Abstract
Nutrition labeling on food packages is increasingly found to promote healthier food choices associated with lower risk of chronic kidney disease (CKD). To examine associations between nutrition labels use and CKD risk, we conducted a nationally representative cross-sectional study of 32,080 adults from the 2008–2019 Korean National Health and Nutrition Examination Survey. Nutrition labels use was collected via self-reported questionnaires. Ascertainment and severity of CKD was determined by estimated glomerular filtration rate or proteinuria. In multivariable-adjusted (MV) logistic regression models, increasing awareness and use of nutrition labels was significantly associated with lower CKD risk (MV-adjusted OR “nutrition labels aware and use” group vs. “nutrition labels unaware” group [95% CIs]: 0.75 [0.59–0.95], Ptrend:0.03). This inverse association varied with CKD’s risk of progression, with 21% and 42% reduced risk observed for CKD subtypes with “moderate” and “high” risk of progression, respectively (all Ptrend ≤ 0.04). Furthermore, the nutrition labels use and CKD risk association significantly differed by age, with 35% reduced risk observed in the older group aged 49 years or older, but not in the younger group (Pinteraction < 0.001). Our results suggest increasing perception and use of nutrition labels may contribute to CKD prevention and its early asymptomatic progression, especially in older adults.
1. Introduction
Chronic kidney disease (CKD) is a long-term condition associated with gradual loss of kidney function [1] and is an increasing public health problem resulting in considerable morbidity and healthcare costs [2,3]. Nearly 700 million people suffer from CKD globally [4], and there was a 29.3% increase in prevalence from 1990 to 2017 [3]. Furthermore, CKD is a clinically silent and asymptomatic disease that is often not detected until later stages, when patients need dialysis or a kidney transplant [5] and are at significantly higher risk of death [6,7]. Thus, primary prevention of CKD, especially by identifying easily modifiable lifestyle factors that inhibit the onset and progression of CKD in the general population poses an effective strategy to reduce its burden [8,9,10].
Nutrition labeling, also known as nutrition (or food) facts labels, is placed on food containers or packaging to provide detailed information about serving size, nutrient content, and the daily percentage values of a food item, including fat, carbohydrate, sugar, sodium, protein, and total energy [11,12]. Since their introduction in the late 1980s to assist in quick, informed, healthier food choices at the time of purchasing food, nutrition labels have rapidly proliferated as a nutritional policy tool in North America, Europe, Asia, and Australasia [13,14,15]. According to the European Food Information Council, a growing number of countries are now mandating nutrition labelling on a much broader range of processed foods and beverage products to promote a healthy food environment [13,14,15]. Indeed, two recent systemic reviews [16,17], a large randomized controlled trial [18], and an international experimental study across 12 countries [19] consistently reported greater perceived healthfulness of food products, healthier food purchasing choices, and greater consumption of better-quality diets high in fruits and vegetables and low in fat, sugar, sodium, and energy among users of nutrition labels, compared with non-users. Such diet consumption has been consistently found to be associated with lower risk of well-known risk factors of CKD [5,20], including obesity [21,22], hypertension [23], and diabetes [24]. Furthermore, recent meta-analyses of 17 prospective cohort studies reported a 30% and 23% lower risk of CKD and albuminuria, the early indicators of kidney damage, among individuals adhering to a healthy diet [25]. Thus, the use of nutrition labels is likely to be associated with lower risk of CKD mediated by its influence on promoting a healthy diet, possibly opening up a novel direction for the development of effective prevention against CKD.
However, to date, only one cross-sectional study of diabetic patients has examined the association between the awareness and use of nutrition labels and renal function, reporting better renal function with greater awareness and use of nutrition labeling [26]. No studies have examined the association of CKD risk with use of nutrition labels among the general population. Therefore, the objective of the present study is to examine our hypothesis that the use of nutrition labels is associated with lower risk of CKD by using data on nutrition labeling awareness and use, demographics, lifestyle, and medical conditions collected by the Korea National Health and Nutrition Survey (KNHANES), a nationally representative cross-sectional survey of Koreans [27]. We have also sought to explore whether the association of nutrition labels use and risk of CKD varies according to CKD subtypes defined by its risk of progression and population characteristics as a secondary analysis.
2. Methods
2.1. Study Design and Population
This study was conducted using data from the KNHANES from 2008 to 2019 [27]. In brief, the KNHANES is a continuous cross-sectional survey of non-institutionalized South Koreans of all ages that has been conducted by the Korea Centers for Disease Control (KCDC) since 1998. The KNHANES uses a multi-stage clustered probability sampling design to obtain data on demographic and socioeconomic status, health-related lifestyles, and medical conditions from a nationally representative population and monitors trends in health risk factors and the prevalence of major chronic diseases in South Korea [27]. The KNHANES combines health interviews and nutrition surveys with physical examinations and laboratory tests of biochemical markers. Written informed consent is obtained from all participants prior to their enrollment in the survey. The institutional review boards of KCDC have approved all KNHANES protocols. The present study was exempt from review by the institutional review board of Ewha Womans University because it uses de-identified and publicly available data (IRB no. 202112-0001-01).
For the present analyses, we pooled the annual KNHANES data collected cross-sectionally between 2008 and 2009. Of 101,138 individuals who participated in the KNHANES between 2008 and 2019, we excluded those who: (1) were under 19 years of age (N = 22,439), (2) were pregnant or lactating (N = 27,763), (3) had no serum creatinine measurements or urine dipstick protein results necessary for CKD ascertainment (N = 7506), (4) did not answer nutrition label questions (N = 45), (5) had implausible energy intake (<500 kcal/d or >5000 kcal/d; N = 8021), and (6) had missing data on covariates included in a primary multivariable model (N = 3284) (Figure 1). Consequently, the final sample size for the present analysis was 32,080 adults.
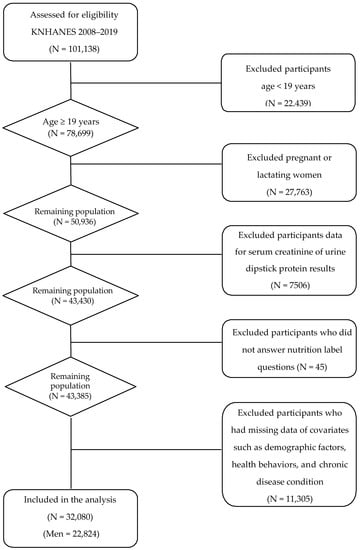
Figure 1.
A flow diagram of the study subjects.
2.2. Assessment of Use of Nutrition Labels
Data on the use of nutrition labels was collected via self-reported questionnaires. The questionnaire specifically asked: “Are you aware of the nutrition labels?” Those who answered “Yes” to the question were asked, as follow-up: “Do you read nutrition labels when you purchase food items?” Based on the responses to the questions, we classified the participants into three groups, each with increasing awareness and use of nutrition labels. Those who answered “No” to the first question, on nutrition labeling awareness, were classified into the “Nutrition labels unaware” group (the lowest level of nutrition labels use). Those who answered “yes” to the first question but “No” to the follow-up question on nutrition labels use at the time of purchasing a food product were classified into the “Nutrition labels aware only” group. Those who answered “yes” to both questions were classified into the “Nutrition labels aware and use” group (the highest level of nutrition labels use).
2.3. Ascertainment of CKD
CKD status was defined using measures of kidney function or kidney damage as recommended by “Kidney Disease: Improving Global Outcomes (KDIGO)” [5]. Glomerular filtration rate is the flow rate of filtered fluid through the kidney and serves as the best indicator of overall kidney function. We estimated GFR (eGFR), using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [28,29,30], which incorporates sex, age, race, and serum level of creatinine. The eGFR values were classified into six eGFR categories as follows: G1: ≥90 mL/min/1.73 m2; G2: 60–89 mL/min/1.73 m2; G3a: 45–59 mL/min/1.73 m2; G3b: 30–44 mL/min/1.73 m2; G4; 15–29 mL/min/1.73 m2; and G5; <15 mL/min/1.73 m2 [5]. Proteinuria serves as a surrogate marker for kidney damage and kidney disease progression [31]. The presence and levels of proteinuria were determined based on urine dipstick results. The values of “negative to trace” protein results were defined as “normal to mild increase” proteinuria (A1); “trace to +” as “moderate increase” proteinuria (A2); and “+ or greater” as “severe increase” proteinuria (A3) [5,32].
Then, following KDIGO guidelines [5], CKD cases were confirmed if participants belonged to G3a, G3b, G4, or G5 eGFR groups (eGFR < 60 mL/min per 1.73 m2) or A2 or A3 proteinuria groups (as “moderate increase” or “severe increase” proteinuria) [5]. CKD cases were further classified into three groups, each reflecting their prognostic risk for progression, morbidity, and mortality based on eGFR and proteinuria levels [5]. These were “moderately increased risk” (G3a and A1; G1–G2 and A2), “high risk” (G3b and A1; G3a and A2; or G1–G2 and A3), and “very high risk” (G4–G5; G3b and A2–A3; or G3a and A3). CKD cases were also grouped following the conventional staging definition, namely stage I (G1 and A2 or A3), stage II (G2 and A2 or A3), stage III (G3), stage IV (G4), and stage V (G5). CKD cases with stages I, II, and III were defined as non-advanced CKD, whereas CKD cases with stages IV and V were defined as advanced CKD [33,34,35,36]. As a result, we ascertained a total of 1437 CKD cases, of which 1015 were “moderately increased risk”, 299 were “high risk”, and 123 were “very high risk” cases, while1376 were non-advanced and 61 were advanced CKD cases.
2.4. Assessment of Covariates
Information on participants’ sociodemographics (sex, age, household income, region, education), health-related lifestyle (smoking status and drinking behavior), and medical conditions was collected via standardized self-reported questionnaires. Physical activity was measured using the international physical activity questionnaire—short form [37,38]. Participants were defined as being active if engaging in ≥150 min moderate physical activity or ≥75 min vigorous physical activity per week; otherwise, they were defined as being inactive. Body weight and height were measured by a trained examiner, and body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m2). Dietary data were collected via 24 h recalls assisted by trained interviewers, and daily nutrients intake from food consumed was calculated based on the national standard food composition table [39]. Blood pressure level was measured with a standard mercury sphygmomanometer. Hypertension was defined as: (1) ≥130 mm Hg systolic or ≥80 mm Hg diastolic blood pressure from an average of two blood pressure readings [40]; or (2) use of antihypertensive medication. Diabetes was defined as: (1) fasting plasma glucose level ≥126 mg/dl or hemoglobin A1C ≥ 6.5%; or (2) use of oral hypoglycemic agents. Hyperglycemia was determined if fasting plasma glucose ≥ 126 mg/dL. Hypercholesterolemia was defined as: (1) fasting total serum cholesterol level ≥240 mg/mL; or (2) use of lipid-lowering medications.
2.5. Statistical Analyses
To account for the multistage sampling design of KNHANES and produce unbiased national estimates, the sampling weights were applied in all analyses. Study population characteristics according to the use of nutrition labels were summarized as mean ± standard deviation (SD) by using the SAS SURVEYMEANS procedure for continuous variables and numbers and percentages by using the SAS SURVEYFREQ procedure for categorical variables.
Logistic regression models were used to calculate odds ratios (OR) and 95% confidence intervals (95% CI) to evaluate the association between use of nutrition labels and risk of overall CKD and CKD subtypes, defined by progression risk, using the SAS SURVERYLOGISTIC procedure. The multivariable model was adjusted for potential confounding factors of well-known risk factors for CKD [6,20,41] as follows: age (years, continuous), sex (men, women), household income (low, middle-low, middle-high, high), region (urban, rural), educational level (less than elementary school, middle school, high school, college or higher), smoking status (not a current smoker, current smoker), high-risk drinking (not a binge drinker, binge drinker), physical activity (inactive, active), obesity status (BMI < 25.0 kg/m2, BMI ≥ 25.0 kg/m2) [42], hypertension (no hypertension, hypertension), diabetes (no diabetes, diabetes), hypercholesterolemia (no hypercholesterolemia, hypercholesterolemia), hyperglycemia (no hyperglycemia, hyperglycemia), and intake of total energy (kcal/d, continuous). Our primary model did not adjust for fruit and vegetable consumption, since this may be on the causal pathway between use of nutrition labels and risk of CKD. However, we tested the robustness of results to additionally include fruit and vegetable consumption in sensitivity analyses. Other sensitivity analyses conducted were including additional comorbid conditions in the model and excluding cases with end-stage renal condition, defined as stage V. A test for trend was conducted by including the ordinal variable of nutrition labels use as a continuous term and evaluating its statistical significance using a Wald test. To examine whether associations varied by population characteristics, we also conducted analyses stratified by sex [43,44,45,46,47,48], age [26,49], obesity status [50,51,52], hypertension [52,53], and diabetes [52,54]. The statistical significance of the heterogeneity of associations was evaluated using the Wald test for the cross-product term between use of nutrition labels and stratification factors.
Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-sided at a significance level <0.05.
3. Results
Table 1 presents the characteristics of the study population. Mean age and total energy intake of the study population was 43.2 ± 0.1 years and 2191.2 ± 6.7 kcal/d. The majority of the study population were men (77.2%), had at least middle-high income level (62.7%), lived in an urban area (83.9%), were married (62.3%), and were not currently smoking (63.9%) or binge drinking (83.3%), although many were physically inactive (62.1%). In addition, the majority were not overweight (65.3%) and did not have hypertension (59.5%) or diabetes (91.0%). With respect to the level of awareness and use of nutrition labels, 8835 (20.3%) were in the “Unaware group”, 16,716 (56.3%) were in the “Aware only” group, and 6529 (23.4%) were in the “Aware and use” group. Participants in the “Aware and use” group were more likely to be younger and female and had greater income and higher education, and lived in urban areas, compared to those in the “Unaware group”. In terms of lifestyle and medical conditions, those in the “Aware and use” group were less likely to smoke and binge drink, were more physically active, and had less hypertension or diabetes, compared to those in the “Unaware group”.

Table 1.
Characteristics # of study participants (N = 32,080).
Associations between use of nutrition labels and risk of CKD were similar in age-adjusted and multivariable-adjusted models, and suggested significantly lower risk of CKD with increasing awareness and use of nutrition labels (all P-trend ≤ 0.03) (Table 2). The multivariable-adjusted OR (95% CI) were 0.80 (0.70–0.93) for the “Aware only” group and 0.75 (0.59–0.95) for the “Aware and use” group compared to “Unaware” group. Further adjustment for fruit and vegetable consumption or comorbid conditions did not alter the results materially (data not shown). Restricting analyses to those without end-stage renal disease also yielded similar results (data not shown).

Table 2.
Multivariable-adjusted $ odds ratio (95% confidence intervals) of overall CKD by use of nutrition labels.
CKD progression varies according to levels of eGFR and albuminuria [5]. We, therefore, examined whether the observed lower risk of CKD with the use of nutrition labels differed by the severity of CKD. Table 3 presents the results of the analyses with CKD cases classified by the potential for CKD progression using the new composite ranking system of KDIGO [5]. There was a suggestion of a stronger inverse association with CKD cases with moderate or high risk of progression (all P-trend ≤ 0.04) compared to CKD cases with very high risk (Table 3). The multivariable-adjusted OR (95% CI) comparing the “Aware and use” group to the “Unaware” group was 0.79 (0.59–1.05) for CKD cases with moderate risk of progression and 0.58 (0.35–0.96) for CKD cases with high risk of progression, whereas there was no association with CKD cases with very high risk of progression. Results from additional analyses examining the associations with CKD subgroups defined by the conventional staging system, which uses eGFR levels alone [33,34,35,36], were also similar, showing the apparent inverse association of nutrition label use with CKD cases with lower stages than those with higher stages (Supplementary Material Table S1).

Table 3.
Multivariable-adjusted $ odds ratio (95% confidence intervals) of CKD subtypes defined by its risk of progression by use of nutrition labels.
In analyses stratified by population characteristics (Table 4), obesity, hypertension, and diabetes status did not significantly modify the association between use of nutrition labels and risk of CKD. Sex also did not significantly modify the association; the results did not change materially when we further explored effect modification by sex across age groups defined by using the age 55 years cut-off (Supplementary Material Table S2) [55]. However, age significantly modified the association between nutrition label use and risk of CKD (P-interaction: <0.001). Specifically, in the group of 49 years old or older, the multivariable OR (95% CIs) was 0.80 (0.67–0.94) for the “Aware only” group and 0.65 (0.46–0.92) for the “Aware and use” group, compared to the “Unaware” group (P-trend: 0.002), whereas no significant associations were observed in younger adults.

Table 4.
Multivariable-adjusted $ odds ratio (95% confidence intervals) of overall CKD by use of nutrition labels according to population characteristics.
4. Discussion
In this cross-sectional study of nationally representative data in Korea, we observed a significantly lower risk of CKD with increasing awareness and use of nutrition labels. This inverse association was found to be more pronounced with CKD cases with moderate and high risk of progression, compared with CKD cases with very high risk of progression. Furthermore, the association between the use of nutrition labels and risk of CKD varied by age with a strong, significant inverse association observed in the older group, but not in the younger group. None of the other factors examined, including sex and obesity, hypertension, and diabetes status, significantly modified the association between use of nutrition labels and CKD risk. To the best of our knowledge, the present study is the first comprehensive, large epidemiologic analysis to examine the association between use of nutrition labels and risk of CKD overall and CKD subtypes defined by potential for progression and by subgroup characteristics. Our results suggest that diet modification, possibly through use of nutrition labels, may contribute to the prevention of CKD overall and its early asymptomatic progression, especially in older individuals.
To date, there has been sparse evidence for the association between nutrition labels use and the risk of CKD. Since the inception of nutrition labeling in the late 1980s, the majority of previous studies examining nutrition labels have primarily focused on demonstrating their impact on healthier dietary behavior [56] and improvements of diet quality [57,58,59,60]. Relatively few epidemiologic studies have examined the disease risk associated with the use of nutrition labels [26,51,60,61,62,63,64,65,66], and most examined associations with metabolic conditions [60,63,64,65,66,67,68] and diabetes [51,62,65]. To our knowledge, only one cross-sectional study of diabetic patients reported an association between the use of nutrition labels and renal function decline [26]. Consistent with our results, the study reported that unawareness of nutrition labeling is associated with significantly greater loss of renal function [26].
In addition, increasing evidence strongly indicates that diet affects kidney function and CKD development via its effects on oxidative stress, inflammation, lipid and glucose metabolism, and known comorbid risk factors of CKD such as hypertension and diabetes [25,69,70]. Promotion of a healthy diet by nutrition labelling [16] supports the plausibility of the inverse association between nutrition labels use and risk of CKD that we observed. Nutrition labeling in Korea, as in other countries, displays the contents, serving size, and percent of daily values of nutrient profiles to limit or encourage consumption, recommended by the WHO, for sodium, saturated fat, trans-fatty acids, sugar, cholesterol, and protein on the packaging of food items. Substantial evidence indicates that being informed of a food’s nutritional quality via the use of nutrition labels may direct food purchase intentions towards healthier food choices [16,17,71]. Indeed, in large national studies in Korea [58] and the USA [59,60,72], nutrition label users were found to consume lower amounts of total energy, total fat, saturated fat, cholesterol, sodium, and sugars and greater amounts of fiber and more fruits, vegetables, and whole grains, and fewer sugar-sweetened beverages, all of which characterize a healthy diet quality, compared with non-users. Greater adherence to such a healthy diet [21,22,23,24] and use of nutrition labels [51,61,62,63,64,65,67,68] have been associated with lower risk of CKD-related clinical factors, including obesity [21,22,67], metabolic syndrome [63,68], dyslipidemia [64], diabetes [24,51,61,62], and hypertension [23,65]. Furthermore, a recent large meta-analysis of 18 prospective cohort studies involving 630,108 adults reported that a healthy dietary pattern is associated with 30% and 23% lower risk of CKD and albuminuria, a marker of kidney damage [25], respectively. Our current findings on CKD extend the increasing population-based evidence supporting the health benefits of nutrition labeling for prevention and management of CKD in addition to diet-related chronic diseases [26,51,60,61,62,63,64,65,66].
CKD is asymptomatic and progresses silently to advanced stages, often being detected too late at fatal stages [5]. Identification of risk factors associated with CKD cases with varied potential of progression may thus open a novel venue for the primary prevention of CKD as well as the development of strategic planning to delay its progression, but it has been an understudied area. To date, no prior studies have examined the association between use of nutrition labeling and risk of CKD progression, as our study has, but there are several lines of evidence that the use of nutrition labeling may preserve existing renal function and delay CKD progression. For example, a cross-sectional study previously reported a dose-response trend with use of nutrition labels and eGFR levels, a strong indicator of CKD progression [26]. Furthermore, several prospective studies of healthy diet, possibly being promoted by nutrition labeling, consistently reported attenuated eGFR decline over study follow-up [73,74,75] or lower risk of CKD progression to end-stage renal disease or mortality [73].
Similarly, we observed a downward trend in the association, with 21% and 41% reduced risk observed with CKD subtypes with increasing potential for “moderate” to “high” risk progression, respectively, among the “Aware and use group” compared with the “Unaware group”. However, the association was not observed with very high risk of progression, which was unexpected. One possible explanation for this is that individuals with CKD subtypes with very high risk of progression are likely to suffer from other complications, such as acute kidney injuries or kidney stones, undergoing rapid deterioration of kidney function [76] and possibly being at stages too late for having health benefits derived from diet. Alternatively, individuals tend to change their diets consciously, regardless of use of nutrition labels, as CKD progresses to end stage with increasing symptoms and clinical diagnosis, attenuating the association. Despite these possibilities, we had a relatively small number of CKD cases with very high risk for progression limiting power and the results could be due to chance. A larger prospective study with a long period of follow-up is necessary to better elucidate the association between nutrition labels use and the risk of CKD subtypes with varying potential to progress.
Interestingly, we observed a stronger inverse association between nutrition labels use and the risk of CKD in the older group than in the younger group. The mechanisms of such greater possible benefit of nutrition labels use observed in the older population remain unclear. Nonetheless, age-based disparities in CKD risk, although limited, were previously reported, possibly suggesting biologic heterogeneity of CKD cases by age [26,49]. For example, our results are consistent with the study of diabetic patients that found less loss of renal function associated with nutritional labels use among older rather than younger diabetic populations [26]. Similarly, water intake [49] was more strongly associated with the risk of renal impairment in the older group than in the younger group. Several possibilities may explain these stronger associations of diet-related factors with CKD risk in the older group. With aging, the kidney is known to experience progressive structural changes and functional declines, characterized as kidney cyst formation, renal volume decrease, nephrosclerosis, glomerular basement membrane thickening, and nephron loss [77]. It is possible that the older population, compared to the younger population, is more susceptible to dietary insults, suggestively explaining the greater benefit of healthy diet, and thus the use of nutrition labels with aging. In addition, considerable evidence reported heterogeneity in the etiology, risk factors, and progression of CKD by age [78,79,80]. Notably, CKD occurring at younger ages is more likely due to congenital anomalies of the kidney and urinary tract rather than diet-related CKD-related clinical factors, including diabetic nephropathy or hypertension. Our observation may reflect greater hereditary influence on the development of CKD at younger ages than other lifestyle factors, including diet. Alternatively, our result might also arise from more active and frequent uses of nutrition labels while purchasing foods among the older rather than younger population, but we did not have such data to explore this hypothesis. Finally, our results could also be due to chance given the limited number of young CKD cases resulting in low power. Large cohort studies with more detailed information on nutrition labels use across various domains, such as frequency, duration, and impact on decision-making at the time of food purchasing, are needed to further explore differences in associations by age.
Our study had several limitations that warrant discussion. First, the cross-sectional nature of the study design limits drawing causal inferences from the association between nutrition labels use and the risk of CKD that we observed. Second, the self-reported data of awareness and use of nutrition labels in the KHANES do not provide information on frequency, as in other studies [60,81], or the specific use of information on nutrition labels, such as use of nutrition facts panel, serving size, and percent daily values, thereby limiting the identification of the most important elements on nutrition labels associated with CKD risk. Third, we cannot rule out that use of nutrition labels serves as a proxy for health-conscious behavior. Nonetheless, our model fully adjusted for known health-related lifestyle factors. Additional adjustment for fruit and vegetable consumption also did not materially change our results. Fourth, the clinical definition of CKD is kidney damage or abnormalities of kidney function present for >3 months. With a single measurement of creatinine, we were not able to measure chronicity of CKD, possibly resulting in an overestimation of CKD cases. However, the KNHANES is a study of a community-dwelling population whose proportion with acute kidney injury is likely to be low [82]. We assessed kidney damage by determining proteinuria, alternative to albuminuria—a gold standard measure for kidney damage—using dipstick results. Nonetheless, KDIGO has long supported the use of proteinuria results as a substitute for albuminuria [5,41]. Furthermore, aligning with the KDIGO guideline, the results of recent international collaborative meta-analyses of 919,833 individuals from 33 cohorts also support the consistency between protein dipstick categories and albumin–creatinine ratio categories [32]. Finally, we cannot rule out residual and unmeasured confounding, including family history of CKD. Nonetheless, given the approximately 13% prevalence of CKD in South Korea, our results from KNHANES, a nationally representative sample, are likely to be driven mostly by participants without a family history of CKD and the confounding by such a factor is expected to be low.
Our study also had several strengths. It is the first to examine the association between use and awareness of nutrition labels and risk of CKD in a large, nationally representative sample of the general population in South Korea. Furthermore, our analysis is distinct from other prior studies of CKD as it applied the most recent, novel classification of CKD defined by KIDO, namely “moderate increased risk,” “high risk,” and “very high risk,” that best represents its progression potential to adverse outcome [83]. With this approach, we were the first to estimate association of nutrition labels use across multiple levels of CKD progression potential. Taking advantage of the rich, high-quality data on lifestyle, anthropometrics, and medical histories collected from standardized protocols in the KNHANES, we were also able to comprehensively adjust for possible confounding factors known to be associated with CKD and conduct detailed analyses of the association overall and by subtypes of CKD, as well as by population characteristics. Through several sensitivity analyses, we demonstrated the robustness of our results. Finally, our findings are of high public health significance, being generalizable to Korean adults. They also add to the increasing scientific evidence of the health benefit of nutrition labeling, and underscore the great potential of nutrition labeling policy.
5. Conclusions
In this first nationally representative cross-sectional study of the general population in South Korea, the use of nutrition labels was found to be associated with lower risk of CKD with stronger associations observed in CKD cases with moderate and high risk of progression and among older adults. With increasing evidence that strongly supports the favorable impact of nutrition labeling on behavioral change towards healthier food purchasing [84], nutrition labels currently appear on the majority of food packages and are expected to be further extended as a global key regulatory nutritional policy. At this junction of nutrition labeling expansion, our results further provide novel insights on nutrition labeling as a simple, effective tool in the prevention and management of CKD. Further prospective cohort studies with detailed uses of the information on nutrition labels are warranted to confirm our observed associations between the use of labeling and CKD risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14091731/s1, Table S1: Multivariable-adjusted$ odds ratio (95% confidence intervals) of CKD defined by stages by use of nutrition labels, Table S2: Multivariable-adjusted$ odds ratio (95% confidence intervals) of overall CKD by use of nutrition labels according to sex and age.
Author Contributions
J.K. analyzed and interpreted the data and wrote the manuscript. S.J. conceived of the study design, secured funding, contributed to the interpretation, and critically reviewed and revised the manuscript. J.F.D., H.K., O.K., Y.K. (Yangha Kim), Y.K. (Yuri Kim), K.S.K., Y.J.P. and H.P. critically reviewed the manuscript and contributed to the interpretation of data. S.J. had primary responsibility for final content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Research Foundation of Korea [2021R1A2C2095343 to S. Jung].
Institutional Review Board Statement
The KNHANES studies were conducted according to the principles of the Declaration of Helsinki recommendations and were approved by the Institutional Review Boards of KCDC. The present study was exempt from review by the institutional review board of Ewha Womans University because it uses de-identified and publicly available data (protocol code 202112-0001-01 and date of approval 2021.12.01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the KNHANES study.
Data Availability Statement
The data from the KNHANES are available to the public and can be obtained after request from the website https://knhanes.kdca.go.kr/knhanes/main.do, accessed on 14 April 2022.
Conflicts of Interest
Authors declare no conflict of interest.
Abbreviations
BMI: body mass index; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; KNHANES, the Korea National Health and Nutrition Survey.
References
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Cockwell, P.; Fisher, L.-A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef]
- Lancet. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary pattern and incidence of chronic kidney disease among adults: A population-based study. Nutr. Metab. 2018, 15, 88. [Google Scholar] [CrossRef]
- Group, K.C.W. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Elsharif, M.E. Mortality rate of patients with end stage renal disease on regular hemodialysis: A single center study. Saudi J. Kidney Dis. Transplant. 2011, 22, 594. [Google Scholar]
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Amouzegar-Bahambari, P.; Azizi, F. Adherence to low-sodium Dietary Approaches to Stop Hypertension-style diet may decrease the risk of incident chronic kidney disease among high-risk patients: A secondary prevention in prospective cohort study. Nephrol. Dial. Transplant. 2018, 33, 1159–1168. [Google Scholar] [CrossRef]
- Dunkler, D.; Kohl, M.; Teo, K.K.; Heinze, G.; Dehghan, M.; Clase, C.M.; Gao, P.; Yusuf, S.; Mann, J.F.; Oberbauer, R. Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol. Dial. Transplant. 2015, 30, iv76–iv85. [Google Scholar] [CrossRef]
- Division of Nutrition, Physical Activity, and Obesity (DNPAO), National Center for Chronic Disease Prevention and Health Promotion(CDC). Learn How the Nutrition Facts Label Can Help You Improve Your Health. Nutrition 2021. Available online: https://www.cdc.gov/nutrition/strategies-guidelines/nutrition-facts-label.html (accessed on 31 January 2021).
- Ministry of Food and Drug Safety. Labeling Standards of Foods, Etc.; Ministry of Food and Drug Safety: Cheongju-si, Korea, 2019.
- Earl, R.; Porter, D.V.; Wellman, N.S. Nutrition labeling: Issues and directions for the 1990s. J. Am. Diet. Assoc. 1990, 90, 1599–1601. [Google Scholar] [CrossRef]
- Perez, R.; Edge, M.S. Global nutrition labeling: Moving toward standardization? Nutr. Today 2014, 49, 77–82. [Google Scholar] [CrossRef]
- EUFIC Global Update on Nutrition Labelling. 2018. Available online: https://www.eufic.org/images/uploads/healthy-living/Executive-Summary-GUNL-2018-V2.pdf. (accessed on 31 January 2021).
- Anastasiou, K.; Miller, M.; Dickinson, K. The relationship between food label use and dietary intake in adults: A systematic review. Appetite 2019, 138, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Christoph, M.J.; An, R. Effect of nutrition labels on dietary quality among college students: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Egnell, M.; Boutron, I.; Péneau, S.; Ducrot, P.; Touvier, M.; Galan, P.; Buscail, C.; Porcher, R.; Ravaud, P.; Hercberg, S.; et al. Front-of-Pack Labeling and the Nutritional Quality of Students’ Food Purchases: A 3-Arm Randomized Controlled Trial. Am. J. Public Health 2019, 109, 1122–1129. [Google Scholar] [CrossRef]
- Egnell, M.; Talati, Z.; Hercberg, S.; Pettigrew, S.; Julia, C. Objective Understanding of Front-of-Package Nutrition Labels: An International Comparative Experimental Study across 12 Countries. Nutrients 2018, 10, 1542. [Google Scholar] [CrossRef]
- Park, J.I.; Baek, H.; Jung, H.H. Prevalence of Chronic Kidney Disease in Korea: The Korean National Health and Nutritional Examination Survey 2011–2013. J. Korean Med. Sci. 2016, 31, 915–923. [Google Scholar] [CrossRef]
- Abiri, B.; Valizadeh, M.; Nasreddine, L.; Hosseinpanah, F. Dietary determinants of healthy/unhealthy metabolic phenotype in individuals with normal weight or overweight/obesity: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef]
- Joo, J.H.; Lee, D.W.; Choi, D.W.; Park, E.C. Association between Food Label Unawareness and Loss of Renal Function in Diabetes: A Cross-Sectional Study in South Korea. Int. J. Environ. Res. Public Health 2020, 17, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Teo, B.W.; Xu, H.; Wang, D.; Li, J.; Sinha, A.K.; Shuter, B.; Sethi, S.; Lee, E.J. GFR estimating equations in a multiethnic Asian population. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 58, 56–63. [Google Scholar] [CrossRef]
- Jeong, T.D.; Lee, W.; Chun, S.; Lee, S.K.; Ryu, J.S.; Min, W.K.; Park, J.S. Comparison of the MDRD study and CKD-EPI equations for the estimation of the glomerular filtration rate in the Korean general population: The fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1), 2010. Kidney Blood Press. Res. 2013, 37, 443–450. [Google Scholar] [CrossRef]
- Inker, L.A.; Levey, A.S.; Pandya, K.; Stoycheff, N.; Okparavero, A.; Greene, T. Early change in proteinuria as a surrogate end point for kidney disease progression: An individual patient meta-analysis. Am. J. Kidney Dis. 2014, 64, 74–85. [Google Scholar] [CrossRef]
- Sumida, K.; Nadkarni, G.N.; Grams, M.E.; Sang, Y.; Ballew, S.H.; Coresh, J.; Matsushita, K.; Surapaneni, A.; Brunskill, N.; Chadban, S.J.; et al. Conversion of Urine Protein-Creatinine Ratio or Urine Dipstick Protein to Urine Albumin-Creatinine Ratio for Use in Chronic Kidney Disease Screening and Prognosis: An Individual Participant-Based Meta-analysis. Ann. Intern. Med. 2020, 173, 426–435. [Google Scholar] [CrossRef]
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Serrano, A.; Huang, J.; Ghossein, C.; Nishi, L.; Gangavathi, A.; Madhan, V.; Ramadugu, P.; Ahya, S.N.; Paparello, J.; Khosla, N.; et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: A two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv. Chronic Kidney Dis. 2007, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef]
- Wong, J.; Zhang, Y.; Swift, O.; Finkelman, M.; Patidar, A.; Ramanarayanan, S.; Vilar, E.; Farrington, K. Beta-glucans in advanced CKD: Role in endotoxaemia and inflammation. BMC Nephrol. 2020, 21, 118. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J. Korean Acad. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Korean National Academy of Agricultural Science. Food Composition Table, 7th ed.; Rural Development Administration, Republic of Korea: Suwon, Korea, 2006. (In Korean) [Google Scholar]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef]
- Graham, S.E.; Nielsen, J.B.; Zawistowski, M.; Zhou, W.; Fritsche, L.G.; Gabrielsen, M.E.; Skogholt, A.H.; Surakka, I.; Hornsby, W.E.; Fermin, D.; et al. Sex-specific and pleiotropic effects underlying kidney function identified from GWAS meta-analysis. Nat. Commun. 2019, 10, 1847. [Google Scholar] [CrossRef]
- Satoh, M.; Hirose, T.; Nakayama, S.; Murakami, T.; Takabatake, K.; Asayama, K.; Imai, Y.; Ohkubo, T.; Mori, T.; Metoki, H. Blood Pressure and Chronic Kidney Disease Stratified by Gender and the Use of Antihypertensive Drugs. J. Am. Heart Assoc. 2020, 9, e015592. [Google Scholar] [CrossRef]
- Wu, J.; Jia, J.; Li, Z.; Pan, H.; Wang, A.; Guo, X.; Wu, S.; Zhao, X. Association of estimated glomerular filtration rate and proteinuria with all-cause mortality in community-based population in China: A Result from Kailuan Study. Sci. Rep. 2018, 8, 2157. [Google Scholar] [CrossRef] [PubMed]
- Swartling, O.; Rydell, H.; Stendahl, M.; Segelmark, M.; Trolle Lagerros, Y.; Evans, M. CKD Progression and Mortality Among Men and Women: A Nationwide Study in Sweden. Am. J. Kidney Dis. 2021, 78, 190–199.e191. [Google Scholar] [CrossRef] [PubMed]
- Weldegiorgis, M.; Woodward, M. The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 506. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Chiodini, P.; Provenzano, M.; Borrelli, S.; Garofalo, C.; Bellizzi, V.; Russo, D.; Conte, G.; De Nicola, L. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am. J. Kidney Dis. 2020, 75, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Kim, J.S.; Jo, M.J.; Cho, E.J.; Ahn, S.Y.; Ko, G.J.; Kwon, Y.J.; Kim, J.E. Impact of water consumption on renal function in the general population: A cross-sectional analysis of KNHANES data (2008–2017). Clin. Exp. Nephrol. 2021, 25, 376–384. [Google Scholar] [CrossRef]
- Olivo, R.E.; Davenport, C.A.; Diamantidis, C.J.; Bhavsar, N.A.; Tyson, C.C.; Hall, R.; Bidulescu, A.; Young, B.; Mwasongwe, S.E.; Pendergast, J.; et al. Obesity and synergistic risk factors for chronic kidney disease in African American adults: The Jackson Heart Study. Nephrol. Dial. Transplant. 2018, 33, 992–1001. [Google Scholar] [CrossRef]
- Han, K.T.; Kim, S.J.; Kim, D.J.; Kim, S.J. Does the active use of nutrition labeling reduce the risk of diabetes mellitus? Results of insulin resistance using Korean National Health and Nutrition Examination Survey. Prim. Care Diabetes 2018, 12, 445–452. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R., 3rd; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Mahmoodi, B.K.; Matsushita, K.; Woodward, M.; Blankestijn, P.J.; Cirillo, M.; Ohkubo, T.; Rossing, P.; Sarnak, M.J.; Stengel, B.; Yamagishi, K.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: A meta-analysis. Lancet 2012, 380, 1649–1661. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Tuot, D.S.; Pavkov, M.E.; Burrows, N.R.; Stack, A.G.; Saran, R.; Bragg-Gresham, J.; Powe, N.R. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019, 95, 1433–1442. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Q.; Jiang, Y.; Wang, N.; Liu, X.; Qiu, Y.; Zhu, J.; Tong, X.; Cui, S.; Zaid, M.; et al. Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020). Int. J. Environ. Res. Public Health 2021, 18, 10451. [Google Scholar] [CrossRef] [PubMed]
- Drichoutis, A.C.; Lazaridis, P.; Nayga, R.M., Jr. Consumers’ use of nutritional labels: A review of research studies and issues. Acad. Mark. Sci. Rev. 2006, 2006, 1. [Google Scholar]
- Kim, S.-Y.; Nayga, R.M.; Capps, O. Food label use, self-selectivity, and diet quality. J. Consum. Aff. 2001, 35, 346–363. [Google Scholar] [CrossRef]
- Kim, M.G.; Oh, S.W.; Han, N.R.; Song, D.J.; Um, J.Y.; Bae, S.H.; Kwon, H.; Lee, C.M.; Joh, H.K.; Hong, S.W. Association between Nutrition Label Reading and Nutrient Intake in Korean Adults: Korea National Health and Nutritional Examination Survey, 2007-2009 (KNHANES IV). Korean J. Fam. Med. 2014, 35, 190–198. [Google Scholar] [CrossRef][Green Version]
- Ollberding, N.J.; Wolf, R.L.; Contento, I. Food label use and its relation to dietary intake among US adults. J. Am. Diet. Assoc. 2010, 110, 1233–1237. [Google Scholar] [CrossRef]
- Kollannoor-Samuel, G.; Shebl, F.M.; Hawley, N.L.; Pérez-Escamilla, R. Nutrition facts panel use is associated with higher diet quality and lower glycated hemoglobin concentrations in US adults with undiagnosed prediabetes. Am. J. Clin. Nutr. 2016, 104, 1639–1646. [Google Scholar] [CrossRef]
- Fitzgerald, N.; Damio, G.; Segura-Pérez, S.; Pérez-Escamilla, R. Nutrition knowledge, food label use, and food intake patterns among Latinas with and without type 2 diabetes. J. Am. Diet. Assoc. 2008, 108, 960–967. [Google Scholar] [CrossRef]
- Kollannoor-Samuel, G.; Shebl, F.M.; Hawley, N.L.; Pérez-Escamilla, R. Nutrition label use is associated with lower longer-term diabetes risk in US adults. Am. J. Clin. Nutr. 2017, 105, 1079–1085. [Google Scholar] [CrossRef]
- Jin, H.S.; Choi, E.B.; Kim, M.; Oh, S.S.; Jang, S.I. Association between Use of Nutritional Labeling and the Metabolic Syndrome and Its Components. Int. J. Environ. Res. Public Health 2019, 16, 4486. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kweon, K.H.; Kim, M.J.; Park, E.C.; Jang, S.Y.; Kim, W.; Han, K.T. Is nutritional labeling associated with individual health? The effects of labeling-based awareness on dyslipidemia risk in a South Korean population. Nutr. J. 2016, 15, 81. [Google Scholar] [CrossRef]
- Hong, S.W.; Oh, S.W.; Lee, C.; Kwon, H.; Hyeon, J.H.; Gwak, J.S. Association between nutrition label use and chronic disease in Korean adults: The Fourth Korea National Health and Nutrition Examination Survey 2008–2009. J. Korean Med. Sci. 2014, 29, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Kye, S.Y.; Han, K.T.; Jeong, S.H.; Choi, J.Y. Nutrition Labeling Usage Influences Blood Markers in Body-Size Self-Conscious Individuals: The Korean National Health and Nutrition Examination Survey (KNHANES) 2013–2018. Int. J. Environ. Res. Public Health 2020, 17, 5769. [Google Scholar] [CrossRef]
- Kim, S.D. Relationship between awareness and use of nutrition labels and obesity. Biomed. Res. 2018, 29, 2238–2242. [Google Scholar] [CrossRef]
- Kang, H.T.; Shim, J.Y.; Lee, Y.J.; Linton, J.A.; Park, B.J.; Lee, H.R. Reading nutrition labels is associated with a lower risk of metabolic syndrome in Korean adults: The 2007-2008 Korean NHANES. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 876–882. [Google Scholar] [CrossRef] [PubMed]
- He, L.Q.; Wu, X.H.; Huang, Y.Q.; Zhang, X.Y.; Shu, L. Dietary patterns and chronic kidney disease risk: A systematic review and updated meta-analysis of observational studies. Nutr. J. 2021, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Causal effects of relative fat, protein, and carbohydrate intake on chronic kidney disease: A Mendelian randomization study. Am. J. Clin. Nutr. 2021, 113, 1023–1031. [Google Scholar] [CrossRef]
- Oostenbach, L.H.; Slits, E.; Robinson, E.; Sacks, G. Systematic review of the impact of nutrition claims related to fat, sugar and energy content on food choices and energy intake. BMC Public Health 2019, 19, 1296. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Wang, G.; Moran, A.E.; Pagán, J.A. Nutrition Label Use and Sodium Intake in the U.S. Am. J. Prev. Med. 2017, 53, S220–S227. [Google Scholar] [CrossRef]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef]
- Cai, Q.; Dekker, L.H.; Vinke, P.C.; Corpeleijn, E.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Diet quality and incident chronic kidney disease in the general population: The Lifelines Cohort Study. Clin. Nutr. 2021, 40, 5099–5105. [Google Scholar] [CrossRef]
- Choi, Y.; Steffen, L.M.; Chu, H.; Duprez, D.A.; Gallaher, D.D.; Shikany, J.M.; Schreiner, P.J.; Shroff, G.R.; Jacobs, D.R. A Plant-Centered Diet and Markers of Early Chronic Kidney Disease during Young to Middle Adulthood: Findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Cohort. J. Nutr. 2021, 151, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Quinn, R.R.; Lam, N.N.; Al-Wahsh, H.; Sood, M.M.; Tangri, N.; Tonelli, M.; Ravani, P. Progression and Regression of Chronic Kidney Disease by Age Among Adults in a Population-Based Cohort in Alberta, Canada. JAMA Netw. Open 2021, 4, e2112828. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; O’Hare, A.M. Interaction of aging and chronic kidney disease. Semin. Nephrol. 2009, 29, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Chen, Y.C.; Lo, C.W.; Wang, W.S.; Lo, S.S.; Tang, G.J.; Thien, P.F. Incidence and renal survival of ESRD in the young Taiwanese population. Clin. J. Am. Soc. Nephrol. 2014, 9, 302–309. [Google Scholar] [CrossRef]
- An, R. Diabetes diagnosis and nutrition facts label use among US adults, 2005–2010. Public Health Nutr. 2016, 19, 2149–2156. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.L.; Jin, H.; Lim, J.Y.; Lee, S.Y. Relationship Between Low Handgrip Strength and Chronic Kidney Disease: KNHANES 2014-2017. J. Ren. Nutr. 2021, 31, 57–63. [Google Scholar] [CrossRef]
- Group, K.C.W. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Song, J.; Brown, M.K.; Tan, M.; MacGregor, G.A.; Webster, J.; Campbell, N.R.C.; Trieu, K.; Ni Mhurchu, C.; Cobb, L.K.; He, F.J. Impact of color-coded and warning nutrition labelling schemes: A systematic review and network meta-analysis. PLoS Med. 2021, 18, e1003765. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).