Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss?
Abstract
:1. Introduction
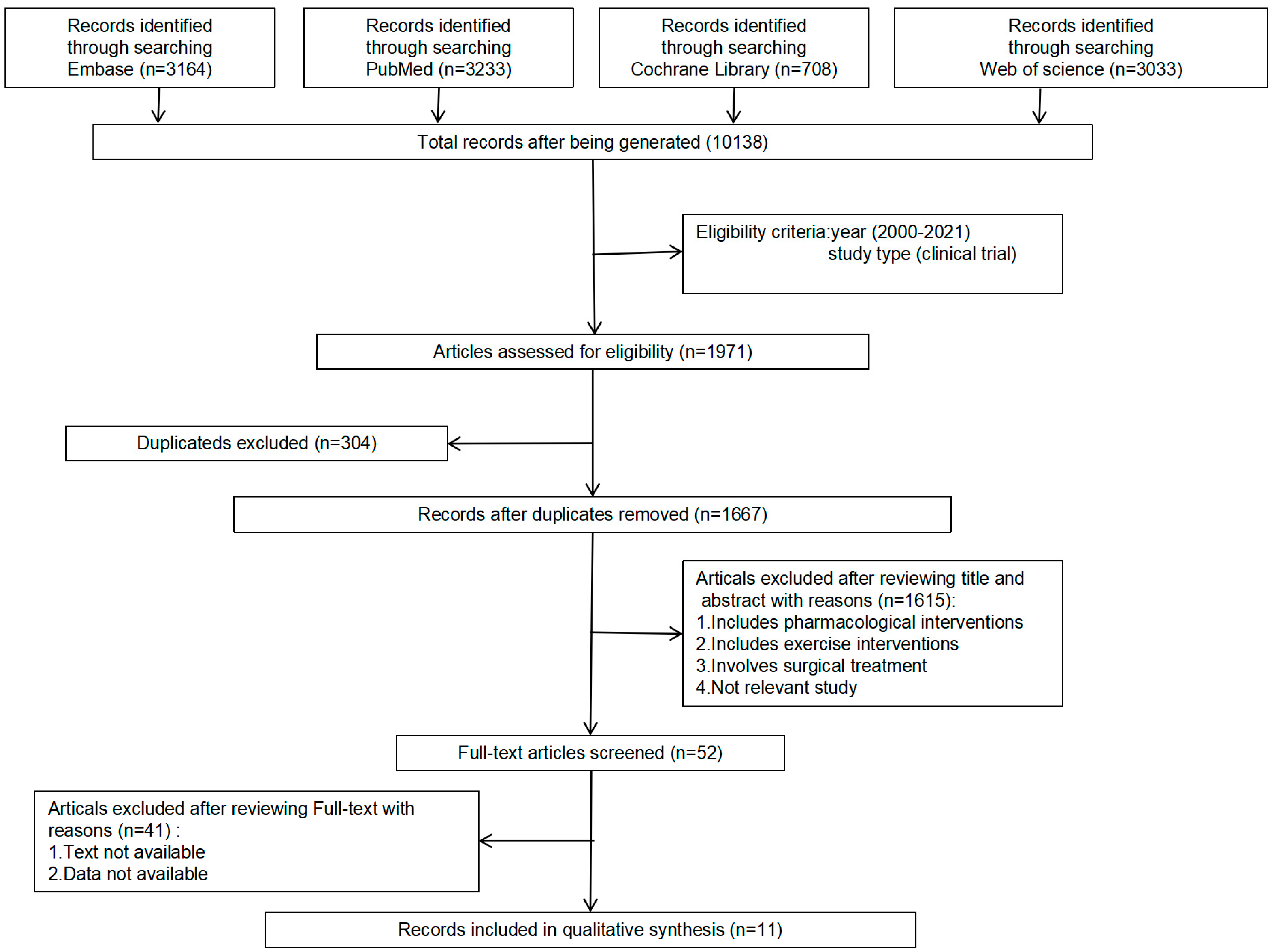
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
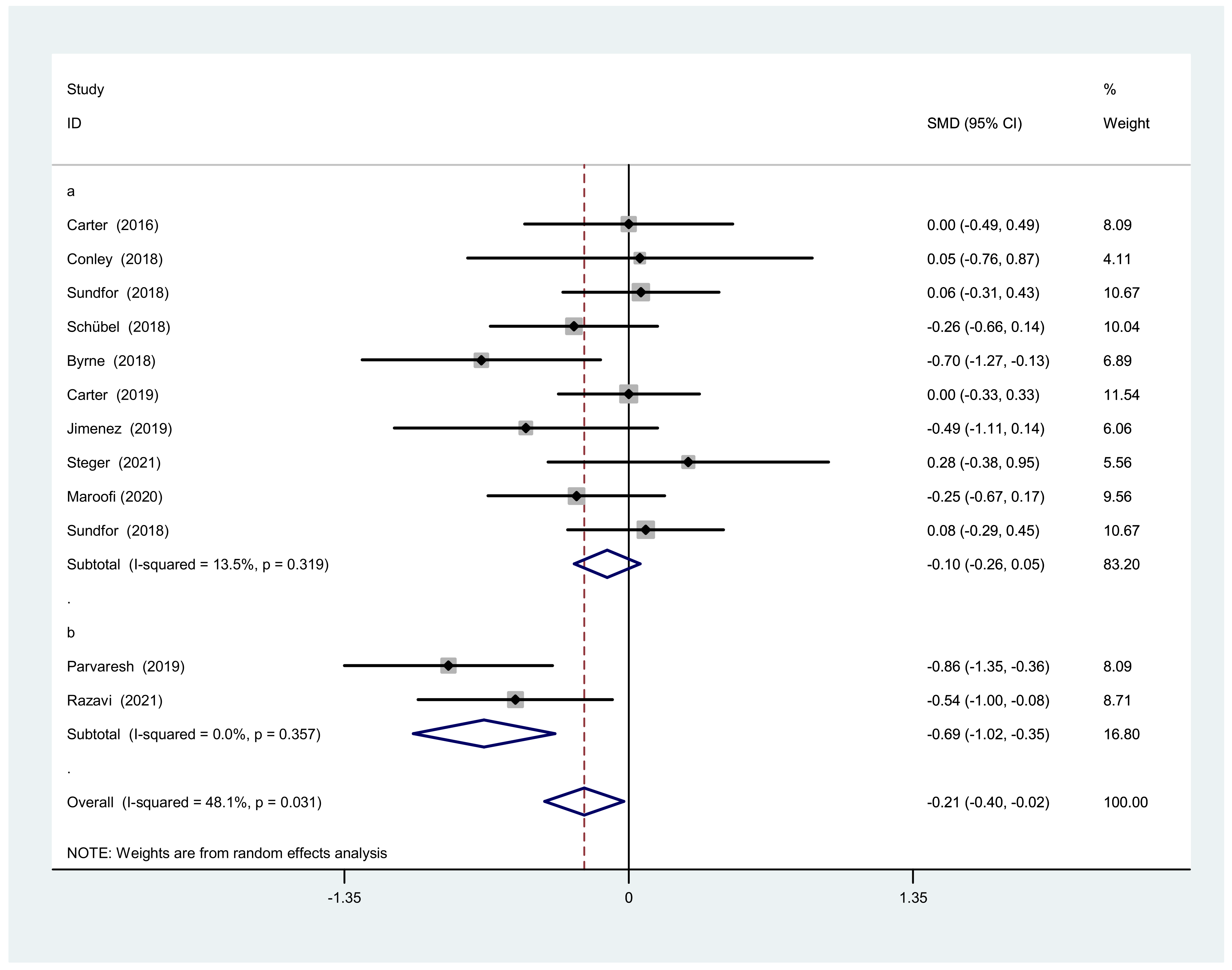
3.3. Meta-Analysis
3.3.1. BMI after IF versus CCR
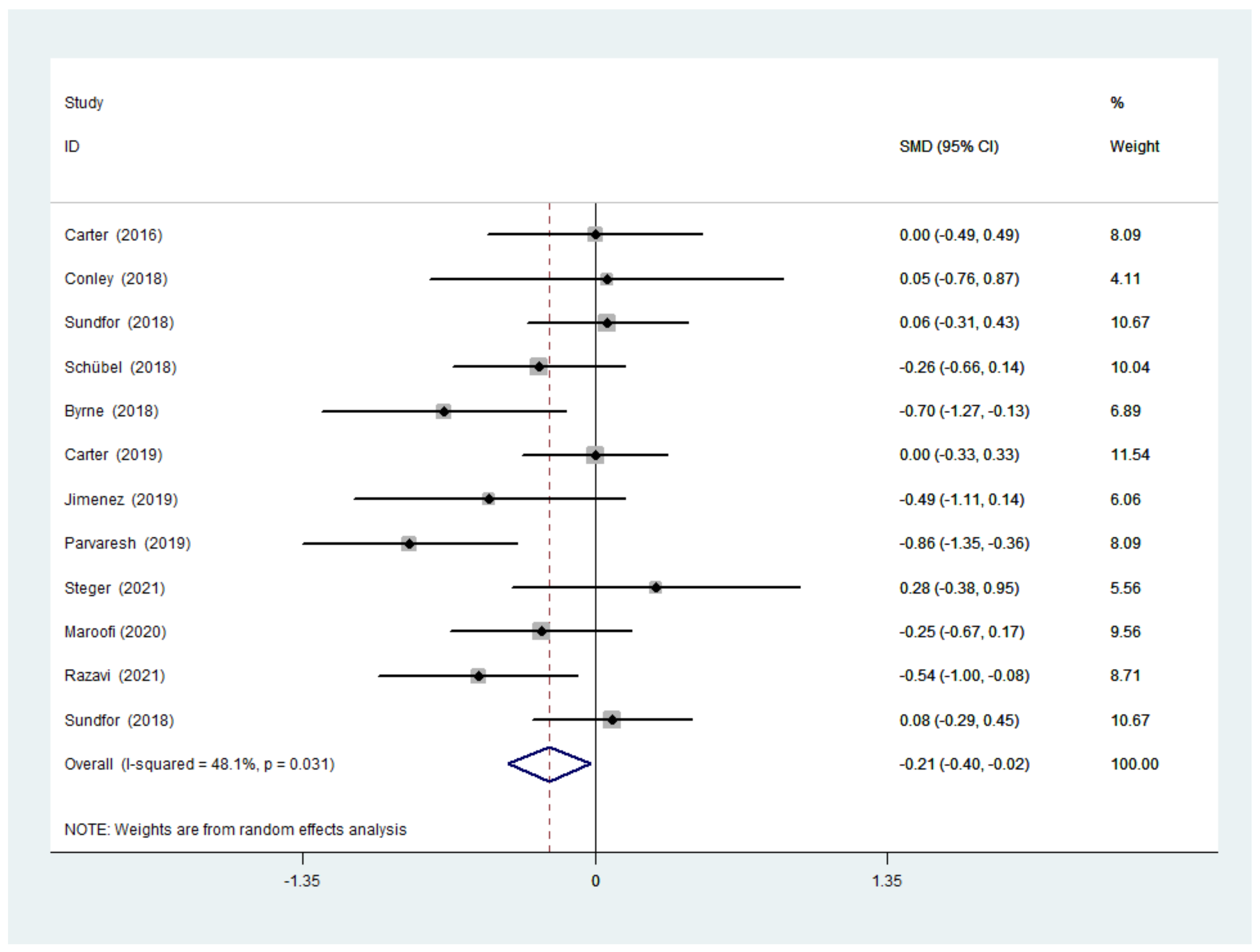
3.3.2. Body Weight after IF Versus CCR
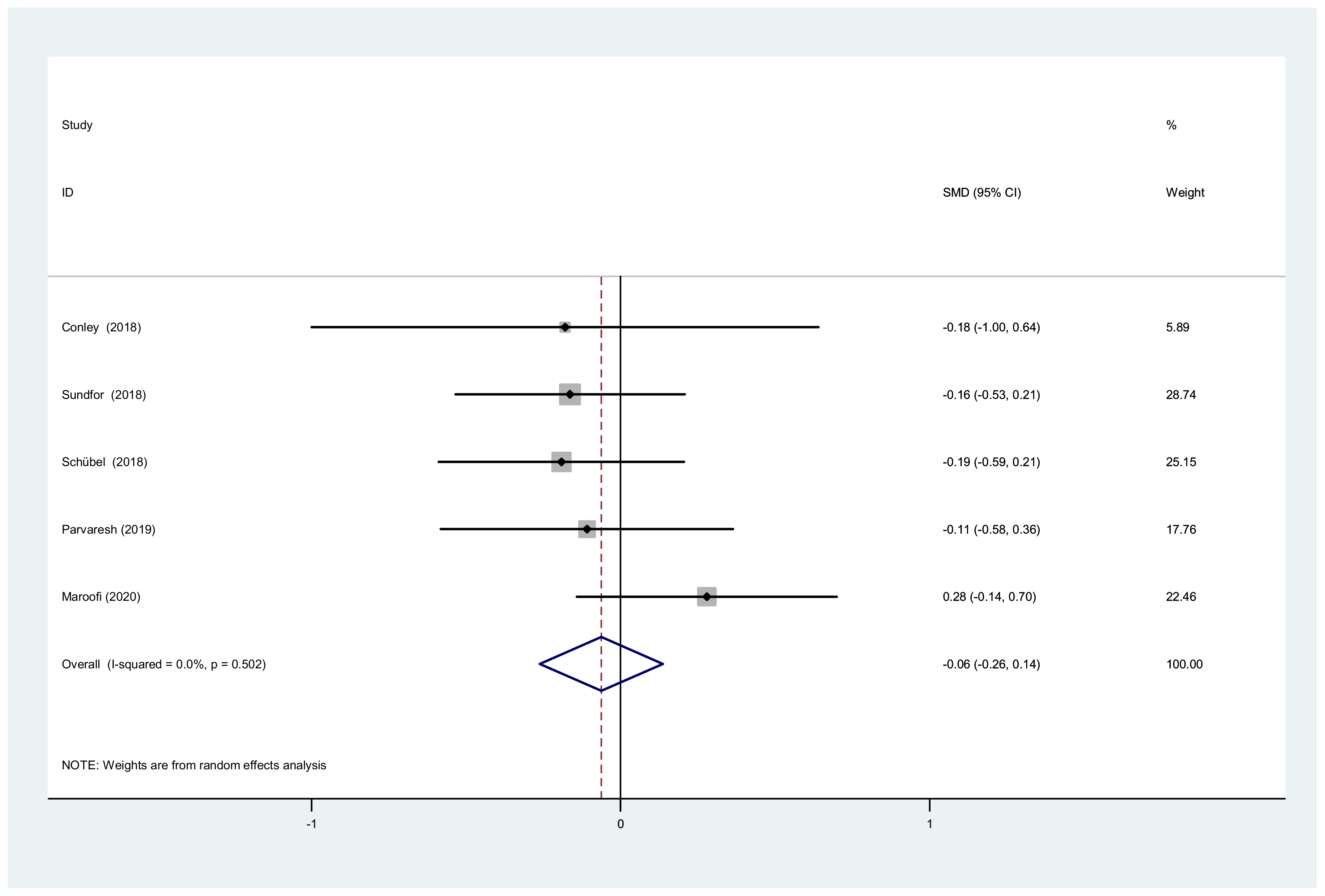
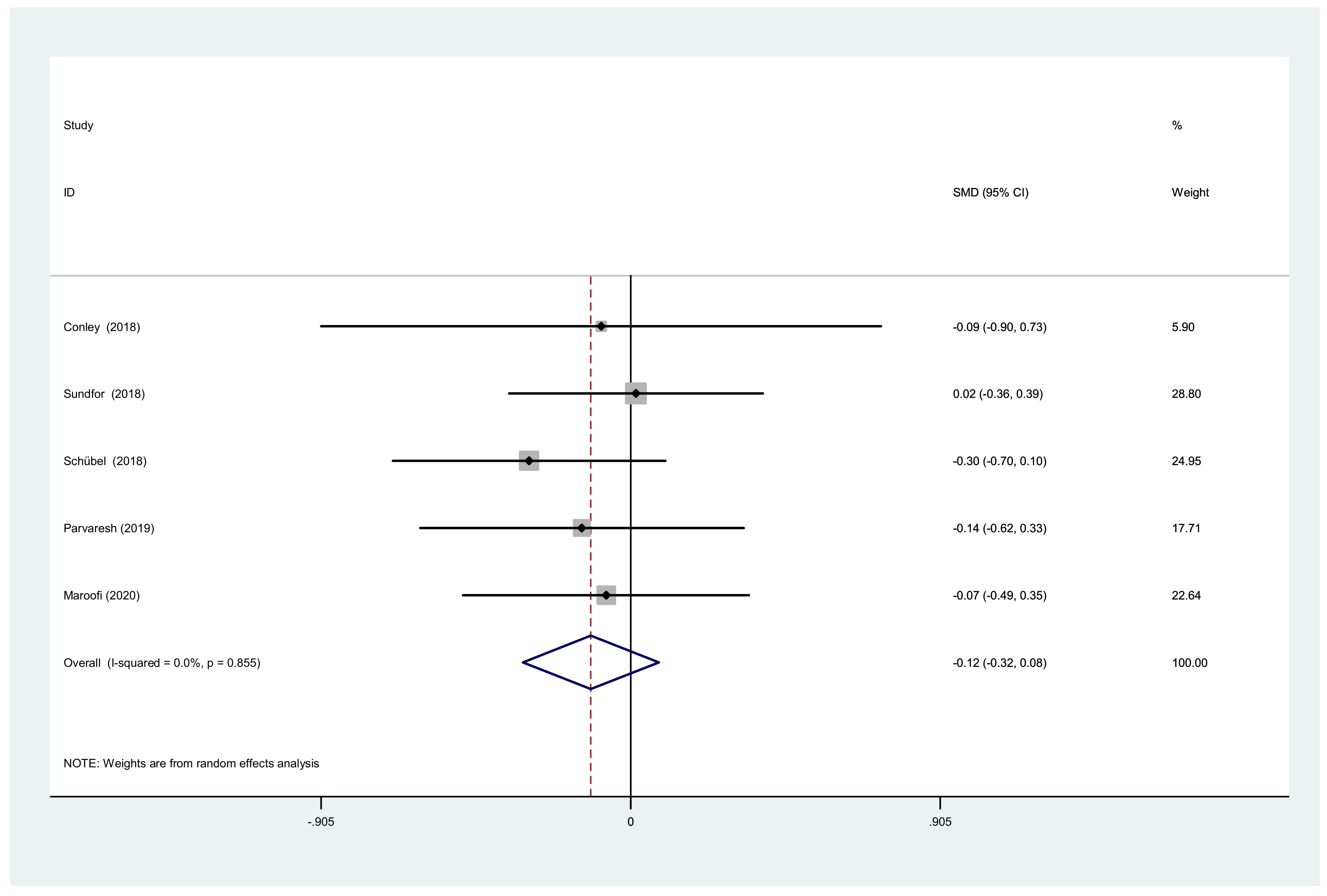
3.3.3. TC, TG and Waist Circumference after IF versus CCR
3.4. Subgroup Analysis
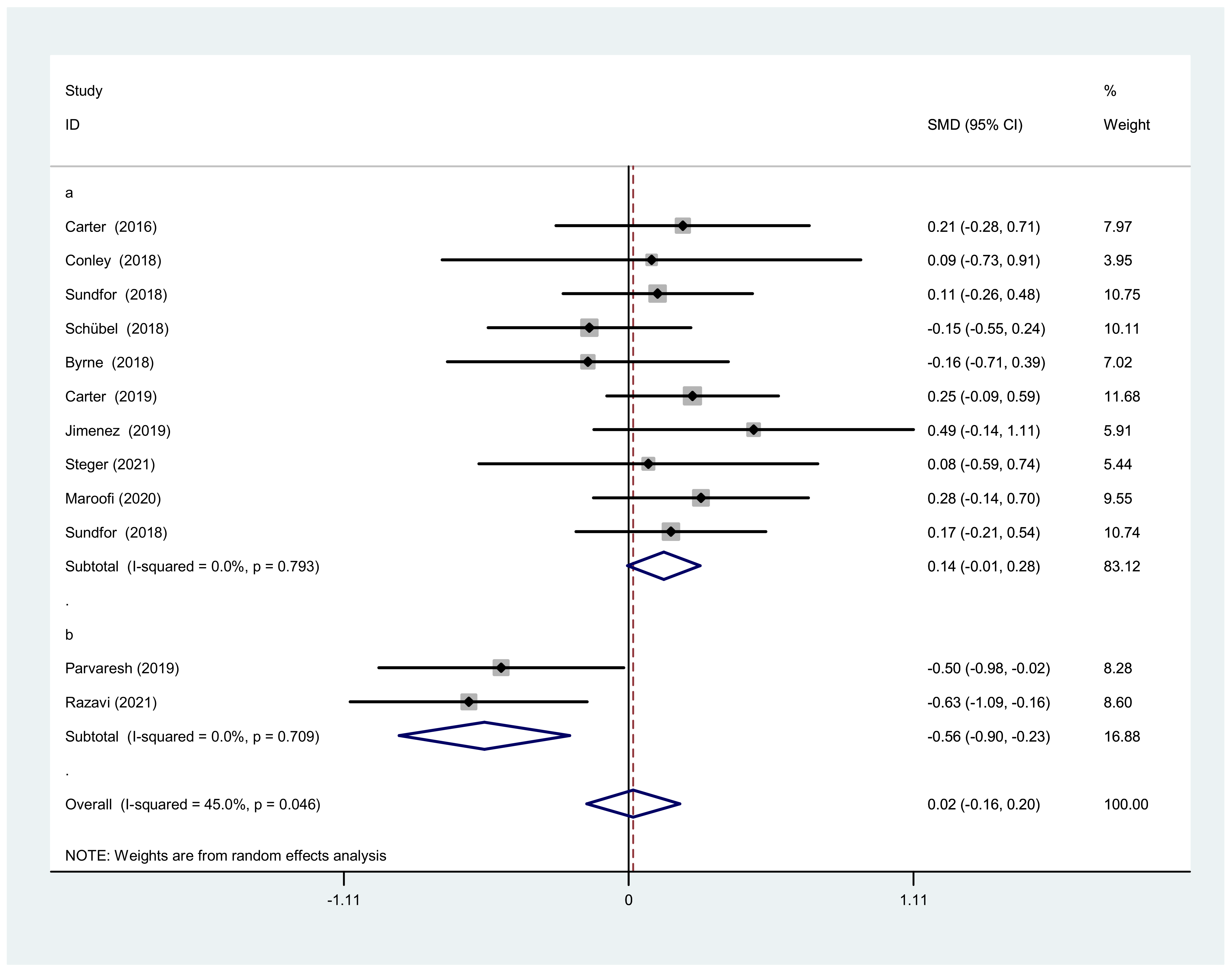
3.4.1. Subgroup Analysis of BMI
3.4.2. Subgroup Analysis of Body Weight
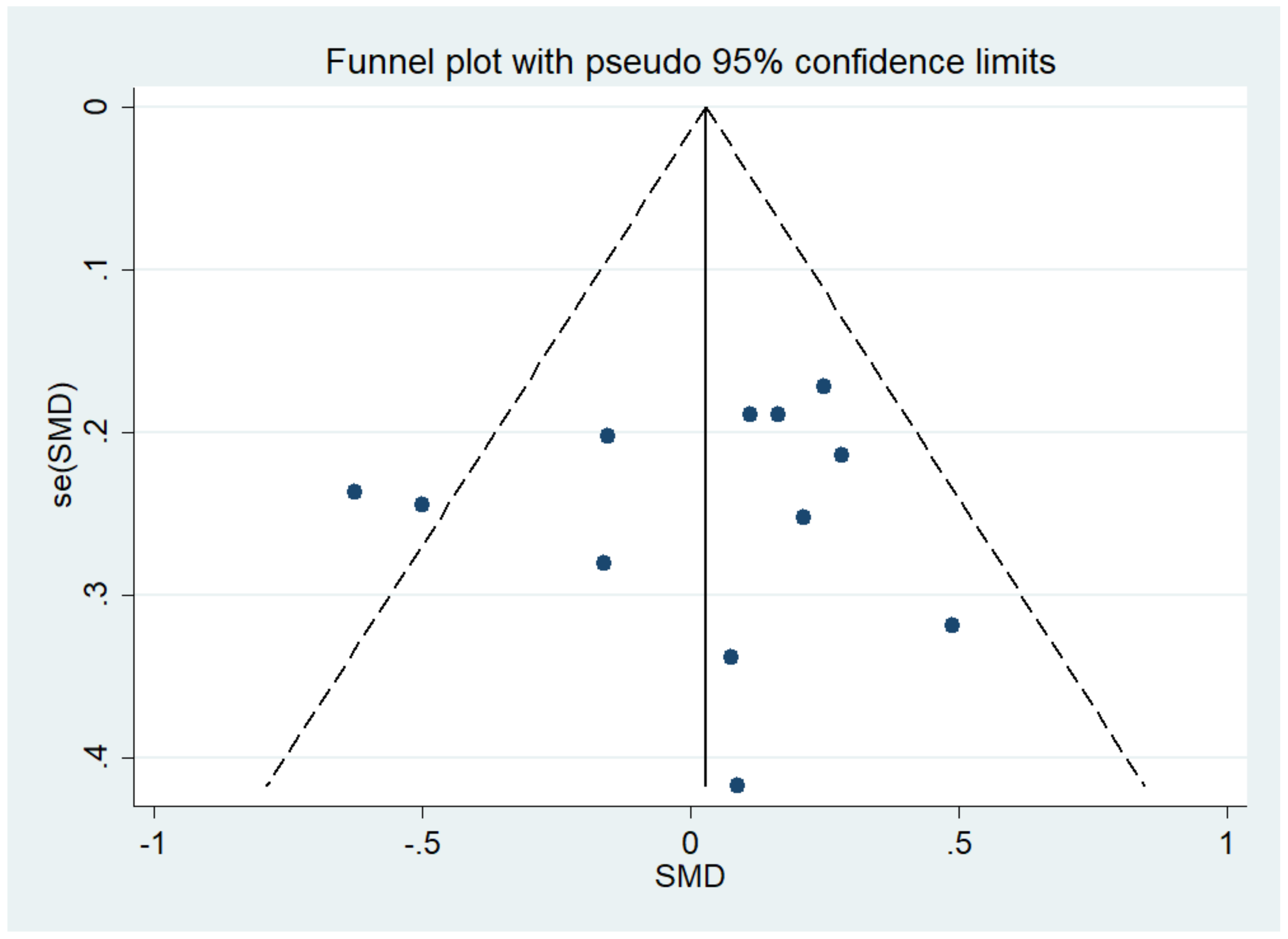
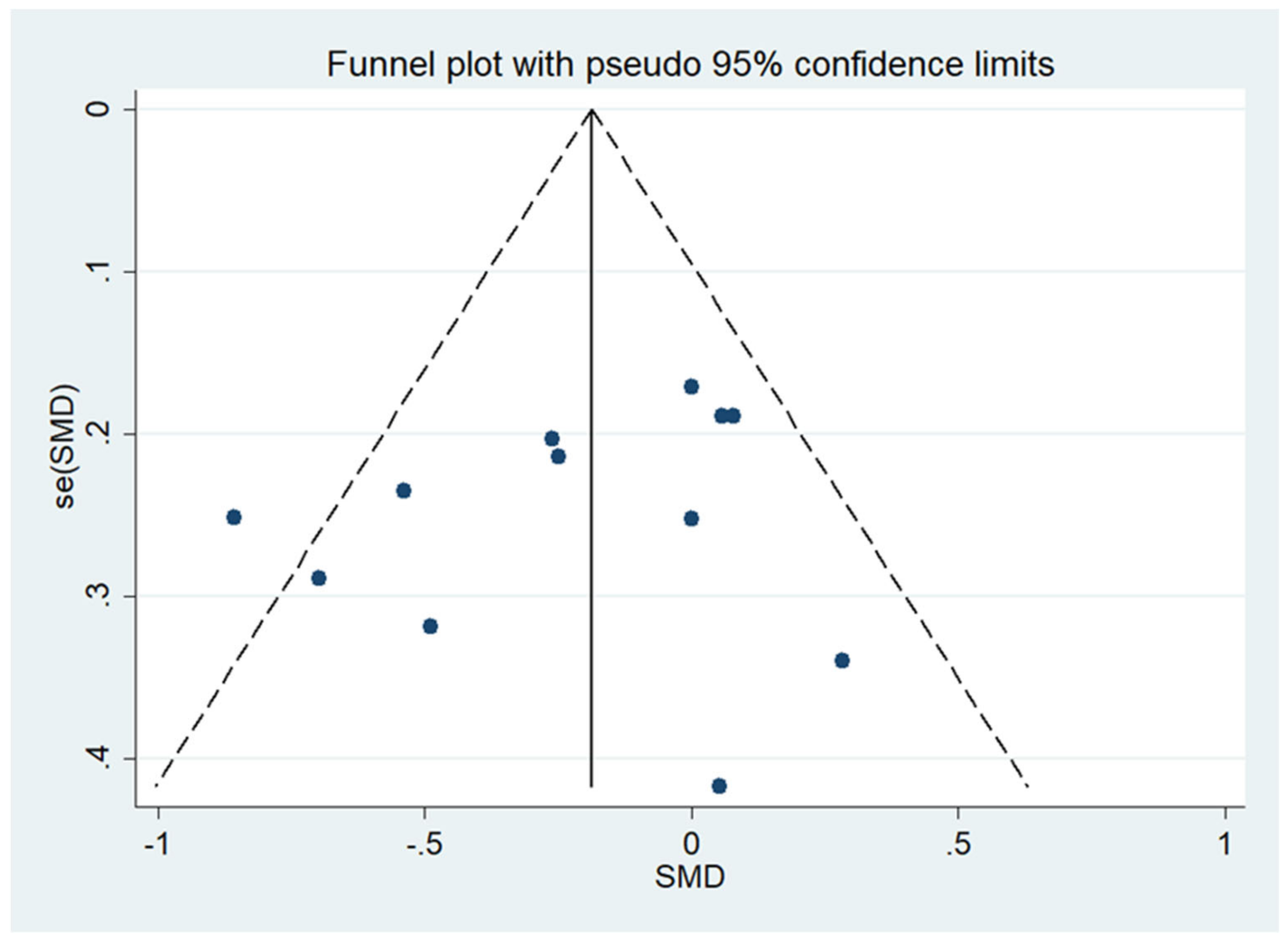
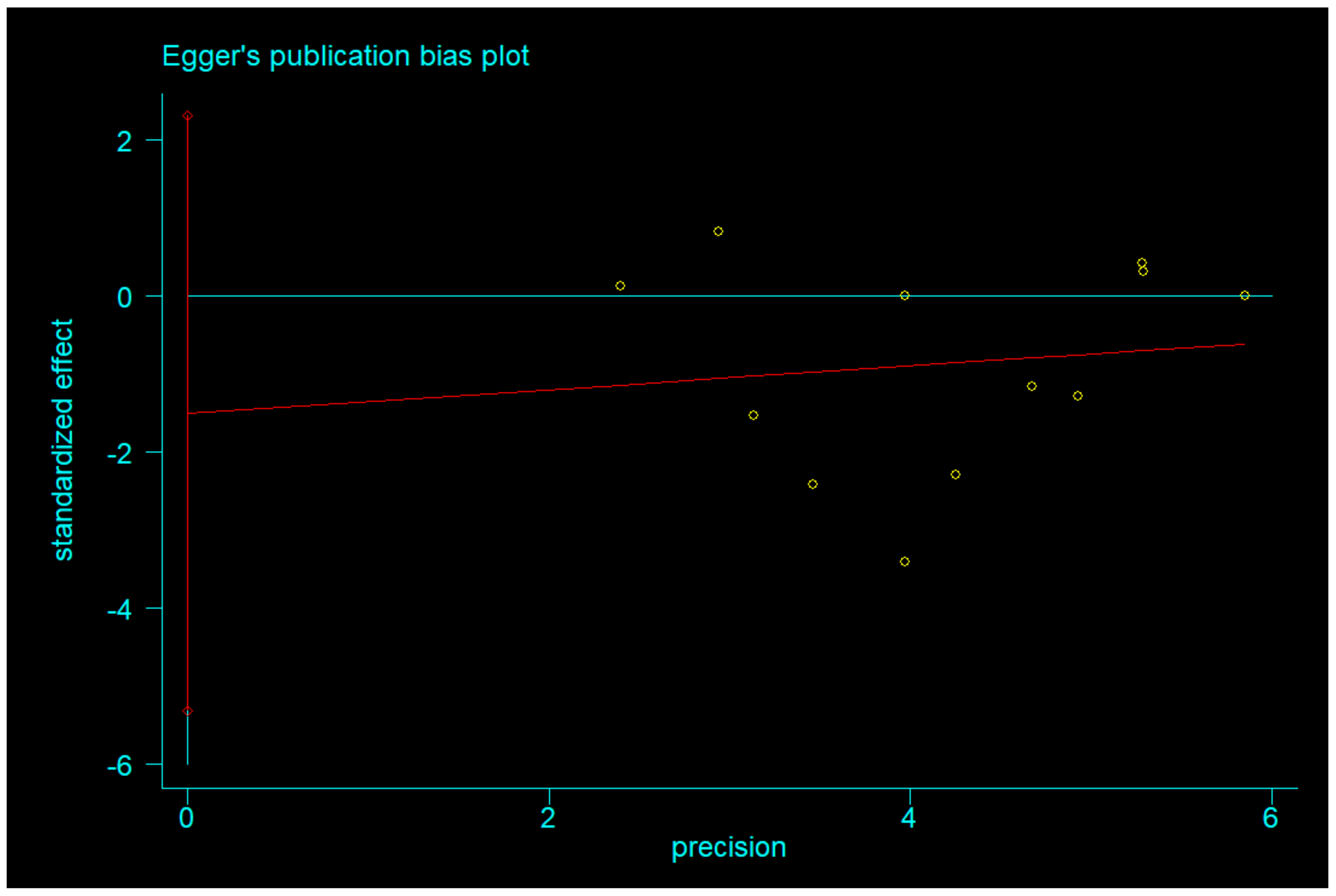
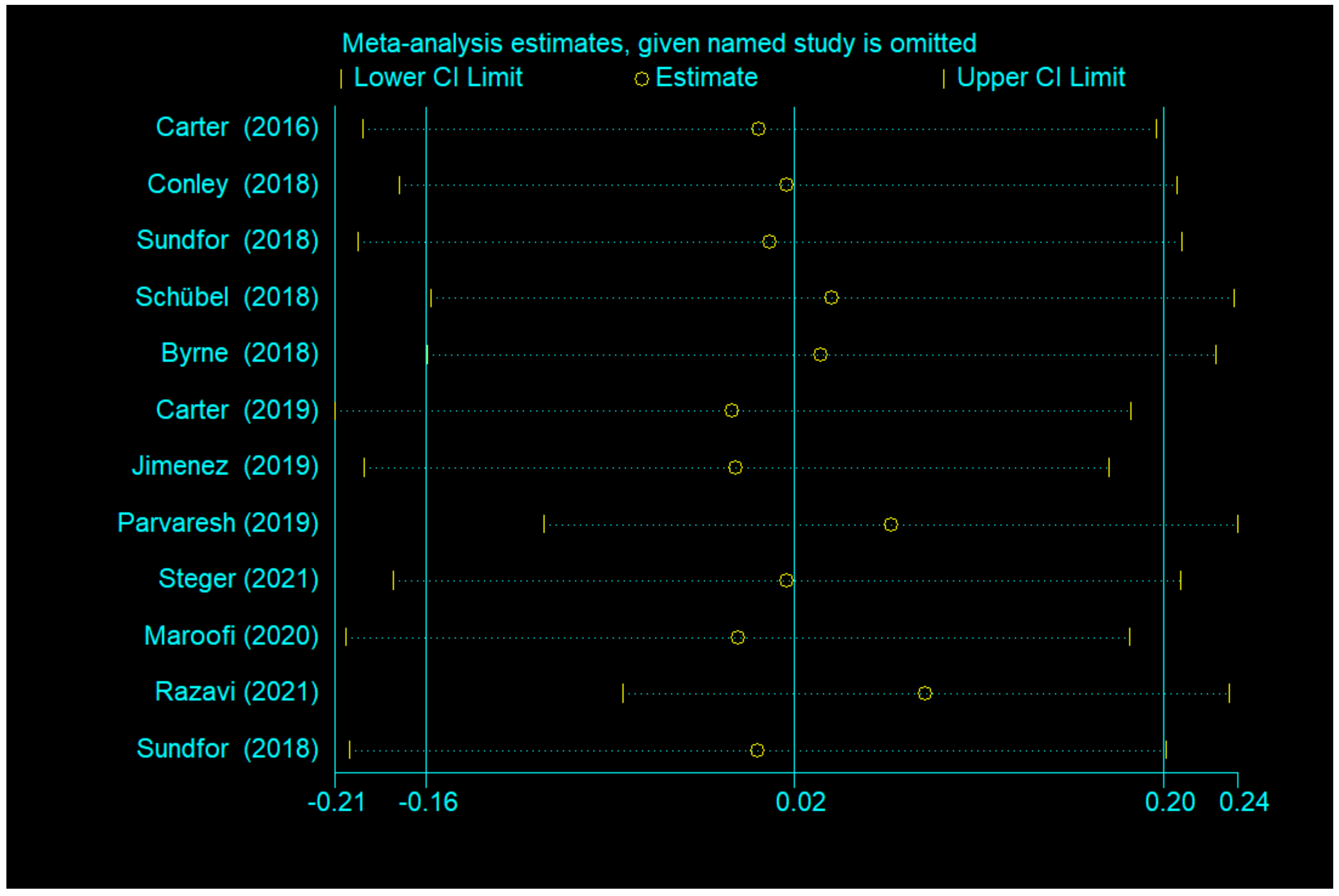
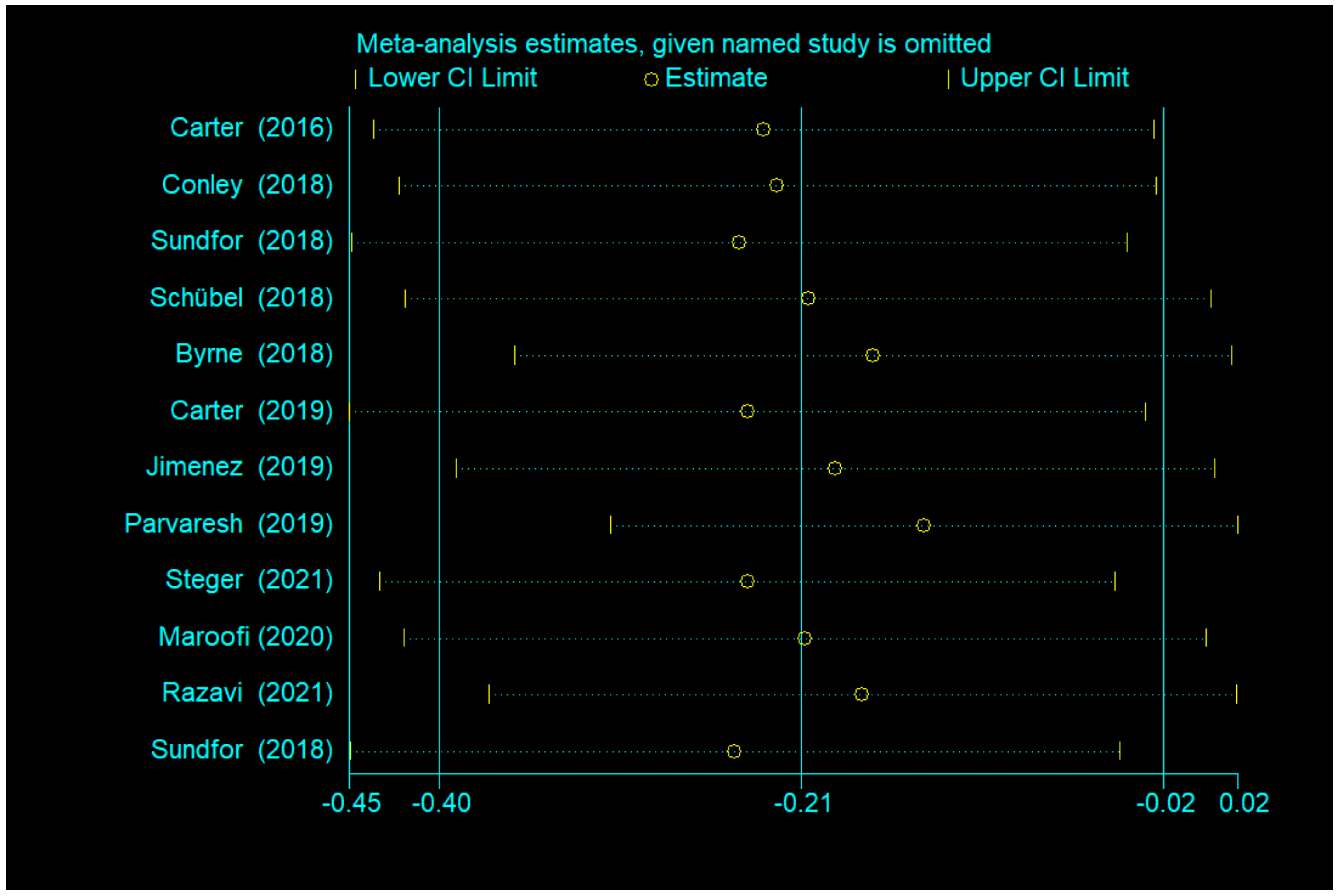
3.4.3. Publication Bias and Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Shin, S.; El-Sabbagh, A.S.; Lukas, B.E.; Tanneberger, S.J.; Jiang, Y. Adipose stem cells in obesity: Challenges and opportunities. Biosci. Rep. 2020, 40, BSR20194076. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. Tomaselli, 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef] [Green Version]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Schübel, R.; Graf, M.E.; Nattenmüller, J.; Nabers, D.; Sookthai, D.; Gruner, L.F.; Johnson, T.; Schlett, C.L.; von Stackelberg, O.; Kirsten, R.; et al. The effects of intermittent calorie restriction on metabolic health: Rationale and study design of the HELENA Trial. Contemp. Clin. Trials 2016, 51, 28–33. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res. Clin. Pract. 2019, 151, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Conley, M.; Fevre, L.L.; Haywood, C.; Proietto, J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. 2018, 75, 65–72. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Byrne, N.M.; Sainsbury, A.; King, N.A.; Hills, A.P.; Wood, R.E. Intermittent energy restriction improves weight loss efficiency in obese men: The MATADOR study. Int. J. Obes. 2018, 42, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Jimenez, A.M.; Oliva, S.L.; Vilar, E.G.; de Cuevillas, B.; Moreno, M.D.C.M.; de Prado, J.G.; Diaz, E.A.; Martin, I.S.M. The Mediterranean diet pattern with intermittent semi-fasting may facilitate weight loss: Randomised controlled trial. Mediterr. J. Nutr. Metab. 2019, 12, 153–161. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef]
- Steger, F.L.; Donnelly, J.E.; Hull, H.R.; Li, X.; Hu, J.; Sullivan, D.K. Intermittent and continuous energy restriction result in similar weight loss, weight loss maintenance, and body composition changes in a 6 month randomized pilot study. Clin. Obes. 2021, 11, e12430. [Google Scholar] [CrossRef] [PubMed]
- Razavi, R.; Parvaresh, A.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Clark, C.C.T.; Safavi, S.M. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int. J. Vitam. Nutr. Res. 2021, 91, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Maroofi, M.; Nasrollahzadeh, J. Effect of intermittent versus continuous calorie restriction on body weight and cardiometabolic risk markers in subjects with overweight or obesity and mild-to-moderate hypertriglyceridemia: A randomized trial. Lipids Health Dis. 2020, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.E.; Martín, I.S.M.; Vilar, E.G.; Martín, M.A.C. Effectiveness of an intermittent fasting diet versus continuous energy restriction on anthropometric measurements, body composition and lipid profile in overweight and obese adults: A meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1024–1039. [Google Scholar] [CrossRef]
- Pignatti, C.; D’Adamo, S.; Stefanelli, C.; Flamigni, F.; Cetrullo, S. Nutrients and Pathways that Regulate Health Span and Life Span. Geriatrics 2020, 5, 95. [Google Scholar] [CrossRef]
- Davis, T.A.; Bales, C.W.; Beauchene, R.E. Differential effects of dietary caloric and protein restriction in the aging rat. Exp. Gerontol. 1983, 18, 427–435. [Google Scholar] [CrossRef]
- Masoro, E.J.; Iwasaki, K.; Gleiser, C.A.; McMahan, C.A.; Seo, E.J.; Yu, B.P. Dietary modulation of the progression of nephropathy in aging rats: An evaluation of the importance of protein. Am. J. Clin. Nutr. 1989, 49, 1217–1227. [Google Scholar] [CrossRef]
- Santos, H.O.; Macedo, R.C.O. Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clin. Nutr. ESPEN 2018, 24, 14–21. [Google Scholar] [CrossRef]
- Marinho, T.S.; Borges, C.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Intermittent fasting benefits on alpha- and beta-cell arrangement in diet-induced obese mice pancreatic islet. J. Diabetes Its Complicat. 2020, 34, 107497. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Tarabra, E.; Toledo, M.; Garcia-Macia, M.; Sahu, S.; Coletto, L.; Batista-Gonzalez, A.; Barzilai, N.; Pessin, J.E.; Schwartz, G.J.; et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab. 2017, 26, 856–871.e855. [Google Scholar] [CrossRef] [Green Version]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Ng, G.Y.; Kang, S.W.; Kim, J.; Alli-Shaik, A.; Baik, S.H.; Jo, D.G.; Hande, M.P.; Sobey, C.G.; Gunaratne, J.; Fann, D.Y.; et al. Genome-Wide Transcriptome Analysis Reveals Intermittent Fasting-Induced Metabolic Rewiring in the Liver. Dose-Response 2019, 17, 1559325819876780. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- de Toledo, F.W.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar]
- Tobias, D.K.; Chen, M.; Manson, J.E.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar]
- Wormser, D.; Kaptoge, S.; di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; Nordestgaard, B.G.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Rong, J.; Wang, Y.; Hu, F.; Bao, C.; Li, X.; Zhao, Y. The relationship between body mass index and hip osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2011, 78, 150–155. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, W.; Wang, Y.; Rong, J.; Bao, C.; Liu, Y.; Zhao, Y.; Wang, C. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2012, 79, 291–297. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.M.; Dyck, J.R. Age-related cardiovascular disease and the beneficial effects of calorie restriction. Heart Fail. Rev. 2012, 17, 707–719. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br. J. Nutr. 2016, 115, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Tonstad, S.; Svendsen, M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur. J. Clin. Nutr. 2019, 73, 1006–1014. [Google Scholar] [CrossRef]
- Wan, R.; Ahmet, I.; Brown, M.; Cheng, A.; Kamimura, N.; Talan, M.; Mattson, M.P. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem. 2010, 21, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
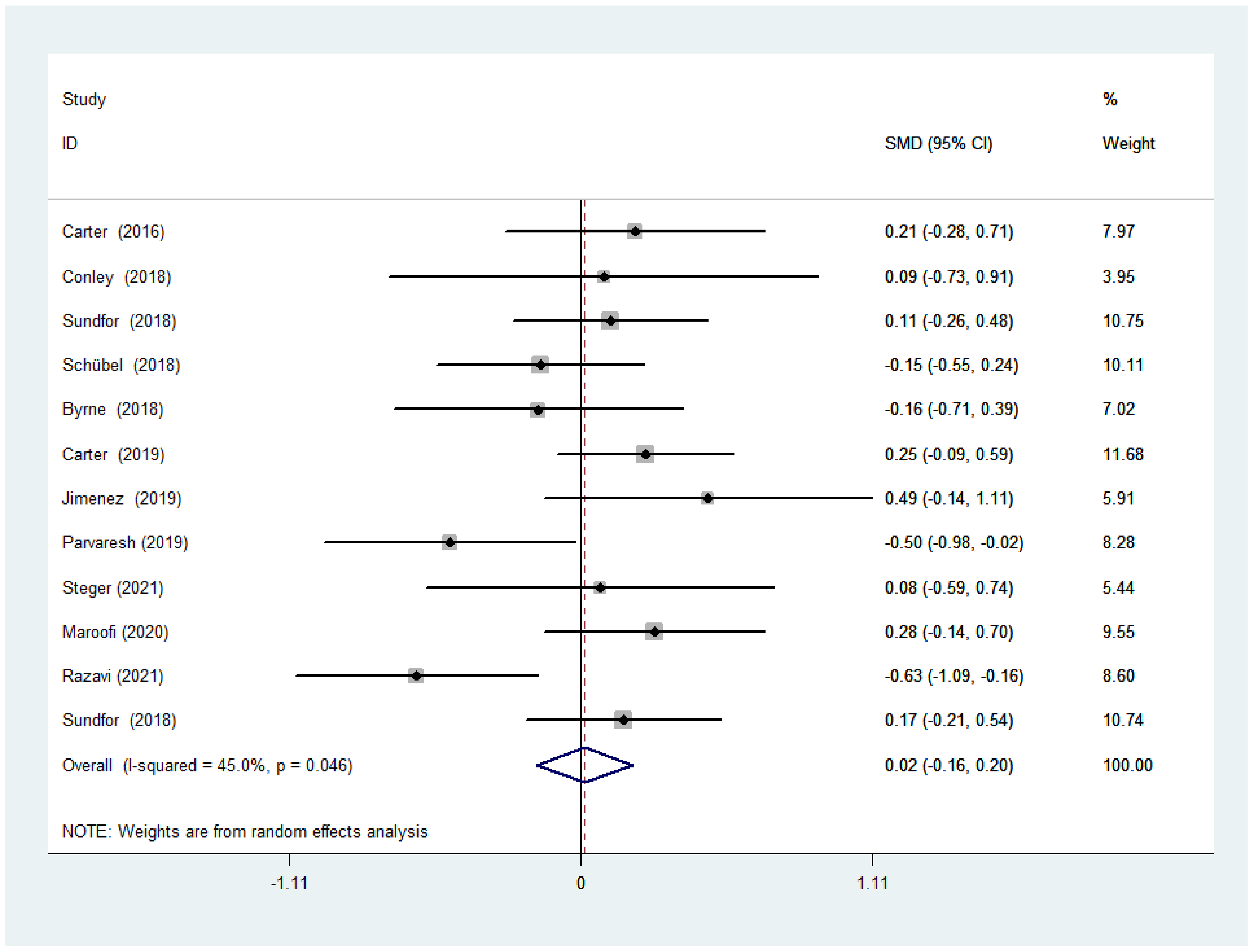


| Author | Year | Country | N | Study Design | Participants | Study Duration | Interventions | Age, y | Body Weight, kg | BMI, kg/m2 | Waist, cm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carter et al. | 2016 | Australia | 63 | a pragmatic pilot trial | adults with overweight or obesity | 12 weeks | IER vs. CER | IER | 61 ± 7.5 | 99 ± 16 | 35 ± 4.8 | - |
| CER | 62 ± 9.1 | 99 ± 15 | 36 ± 5.2 | - | ||||||||
| Conley et al. | 2018 | Australia | 24 | a randomised pilot stugy | obese war veterans | 6 months | twice-weekly vs. CCR | twice-weekly | 68 ± 2.7 | 99.1 ± 7.9 | 33.4 ± 1.8 | 114.2 ± 5.2 |
| CCR | 67.1 ± 3.9 | 107.3 ± 17.1 | 36.2 ± 4.3 | 122.5 ± 10.4 | ||||||||
| Sundfor et al. | 2018 | Norway | 112 | RCT | adults with metabolic syndrome | 6 months | IER vs. CER | IER | 49.9 ± 10.1 | 108.6 ± 16.3 | 35.1 ± 3.9 | 116 ± 10 |
| CER | 47.5 ± 11.6 | 107.5 ± 16.1 | 35.3 ± 3.5 | 116 ± 10 | ||||||||
| Schübel et al. | 2018 | Germany | 150 | RCT | overweight or obese nonsmokers | 48 weeks | ICR vs. CCR | ICR | 49.4 ± 9.0 | 96.4 ± 15.8 | 32.0 ± 3.8 | - |
| CCR | 50.0 ± 8.0 | 92.5 ± 15.7 | 31.2 ± 4.0 | - | ||||||||
| Byrne et al. | 2018 | Australia | 51 | RCT | men with obesity | 16 weeks | ICR vs. CCR | ICR | 39.9 ± 9.2 | 109.8 ± 14.1 | 39.7 ± 6.8 | - |
| CCR | 39.3 ± 6.6 | 111.6 ± 10.0 | 38.9 ± 5.2 | - | ||||||||
| Carter et al. | 2019 | Australia | 137 | RCT | adults with type 2 diabetes | 12 months | IER vs. CER | IER | 61 ± 9.0 | 100 ± 19 | 35 ± 5.8 | - |
| CER | 61 ± 9.2 | 102 ± 17 | 37 ± 5.7 | - | ||||||||
| Jimenez et al. | 2019 | Spain | 42 | RCT | adults with overweight or obesity | 6 weeks | ICR vs. CCR | ICR | 46.32 ± 8.03 | 92.21 ± 13.82 | 32.83 ± 3.73 | 106.24 ± 11.89 |
| CCR | 47.88 ± 7.67 | 97.99 ± 18.05 | 35.92 ± 5.32 | 110.49 ± 14.17 | ||||||||
| Parvaresh et al. | 2019 | Iran | 70 | RCT | adults with metabolic syndrome | 8 weeks | ADF vs. CCR | ADF | 44.6 ± 9.08 | 86.7 ± 10.65 | 31.1 ± 3.35 | 101 ± 9.41 |
| CCR | 46.4 ± 7.94 | 84.2 ± 12.21 | 31.6 ± 3.82 | 103 ± 12.92 | ||||||||
| Steger et al. | 2021 | USA | 35 | RCT | adults with overweight or obesity | 12 weeks | IER vs. CER | IER | 43.4 ± 11 | 87.4 ± 11.5 | 31.1 ± 2.4 | 94.2 ± 8.8 |
| CER | 48 ± 10 | 91.0 ± 9.7 | 31.4 ± 2.5 | 95.9 ± 9.3 | ||||||||
| Maroofi et al. | 2020 | Iran | 88 | RCT | subjects with overweight or obesity and mild-to-moderate HTG | 8 weeks | ICR vs. CCR | ICR | 44.0 ± 8.6 | 83.9 ± 13.7 | 31.6 ± 3.9 | 100.6 ± 9.8 |
| CCR | 45.2 ± 11.7 | 90.1 ± 19.3 | 32.4 ± 4.6 | 104.7 ± 11.0 | ||||||||
| Razavi et al. | 2021 | Iran | 80 | RCT | adults with metabolic syndrome | 4 months | ADF vs. CCR | ADF | 41.3 ± 8.65 | 89.4 ± 7.72 | 31.3 ± 3.12 | 106 ± 9.71 |
| CCR | 43.1 ± 9.26 | 87.1 ± 8.17 | 31.2 ± 3.95 | 104 ± 10.2 |
| Author | Year | Country | Interventions | Fat Mass, kg | Percentage Fat Mass (%) | TC | TG | FBG | Insulin, IU/L | SBP, mmhg | DBP, mmhg | HOMA-IR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carter et al. | 2016 | Australia | IER vs. CER | IER | 38 ± 9.2 | 41 ± 7.9 | - | - | - | - | 134 ± 17 | 84 ± 10 | - |
| CER | 40 ± 10.5 | 42 ± 7.7 | - | - | - | - | 138 ± 15 | 90 ± 11 | - | ||||
| Conley et al. | 2018 | Australia | twice-weekly vs. CCR | twice-weekly | - | - | 3.9 ± 0.9 (mmol/L) | 1.9 ± 0.6 (mmol/L) | - | - | 141.5 ± 13.9 | 84.0 ± 9.5 | - |
| CCR | - | - | 4.3 ± 1.0 (mmol/L) | 2.4 ± 1.7 (mmol/L) | - | - | 149.8 ± 18.3 | 88.1 ± 14.4 | - | ||||
| Sundfor et al. | 2018 | Norway | IER vs. CER | IER | - | - | 4.87 ± 0.90 (mmol/L) | 1.84 ± 0.83 (mmol/L) | 5.8 ± 1.2 (mmol/L) | - | 129 ± 13.4 | 88 ± 8.1 | - |
| CER | - | - | 5.09 ± 0.87 (mmol/L) | 1.55 ± 0.68 (mmol/L) | 5.7 ± 0.7 (mmol/L) | - | 128 ± 13.2 | 86 ± 8.7 | - | ||||
| Schübel et al. | 2018 | Germany | ICR vs. CCR | ICR | - | - | 205.0 ± 30.8 (mg/dL) | 130.0 ± 83.8 (mg/dL) | 92.7 ± 7.5 (mg/dL) | 11.6 ± 5.4 | 139.4 ± 18.7 | 136.0 ± 16.7 | 2.7 ± 1.3 |
| CCR | - | - | 202.9 ± 39.3 (mg/dL) | 121.2 ± 66.3 (mg/dL) | 93.9 ± 7.5 (mg/dL) | 12.6 ± 6.9 | 87.2 ± 9.9 | 87.3 ± 8.7 | 3.0 ± 1.7 | ||||
| Byrne et al. | 2018 | Australia | ICR vs. CCR | ICR | 44.1 ± 11.5 | 39.7 ± 6.8 | - | - | - | - | - | - | - |
| CCR | 43.6 ± 8.5 | 38.9 ± 5.2 | - | - | - | - | - | - | - | ||||
| Carter et al. | 2019 | Australia | IER vs. CER | IER | 40 ± 9.4 | 42 ± 7.3 | 4.6 ± 1.3 (mmol/L) | 1.5 ± 0.7 (mmol/L) | - | 14 ± 20 | - | - | - |
| CER | 42 ± 9.1 | 44 ± 6.6 | 5.0 ± 1.7 (mmol/L) | 1.9 ± 1.4 (mmol/L) | - | 14 ± 21 | - | - | - | ||||
| Jimenez et al. | 2019 | Spain | ICR vs. CCR | ICR | - | 40.76 ± 6.61 | - | - | - | - | - | - | - |
| CCR | - | 44.51 ± 6.40 | - | - | - | - | - | - | - | ||||
| Parvaresh et al. | 2019 | Iran | ADF vs. CCR | ADF | - | - | 177 ± 36.52 (mg/dL) | 199 ± 108.29 (mg/dL) | 102 ± 9.17 (mg/dL) | 13.07 ± 6.34 | 125 ± 9.78 | 84 ± 9.35 | 3.33 ± 1.69 |
| CCR | - | - | 177 ± 37.17 (mg/dL) | 218 ± 115.10 (mg/dL) | 101 ± 7.58 (mg/dL) | 14.28 ± 6.79 | 127 ± 14.03 | 83 ± 6.61 | 3.49 ± 1.86 | ||||
| Steger et al. | 2021 | USA | IER vs. CER | IER | 37.9 ± 6.8 | 45.0 ± 4.7 | - | - | - | - | 119 ± 14 | 70 ± 11 | - |
| CER | 41.5 ± 7.6 | 47.4 ± 6.3 | - | - | - | - | 123 ± 10 | 74 ± 10 | - | ||||
| Maroofi et al. | 2020 | Iran | ICR vs. CCR | ICR | - | 37.5 ± 4.6 | 178.6 ± 30.3 (mg/dL) | 180.5 ± 115 (mg/dL) | - | 18.2 ± 8.1 | - | - | 3.5 ± 3 |
| CCR | - | 35.9 ± 5.8 | 190.1 ± 38.1 (mg/dL) | 165.0 ± 126 (mg/dL) | - | 22.0 ± 9.7 | - | - | 3.7 ± 3 | ||||
| Razavi et al. | 2021 | Iran | ADF vs. CCR | ADF | 37.1 ± 9.25 | - | - | - | - | - | 134 ± 9 | 86 ± 4 | - |
| CCR | 34.2 ± 9.80 | - | - | - | - | - | 137 ± 10 | 85 ± 5 | - |
| Groups | Participants | Random Effect SMD (95% CI) | I2 (%) | p for Heterogeneity | |
|---|---|---|---|---|---|
| Over all | 12 | 905 | 0.02 (−0.16, 0.20) | 45 | 0.046 |
| Subgroup analysis | |||||
| Age | |||||
| ≥60 y | 3 | 223 | 0.22 (−0.04, 0.49) | 0 | 0.937 |
| <60 y | 9 | 682 | −0.04 (−0.27, 0.18) | 53.2 | 0.029 |
| Area | |||||
| Oceania | 4 | 274 | 0.15 (−0.09, 0.39) | 0 | 0.651 |
| Europe | 4 | 364 | 0.10 (−0.11, 0.31) | 5.5 | 0.366 |
| Western Asia | 3 | 232 | −0.27 (−0.85, 0.31) | 79.4 | 0.008 |
| North America | 1 | 35 | 0.08 (−0.59, 0.74) | ||
| Physical condition | |||||
| Obesity or overweight with disease | 6 | 593 | 0.05 (−0.18, 0.27) | 0 | 0.568 |
| Obesity or overweight | 6 | 312 | −0.03 (−0.32, 0.27) | 68.9 | 0.007 |
| Groups | Participants | Random Effect SMD (95% CI) | I2 (%) | p for Heterogeneity | |
|---|---|---|---|---|---|
| Over all | 12 | 905 | −0.21 (−0.40, −0.02) | 48.1 | 0.031 |
| Subgroup analysis | |||||
| Age | |||||
| ≥60 y | 3 | 223 | 0.01 (−0.26, 0.27) | 0 | 0.993 |
| <60 y | 9 | 682 | −0.28 (−0.52, −0.05) | 56.6 | 0.018 |
| Area | |||||
| Oceania | 4 | 274 | −0.15 (−0.48, 0.18) | 38.9 | 0.178 |
| Europe | 4 | 364 | −0.09 (−0.32, 0.14) | 18.5 | 0.298 |
| Western Asia | 3 | 232 | −0.53 (−0.87, −0.19) | 41.1 | 0.183 |
| North America | 1 | 35 | 0.28 (−0.38, 0.95) | ||
| Physical condition | |||||
| Obesity or overweight with disease | 6 | 593 | −0.21 (−0.48, 0.06) | 27.3 | 0.23 |
| Obesity or overweight | 6 | 312 | −0.22 (−0.50, 0.06) | 64.8 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, C.; Wang, H.; Ma, Z.; Liu, D.; Guan, X.; Liu, Y.; Fu, Y.; Cui, M.; Dong, J. Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients 2022, 14, 1781. https://doi.org/10.3390/nu14091781
Zhang Q, Zhang C, Wang H, Ma Z, Liu D, Guan X, Liu Y, Fu Y, Cui M, Dong J. Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients. 2022; 14(9):1781. https://doi.org/10.3390/nu14091781
Chicago/Turabian StyleZhang, Qing, Caishun Zhang, Haidan Wang, Zhengye Ma, Defeng Liu, Xiaohan Guan, Yixin Liu, Yanwen Fu, Mingxuan Cui, and Jing Dong. 2022. "Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss?" Nutrients 14, no. 9: 1781. https://doi.org/10.3390/nu14091781
APA StyleZhang, Q., Zhang, C., Wang, H., Ma, Z., Liu, D., Guan, X., Liu, Y., Fu, Y., Cui, M., & Dong, J. (2022). Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients, 14(9), 1781. https://doi.org/10.3390/nu14091781





