Pillard, F.; Passerieux, E.; Bommart, S.; De Mauverger, E.R.; Hugon, G.; Pincemail, J.; Pietri, S.; et al. Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2012, 53, 1068–1079. [Google Scholar] [CrossRef]
- Nesbitt, V.; Pitceathly, R.D.S.; Turnbull, D.M.; Taylor, R.W.; Sweeney, M.G.; Mudanohwo, E.E.; Rahman, S.; Hanna, M.G.; McFarland, R. The UK MRC Mitochondrial Disease Patient Cohort Study: Clinical phenotypes associated with the m.3243A>G mutation-Implications for diagnosis and management. J. Neurol. Neurosurg. Psychiatry 2013, 84, 936–938. [Google Scholar] [CrossRef]
- Parikh, S.; Karaa, A.; Goldstein, A.; Bertini, E.S.; Chinnery, P.F.; Christodoulou, J.; Cohen, B.H.; Davis, R.L.; Falk, M.J.; Fratter, C.; et al. Diagnosis of possible’ mitochondrial disease: An existential crisis. J. Med. Genet. 2019, 56, 123–130. [Google Scholar] [CrossRef]
- Ng, Y.S.; Turnbull, D.M. Mitochondrial disease: Genetics and management. J. Neurol. 2016, 263, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.J.; Zhao, J.R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.L.; Wan, L.L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 71–85. [Google Scholar] [CrossRef]
- Sharma, R.; Reinstadler, B.; Engelstad, K.; Skinner, O.S.; Stackowitz, E.; Haller, R.G.; Clish, C.B.; Pierce, K.; Walker, M.A.; Fryer, R.; et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J. Clin. Invest. 2021, 131, e136055. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Marzorati, M.; Lanfranconi, F.; Ferri, A.; Longaretti, M.; Stucchi, A.; Vago, P.; Marconi, C.; Morandi, L. Impaired oxygen extraction in metabolic myopathies: Detection and quantification by near-infrared spectroscopy. Muscle Nerve 2007, 35, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A. Metabolic Myopathies. Contin. Lifelong Learn. Neurol. 2016, 22, 1829–1851. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Gadea, G.; Santalla, A.; Ballester-Lopez, A.; Arenas, J.; Martín, M.A.; Godfrey, R.; Pinís, T.; Pintos-Morell, G.; Coll-Cantí, J.; Lucia, A. Exercise and preexercise nutrition as treatment for McArdle disease. Med. Sci. Sports Exerc. 2016, 48, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Roe, C.R.; Yang, B.Z.; Brunengraber, H.; Roe, D.S.; Wallace, M.; Garritson, B.K. Carnitine palmitoyltransferase II deficiency: Successful anaplerotic diet therapy. Neurology 2008, 71, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Negro, M.; Cerullo, G.; Parimbelli, M.; Ravazzani, A.; Feletti, F.; Berardinelli, A.; Cena, H.; D’Antona, G. Exercise, Nutrition, and Supplements in the Muscle Carnitine Palmitoyl-Transferase II Deficiency: New Theoretical Bases for Potential Applications. Front. Physiol. 2021, 12, 1175. [Google Scholar] [CrossRef]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M.; et al. Evidence-Based role of nutrients and antioxidants for chronic pain management in musculoskeletal frailty and sarcopenia in aging. Geriatrics 2020, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Riva, A.; Petrangolini, G.; Iannello, G.; Nichetti, M.; et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phyther. Res. 2020, 34, 2843–2856. [Google Scholar] [CrossRef]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in personalized nutrition: Can you “eat for your genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef]

| Variables | Weariness | V’O2/W Slope | V’O2@VT1 | V’O2max |
|---|---|---|---|---|
| La@rest | −0.021 | 0.048 | −0.242 *** | −0.235 *** |
| La@eVT1 | 0.002 | −0.142 | −0.582 *** | −0.566 *** |
| La@max | −0.023 | −0.221 ** | −0.306 *** | −0.384 *** |
| Pyr@rest | 0.028 | −0.010 | −0.324 *** | −0.313 *** |
| Pyr@eVT1 | 0.048 | −0.101 | −0.663 *** | −0.613 *** |
| Pyr@max | 0.136 | −0.333 *** | −0.492 *** | −0.650 *** |
| La/Pyr@rest | −0.054 | 0.071 | 0.074 | 0.028 |
| La/Pyr@eVT1 | −0.072 | −0.117 | −0.166 * | −0.222 ** |
| La/Pyr@max | −0.185 * | 0.020 | 0.192 ** | 0.276 *** |
| BOH@rest | −0.009 | −0.098 | −0.170 * | −0.196 ** |
| BOH@eVT1 | −0.055 | −0.073 | −0.258 *** | −0.251 *** |
| BOH@max | −0.027 | −0.113 | −0.168 * | −0.224 ** |
| AA@rest | 0.067 | −0.065 | −0.174 ** | −0.197 ** |
| AA@eVT1 | 0.042 | −0.081 | −0.350 *** | −0.324 *** |
| AA@max | 0.078 | −0.122 | −0.194 ** | −0.277 *** |
| BOH/AA@rest | −0.018 | −0.156 | −0.032 | −0.096 |
| BOH/AA@eVT1 | −0.078 | −0.058 | −0.010 | −0.071 |
| BOH/AA@max | −0.053 | −0.128 | −0.024 | −0.092 |
| Nr. of Variables | Variables | R2 | R2 Adjusted | Mallow’s Cp |
|---|---|---|---|---|
| 1 | V’O2@VT1 | 0.527 | 0.521 | 44.62 |
| 2 | V’O2/W slope + V’O2@VT1 | 0.619 | 0608 | 23.85 |
| 3 | V’O2/Wslope + V’O2@VT1 + AA@max | 0.641 | 0.627 | 20.20 |
| 4 | V’O2/Wslope + V’O2@VT1 + BOH@eVT1 + BOH@max | 0.681 | 0.663 | 12.42 |
| 5 | V’O2/Wslope + V’O2@VT1 + Pyr@max + BOH@eVT1 + BOH@max | 0.706 | 0.685 | 8.21 |
| 6 | V’O2/Wslope + V’O2@VT1 + La@max + La/Pyr@rest + La/Pyr@eVT1 + La/Pyr@max | 0.723 | 0.699 | 5.83 |
| 7 | V’O2/Wslope + V’O2@VT1+ La@rest + La@max + Pyr@rest + La/Pyr@eVT1 + La/Pyr@max | 0.735 | 0.708 | 4.82 |
| 8 | V’O2/Wslope + V’O2@VT1 + La@max/ + La/Pyr@rest + La/Pyr@eVT1 + La/Pyr@max + AA@eVT1 + AA@max | 0.750 | 0.721 | 3.08 |
| Healthy Subjects (n = 10) | Muscle Complaint Subjects (n = 230) | Patients with Muscle Disease (n = 9) | p-Value | |
|---|---|---|---|---|
| Sex female (n; %) | 5; 50 | 118; 51.3 | 4; 44.4 | 1.000 |
| Age | 37.1 ± 13.3 | 44.9 ± 15.8 | 36.3 ± 16.0 | 0.115 |
| BMI kg/m2 | 22.7 ± 2.3 | 24.9 ± 5.0 | 24.9 ± 3.2 | 0.343 |
| Lean mass (%) | 51.9 ± 9.2 | 50.6 ± 11.4 | 50.2 ± 13.4 | 0.896 |
| Skeletal Muscle Index (kg/m2) | 8.4 ± 1.4 | 8.8 ± 2.6 | 8.7 ± 2.2 | 0.909 |
| V’O2max% predicted | 100.7 ± 17.0 | 82.8 ± 23.6 | 62.2 ± 22.6 £ | 0.0008 |
| V’O2@VT1% V’O2max | 65.7 ± 17.4 | 57.0 ± 16.3 | 51.3 ± 19.9 | 0.131 |
| Weariness (/10) | 0 ± 0 | 3.9 ± 2.8 $ | 5.6 ± 2.8 £ | <0.0001 |
| V’O2/work rate slope (mL/min/Watt) | 8.7 ± 1.2 | 8.8 ± 2.0 | 8.1 ± 0.4 | 0.577 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillet, P.-E.; Badiou, S.; Lambert, K.; Sutra, T.; Plawecki, M.; Raynaud de Mauverger, E.; Brun, J.-F.; Mercier, J.; Gouzi, F.; Cristol, J.-P. Biomarkers of Redox Balance Adjusted to Exercise Intensity as a Useful Tool to Identify Patients at Risk of Muscle Disease through Exercise Test. Nutrients 2022, 14, 1886. https://doi.org/10.3390/nu14091886
Grillet P-E, Badiou S, Lambert K, Sutra T, Plawecki M, Raynaud de Mauverger E, Brun J-F, Mercier J, Gouzi F, Cristol J-P. Biomarkers of Redox Balance Adjusted to Exercise Intensity as a Useful Tool to Identify Patients at Risk of Muscle Disease through Exercise Test. Nutrients. 2022; 14(9):1886. https://doi.org/10.3390/nu14091886
Chicago/Turabian StyleGrillet, Pierre-Edouard, Stéphanie Badiou, Karen Lambert, Thibault Sutra, Maëlle Plawecki, Eric Raynaud de Mauverger, Jean-Frédéric Brun, Jacques Mercier, Fares Gouzi, and Jean-Paul Cristol. 2022. "Biomarkers of Redox Balance Adjusted to Exercise Intensity as a Useful Tool to Identify Patients at Risk of Muscle Disease through Exercise Test" Nutrients 14, no. 9: 1886. https://doi.org/10.3390/nu14091886
APA StyleGrillet, P.-E., Badiou, S., Lambert, K., Sutra, T., Plawecki, M., Raynaud de Mauverger, E., Brun, J.-F., Mercier, J., Gouzi, F., & Cristol, J.-P. (2022). Biomarkers of Redox Balance Adjusted to Exercise Intensity as a Useful Tool to Identify Patients at Risk of Muscle Disease through Exercise Test. Nutrients, 14(9), 1886. https://doi.org/10.3390/nu14091886









