Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study
Abstract
:1. Introduction
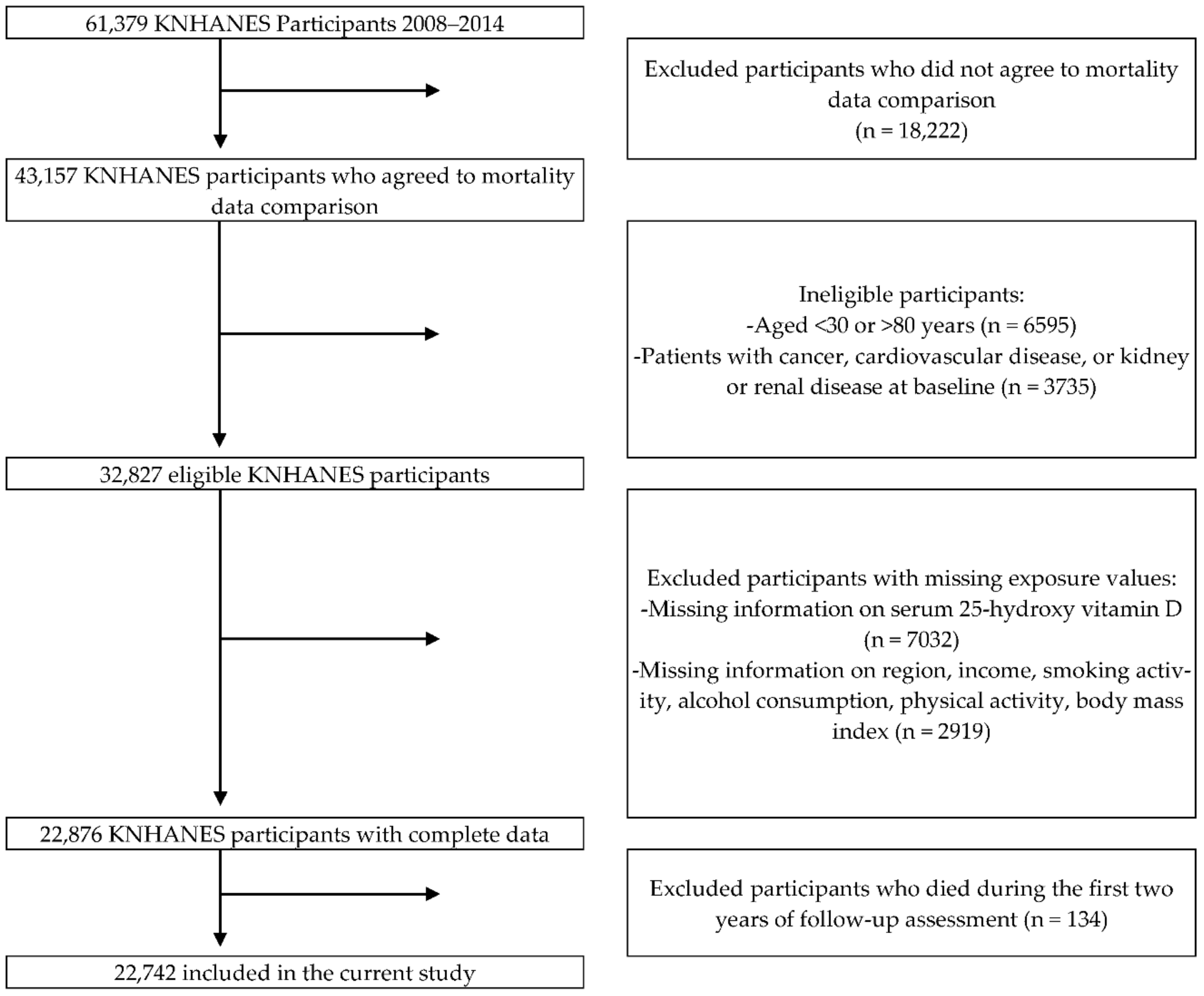
2. Materials and Methods
2.1. Study Population
2.2. Serum 25(OH)D Assessment
2.3. Mortality Assessment
2.4. Other Characteristics
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea. Causes of Death Statistics in 2019; Statistics Korea: Seoul, Korea, 2019.
- Heron, M. National Vital Statistics Reports Volume 68, Number 6, June 24, 2019, Deaths: Leading Causes for 2017; CDC: Atlanta, GA, USA, 2019.
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Hong, I.Y.; Chung, J.W.; Choi, H.S. Vitamin D status in South Korean population. Medicine 2018, 97, e11032. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.E.; Tangpricha, V. Vitamin D Deficiency and Risk for Cardiovascular Disease. Am. J. Med. Sci. 2009, 338, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The Role of Vitamin D in Cancer Prevention. Am. J. Public Health 2006, 96, 252. [Google Scholar] [CrossRef]
- Hsia, J.; Heiss, G.; Ren, H.; Allison, M.; Dolan, N.C.; Greenland, P.; Heckbert, S.R.; Johnson, K.C.; Manson, J.E.; Sidney, S.; et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007, 115, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.A.; MacLennan, G.S.; Avenell, A.; Bolland, M.; Grey, A.; Witham, M.; RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: Trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 746–755. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults (Review). Cochrane Database Syst. Rev. 2014, CD007470. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Fuss, P.; Vickery, E.M.; LeBlanc, E.S.; Sheehan, P.R.; Lewis, M.R.; Dolor, R.J.; Johnson, K.C.; Kashyap, S.R.; Nelson, J.; et al. Vitamin D Supplementation for Prevention of Cancer: The D2d Cancer Outcomes (D2dCA) Ancillary Study. J. Clin. Endocrinol. Metab. 2021, 106, 2767–2778. [Google Scholar] [CrossRef]
- Scragg, R.; Khaw, K.T.; Toop, L.; Sluyter, J.; Lawes, C.M.M.; Waayer, D.; Giovannucci, E.; Camargo, C.A. Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol. 2018, 4, e182178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Grossman, E.; Messerli, F.H.; Boyko, V.; Goldbourt, U. Is there an association between hypertension and cancer mortality? Am. J. Med. 2002, 112, 479–486. [Google Scholar] [CrossRef]
- Ali, M.K.; Jaacks, L.M.; Kowalski, A.J.; Siegel, K.R.; Ezzati, M. Noncommunicable diseases: Three decades of global data show a mixture of increases and decreases in mortality rates. Health Aff. 2015, 34, 1444–1455. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335. [Google Scholar] [CrossRef]
- Otani, T.; Iwasaki, M.; Sasazuki, S.; Inoue, M.; Tsugane, S. Plasma vitamin D and risk of colorectal cancer: The Japan Public Health Center-Based Prospective Study. Br. J. Cancer 2007, 97, 446. [Google Scholar] [CrossRef]
- Budhathoki, S.; Hidaka, A.; Yamaji, T.; Sawada, N.; Tanaka-Mizuno, S.; Kuchiba, A.; Charvat, H.; Goto, A.; Kojima, S.; Sudo, N.; et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: Large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ 2018, 360, 671. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-B.; Abnet, C.C.; Chen, W.; Dawsey, S.M.; Fan, J.-H.; Yin, L.-Y.; Yin, J.; Major, J.M.; Taylor, P.R.; Qiao, Y.-L.; et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: A nested case–control study. Br. J. Cancer 2013, 109, 1997–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zou, H.; Zhao, Y.; Hu, C.; Atanda, A.; Qin, X.; Jia, P.; Jiang, Y.; Qi, Z. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: A systematic review and dose-response meta-analysis. BMJ Open 2019, 9, e030513. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Guo, X.; Yu, X.; Liu, S.; Cui, X.; Zhang, B.; Liang, H. 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2019, 11, 2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Afzal, S.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Millwood, I.Y.; Walters, R.G.; Chen, Y.; Chen, N.; et al. Vitamin D and cause-specific vascular disease and mortality: A Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med. 2019, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.W.; Chen, W.; Fan, J.H.; Dawsey, S.M.; Taylor, P.R.; Qiao, Y.L.; Abnet, C.C. Prospective study of serum 25-hydroxyvitamin d concentration and mortality in a chinese population. Am. J. Epidemiol. 2012, 176, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.; Taylor, C.; Yaktine, A.; Del Valle, H. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Lumley, T. Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalidi, B.; Kuk, J.L.; Ardern, C.I. Lifetime risk of cardiometabolic mortality according to vitamin D status of middle and older-aged adults: NHANES III mortality follow-up. J. Steroid Biochem. Mol. Biol. 2019, 186, 34–41. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective Study of Predictors of Vitamin D Status and Cancer Incidence and Mortality in Men. JNCI J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef]
- Hsu, S.; Hoofnagle, A.N.; Gupta, D.K.; Gutierrez, O.M.; Peralta, C.A.; Shea, S.; Allen, N.B.; Burke, G.; Michos, E.D.; Ix, J.H.; et al. Race, Ancestry, and Vitamin D Metabolism: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2020, 105, e4337–e4350. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Dotto, G.P. Racial Differences in Cancer Susceptibility and Survival: More Than the Color of the Skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, G. Disparities in cardiovascular disease risk in the United States. Curr. Cardiol. Rev. 2015, 11, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van’t Veer, P.; Jansen, M.C.; Klerk, M.; Kok, F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease†. Public Health Nutr. 2000, 3, 103–107. [Google Scholar] [CrossRef] [Green Version]
- OECD. Health at a Glance 2019: OECD Indicators; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Obesity and the Economics of Prevention: Fit Not Fat—Korea Key Facts—OECD. Available online: https://www.oecd.org/els/health-systems/obesityandtheeconomicsofpreventionfitnotfat-koreakeyfacts.htm (accessed on 8 November 2021).
- Zhao, G.; Ford, E.S.; Li, C.; Croft, J.B. Serum 25-hydroxyvitamin D levels and all-cause and cardiovascular disease mortality among US adults with hypertension: The NHANES linked mortality study. J. Hypertens. 2012, 30, 284–289. [Google Scholar] [CrossRef]
- Lin, T.; Song, Y.; Zhang, X.; Guo, H.; Liu, L.; Zhou, Z.; Wang, B.; Tang, G.; Liu, C.; Yang, Y.; et al. Plasma 25-hydroxyvitamin D concentrations and risk of incident cancer in adults with hypertension: A nested case–control study. Clin. Nutr. 2019, 38, 2381–2388. [Google Scholar] [CrossRef]
- Stocks, T.; Van Hemelrijck, M.; Manjer, J.; Bjørge, T.; Ulmer, H.; Hallmans, G.; Lindkvist, B.; Selmer, R.; Nagel, G.; Tretli, S.; et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012, 59, 802–810. [Google Scholar] [CrossRef]
- Rigby, W.F.; Denome, S.; Fanger, M.W. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J. Clin. Investig. 1987, 79, 1659–1664. [Google Scholar] [CrossRef] [Green Version]
- Al-Bayyari, N.; Hailat, R.; Subih, H.; Alkhalidy, H.; Eaton, A. Vitamin D 3 reduces risk of cardiovascular and liver diseases by lowering homocysteine levels: Double-blinded, randomised, placebo-controlled trial. Br. J. Nutr. 2021, 125, 139–146. [Google Scholar] [CrossRef]
- Cheshmazar, E.; Hosseini, A.F.; Yazdani, B.; Razmpoosh, E.; Zarrati, M. Effects of Vitamin D Supplementation on Omentin-1 and Spexin Levels, Inflammatory Parameters, Lipid Profile, and Anthropometric Indices in Obese and Overweight Adults with Vitamin D Deficiency under Low-Calorie Diet: A Randomized Placebo Controlled Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 3826237. [Google Scholar] [CrossRef]
- Chandler, P.D.; Scott, J.B.; Drake, B.F.; Ng, K.; Manson, J.E.; Rifai, N.; Chan, A.T.; Bennett, G.G.; Hollis, B.W.; Giovannucci, E.L.; et al. Impact of Vitamin D Supplementation on Inflammatory Markers in African-Americans: Results of a Four-Arm, Randomized, Placebo-Controlled Trial. Cancer Prev. Res. 2014, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- Beilfuss, J.; Berg, V.; Sneve, M.; Jorde, R.; Kamycheva, E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 2012, 60, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T. The relationship of vitamin D status to risk of cardiovascular disease and mortality. Dan. Med. J. 2015, 62, B5008. [Google Scholar] [PubMed]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Bo Hansen, A.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Pais, P.; Dans, A.L.; Bosch, J.; Xavier, D.; Lopez-Jaramillo, P.; Yusoff, K.; Santoso, A.; Talukder, S.; Gamra, H.; et al. The International Polycap Study-3 (TIPS-3): Design, baseline characteristics and challenges in conduct. Am. Heart J. 2018, 206, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D Deficiency, Smoking, and Lung Function in the Normative Aging Study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Levels of Serum 25(OH)D (ng/mL) | 0–12 (N = 3713) | 12–20 (N = 11,192) | ≥20 (N = 7837) | p-Value |
|---|---|---|---|---|
| Mean follow-up (years) | 8.7 ± 0.1 a | 8.7 ± 0.03 a | 9.0 ± 0.04 b | <0.001 |
| Survey season (%) Spring (March–May) Summer (June–Aug) Autumn (Sept–Nov) Winter (Dec–Feb) | 36.6 14.0 13.5 35.9 | 27.9 23.9 23.1 25.1 | 14.9 36.4 34.5 14.2 | <0.001 |
| Mean serum 25(OH)D (ng/mL) | 9.9 ± 0.04 a | 15.9 ± 0.03 b | 25.4 ± 0.1 c | <0.001 |
| Age (years) | <0.001 | |||
| 30–44 | 52.2 | 48.0 | 35.9 | <0.001 |
| 45–59 | 31.5 | 36.2 | 40.6 | |
| 60–79 | 16.3 | 15.7 | 23.6 | |
| Males (%, N) | 26.4 (979) a | 38.1 (4265) b | 52.1 (4081) c | <0.001 |
| Education (%) Less than high school High school graduate University | 26.0 39.1 34.9 | 27.9 36.8 35.3 | 37.8 33.9 28.3 | <0.001 |
| Household income (%) Lowest Lower middle Upper middle Highest | 14.2 27.1 30.4 28.3 | 12.8 26.0 31.1 30.1 | 16.0 25.7 28.8 29.5 | <0.001 |
| Urban dweller (%, N) | 87.5 (3159) c | 83.0 (8934) b | 72.0 (5243) a | <0.001 |
| Lifestyle | ||||
| Smoking status (%) Never Former Current | 61.7 10.0 28.3 | 54.7 14.4 30.9 | 45.6 16.6 37.8 | <0.001 |
| Current drinker (%, N) | 71.0 (2396) a | 76.9 (7900) b | 79.0 (5633) c | <0.001 |
| Mean METs | 1736.8 ± 67.9 a | 2215.1 ± 45.9 b | 2988.9 ± 78.8 c | <0.001 |
| Health status | ||||
| Obese (%, N) | 30.1 (1089) a | 34.9 (3752) b | 35.4 (2655) c | <0.001 |
| Hypertension (%, N) | 26.9 (1063) a | 27.0 (3266) a | 32.4 (2761) b | <0.001 |
| Diabetes mellitus (%, N) | 8.4 (336) a | 8.7 (1029) a | 9.7 (834) b | <0.001 |
| Biochemistry | ||||
| SBP (mmHg) | 117.6 ± 0.4 | 117.9 ± 0.2 | 120.1 ± 0.3 | <0.001 |
| DBP (mmHg) | 76.8 ± 0.3 | 77.5 ± 0.1 | 78.3 ± 0.2 | <0.001 |
| Glucose (mg/dL) | 97.6 ± 0.5 | 98.5 ± 0.3 | 98.8 ± 0.3 | <0.001 |
| TG (mg/dL) | 145.8 ± 2.8 | 143.3 ± 1.6 | 141.5 ± 1.6 | 0.020 |
| TC (mg/dL) | 189.2 ± 0.8 | 191.8 ± 0.4 | 192.6 ± 0.5 | 0.212 |
| HDL-C (mg/dL) | 48.7 ± 0.3 | 49.0 ± 0.1 | 48.5 ± 0.2 | <0.001 |
| LDL-C (mg/dL) | 117.9 ± 1.5 | 116.5 ± 0.8 | 117.2 ± 1.1 | 0.003 |
| Serum 25(OH)D (ng/mL) | Total | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths/PY | Weighted Deaths/PY | HR (95% CI) | p-Value | Deaths/PY | Weighted Deaths/PY | HR (95% CI) | p-Value | Deaths/PY | Weighted Deaths/PY | HR (95% CI) | p-Value | |
| All-cause mortality | ||||||||||||
| ≥20 | 455/71,323 | 218,715/51,438,932 | 1.00 (ref) | 303/36,761 | 151,351/31,744,736 | 1.00 (ref) | 152/34,562 | 67,364/19,694,196 | 1.00 (ref) | |||
| 12–19 | 442/98,826 | 255,061/75,185,216 | 1.28 (1.06–1.54) | 0.009 | 247/36,913 | 157,288/36,093,644 | 1.44 (1.13–1.83) | 0.003 | 195/61,913 | 97,773/39,091,571 | 1.02 (0.77–1.34) | 0.896 |
| <12 | 173/32,801 | 101,864/25,120,105 | 1.71 (1.32–2.22) | <0.001 | 82/8253 | 53,555/8,823,540 | 2.08 (1.44–2.99) | <0.001 | 91/24,549 | 48,310/16,296,565 | 1.26 (0.89–1.77) | 0.196 |
| Cancer mortality | ||||||||||||
| ≥20 | 159/71,323 | 77,283/51,438,932 | 1.00 (ref) | 106/36,761 | 51,736/31,744,736 | 1.00 (ref) | 53/34,562 | 25,548/19,694,196 | 1.00 (ref) | |||
| 12–19 | 161/98,826 | 92,325/75,185,216 | 1.36 (0.99–1.87) | 0.058 | 91/36,913 | 60,380/36,093,644 | 1.64 (1.10–2.45) | 0.016 | 70/61,913 | 31,945/39,091,571 | 0.93 (0.58–1.50) | 0.773 |
| <12 | 55/32,801 | 34,962/25,120,105 | 1.83 (1.17–2.87) | 0.008 | 26/8253 | 16,814/8,823,540 | 1.90 (1.03–3.52) | 0.041 | 29/24,549 | 18,149/16,296,565 | 1.47 (0.79–2.73) | 0.222 |
| Cardiovascular mortality | ||||||||||||
| ≥20 | 90/71,323 | 39,489/51,438,932 | 1.00 (ref) | 49/36,761 | 22,885/31,744,736 | 1.00 (ref) | 41/34,562 | 16,604/19,694,196 | 1.00 (ref) | |||
| 12–19 | 93/98,826 | 49,866/75,185,216 | 1.29 (0.86–1.94) | 0.218 | 43/36,913 | 25,122/36,093,644 | 1.80 (1.01–3.23) | 0.046 | 50/61,913 | 24,744/39,091,571 | 0.87 (0.50–1.50) | 0.618 |
| <12 | 41/32,801 | 20,824/25,120,105 | 1.72 (1.01–2.93) | 0.046 | 18/8253 | 10,033/8,823,540 | 2.96 (1.28–6.84) | 0.011 | 23/24,549 | 10,791/16,296,565 | 1.06 (0.55–2.04) | 0.852 |
| Serum 25(OH)D (ng/mL) | No Hypertension | Hypertension | ||||||
|---|---|---|---|---|---|---|---|---|
| Deaths/PY | Weighted Deaths/PY | HR (95% CI) | p-Value | Deaths/PY | Weighted Deaths/PY | HR (95% CI) | p-Value | |
| All-cause mortality | ||||||||
| ≥20 | 211/45,317 | 108,690/34,204,328 | 1.00 (ref) | 231/25,010 | 102,253/16,339,236 | 1.00 (ref) | ||
| 12–19 | 191/68,123 | 109,793/53,624,356 | 1.15 (0.87–1.52) | 0.328 | 235/28,685 | 134,893/19,651,392 | 1.46 (1.15–1.86) | 0.002 |
| <12 | 66/22,867 | 43,152/18,024,097 | 1.71 (1.13–2.58) | 0.011 | 97/9276 | 55,236/6,489,899 | 1.74 (1.24–2.44) | 0.001 |
| Cancer mortality | ||||||||
| ≥20 | 82/45,317 | 42,013/34,204,328 | 1.00 (ref) | 75/25,010 | 33,557/16,339,236 | 1.00 (ref) | ||
| 12–19 | 68/68,123 | 42,655/53,624,356 | 1.10 (0.70–1.74) | 0.668 | 89/28,685 | 47,100/19,651,392 | 1.74 (1.12–2.72) | 0.015 |
| <12 | 23/22,867 | 15,357/18,024,097 | 1.52 (0.78–2.98) | 0.222 | 32/9276 | 19,605/6,489,899 | 2.27 (1.22–4.22) | 0.010 |
| Cardiovascular mortality | ||||||||
| ≥20 | 39/45,317 | 17,135/34,204,328 | 1.00 (ref) | 48/25,010 | 20,785/16,339,236 | 1.00 (ref) | ||
| 12–19 | 28/68,123 | 12,317/53,624,356 | 0.82 (0.39–1.73) | 0.605 | 60/28,685 | 34,857/19,651,392 | 1.75 (1.08–2.85) | 0.023 |
| <12 | <10/22,867 | 3742/18,024,097 | 1.01 (0.33–3.11) | 0.982 | 29/9276 | 15,372/6,489,899 | 2.23 (1.17–4.24) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Lee, J.; Park, C.Y.; Shin, M.-J. Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study. Nutrients 2022, 14, 1849. https://doi.org/10.3390/nu14091849
Park D, Lee J, Park CY, Shin M-J. Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study. Nutrients. 2022; 14(9):1849. https://doi.org/10.3390/nu14091849
Chicago/Turabian StylePark, Dahyun, Juhee Lee, Clara Yongjoo Park, and Min-Jeong Shin. 2022. "Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study" Nutrients 14, no. 9: 1849. https://doi.org/10.3390/nu14091849
APA StylePark, D., Lee, J., Park, C. Y., & Shin, M.-J. (2022). Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study. Nutrients, 14(9), 1849. https://doi.org/10.3390/nu14091849






