The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients
Abstract
1. Introduction
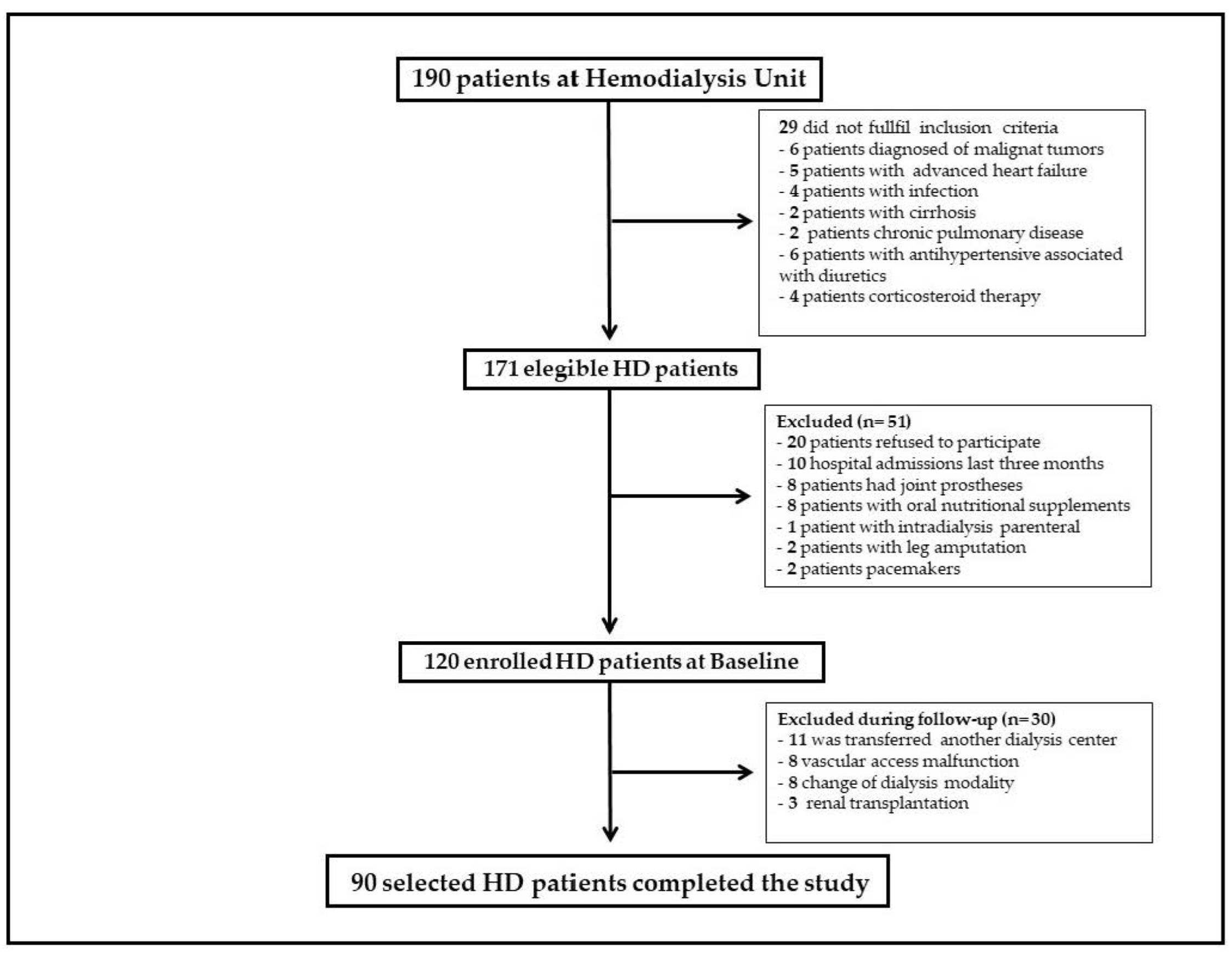
2. Patients and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Anthropometric Measures and Body Composition Analysis
2.4. Laboratory Parameters
2.5. Malnutrition-Inflammation Score Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Global Data and Comparison between Survivor and Non-Survivors
3.2. Analysis of the ECM/BCM Ratio Cut-Off Point
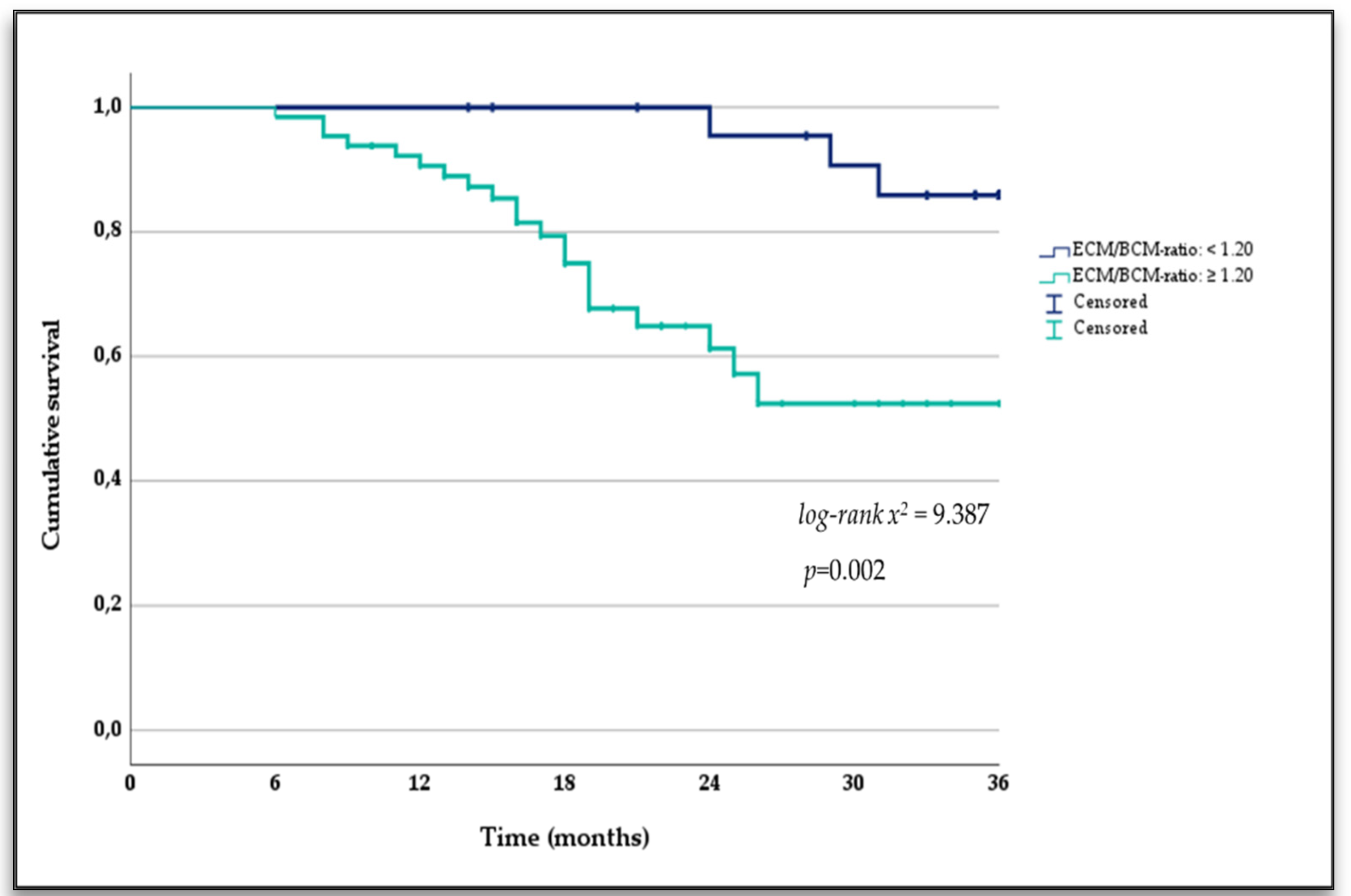
3.3. Extracellular Mass to Body Cell Mass Ratio and Mortality
3.4. Cox proportional Hazards Analysis of Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.S.; Chang, J.W.; Park, Y. Nutritional Status Predicts 10-Year Mortality in Patients with End-Stage Renal Disease on Hemodialysis. Nutrients 2017, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS ONE 2018, 13, e0190410. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Moissl, U.; Genser, B.; Rayner, H.; Dasgupta, I.; Stuard, S.; Stopper, A.; Chazot, C.; Maddux, F.W.; Canaud, B.; et al. Greater fluid overload and lower interdialytic weight gain are independently associated with mortality in a large international hemodialysis population. Nephrol. Dial. Transpl. 2018, 33, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, J.; Zhang, F.; Huang, C.; Wu, Y.; Han, Y.; Zhang, W.; Zhao, Y. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: A systematic review and meta-analysis. J. Nephrol. 2013, 26, 243–253. [Google Scholar] [CrossRef]
- Huang, J.C.; Tsai, Y.C.; Wu, P.Y.; Lee, J.J.; Chen, S.C.; Chiu, Y.W.; Hsu, Y.L.; Chang, J.M.; Chen, H.C. Independent Association of Overhydration with All-Cause and Cardiovascular Mortality Adjusted for Global Left Ventricular Longitudinal Systolic Strain and E/E’ Ratio in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1322–1332. [Google Scholar] [CrossRef]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, W.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol. Dial. Transpl. 2009, 24, 1574–1579. [Google Scholar] [CrossRef]
- Chazot, C.; Wabel, P.; Chamney, P.; Moissl, U.; Wieskotten, S.; Wizemann, V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol. Dial. Transpl. 2012, 27, 2404–2410. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Regidor, D.L.; Kovesdy, C.P.; Van Wyck, D.; Bunnapradist, S.; Horwich, T.B.; Fonarow, G.C. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 2009, 119, 671–679. [Google Scholar] [CrossRef]
- Antlanger, M.; Josten, P.; Kammer, M.; Exner, I.; Lorenz-Turnheim, K.; Eigner, M.; Paul, G.; Klauser-Braun, R.; Sunder-Plassmann, G.; Saemann, M.D.; et al. Blood volume-monitored regulation of ultrafiltration to decrease the dry weight in fluid-overloaded hemodialysis patients: A randomized controlled trial. BMC Nephrol. 2017, 18, 238. [Google Scholar] [CrossRef]
- Nescolarde, L.; Piccoli, A.; Roman, A.; Nunez, A.; Morales, R.; Tamayo, J.; Donate, T.; Rosell, J. Bioelectrical impedance vector analysis in haemodialysis patients: Relation between oedema and mortality. Physiol. Meas. 2004, 25, 1271–1280. [Google Scholar] [CrossRef]
- Chertow, G.M.; Lazarus, J.M.; Lew, N.L.; Ma, L.; Lowrie, E.G. Bioimpedance norms for the hemodialysis population. Kidney Int. 1997, 52, 1617–1621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tabinor, M.; Elphick, E.; Dudson, M.; Kwok, C.S.; Lambie, M.; Davies, S.J. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci. Rep. 2018, 8, 4441. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z. Effect of bioimpedance-defined overhydration parameters on mortality and cardiovascular events in patients undergoing dialysis: A systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211031063. [Google Scholar] [CrossRef] [PubMed]
- Avram, M.M.; Fein, P.A.; Borawski, C.; Chattopadhyay, J.; Matza, B. Extracellular mass/body cell mass ratio is an independent predictor of survival in peritoneal dialysis patients. Kidney Int. Suppl. 2010, 78, S37–S40. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Sanchez-Muniz, F.J.; Barril, G. Extracellular mass to body cell mass ratio as a potential index of wasting and fluid overload in hemodialysis patients. A case-control study. Clin. Nutr. 2020, 39, 1117–1123. [Google Scholar] [CrossRef]
- de Araujo, A.A.; Vannini, F.D.; de Arruda Silveira, L.V.; Barretti, P.; Martin, L.C.; Caramori, J.C. Associations between bioelectrical impedance parameters and cardiovascular events in chronic dialysis patients. Int. Urol. Nephrol. 2013, 45, 1397–1403. [Google Scholar] [CrossRef]
- Cai, Z.; Cai, D.; Yao, D.; Chen, Y.; Wang, J.; Li, Y. Associations between body composition and nutritional assessments and biochemical markers in patients with chronic radiation enteritis: A case-control study. Nutr. J. 2016, 15, 57. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J. Bioelectric impedance and individual characteristics as prognostic factors for post-operative complications. Clin. Nutr. 2005, 24, 830–838. [Google Scholar] [CrossRef]
- Seppel, T.; Kosel, A.; Schlaghecke, R. Bioelectrical impedance assessment of body composition in thyroid disease. Eur. J. Endocrinol. 1997, 136, 493–498. [Google Scholar] [CrossRef]
- Choi, H.N.; Kim, K.A.; Kim, Y.S.; Yim, J.E. Independent Association of Phase Angle with Fasting Blood Glucose and Hemoglobin A1c in Korean Type 2 Diabetes Patients. Clin. Nutr. Res. 2020, 9, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Bruns, F.J.; Saul, M.; Seddon, P.; Zeidel, M.L. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am. J. Med. 2000, 108, 609–613. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- McGinlay, J.M.; Payne, R.B. Serum albumin by dye-binding: Bromocresol green or bromocresol purple? The case for conservatism. Ann. Clin. Biochem. 1988, 25 Pt 4, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Sanchez-Muniz, F.J.; Barril, G. Predictors of protein-energy wasting in haemodialysis patients: A cross-sectional study. J. Hum. Nutr. Diet. 2014, 29, 38–47. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Hecking, M.; Rayner, H.; Wabel, P. What are the Consequences of Volume Expansion in Chronic Dialysis Patients? Defining and Measuring Fluid Overload in Hemodialysis Patients. Semin. Dial. 2015, 28, 242–247. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Fiedler, R.; Jehle, P.M.; Osten, B.; Dorligschaw, O.; Girndt, M. Clinical nutrition scores are superior for the prognosis of haemodialysis patients compared to lab markers and bioelectrical impedance. Nephrol. Dial. Transpl. 2009, 24, 3812–3817. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J. Am. Soc. Nephrol. 2001, 12, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- de, M.R.; Grootendorst, D.C.; Indemans, F.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 2009, 19, 127–135. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Greene, T.; Daugirdas, J.T.; Kimmel, P.L.; Schulman, G.W.; Toto, R.D.; Levin, N.W.; Yan, G. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am. J. Kidney Dis. 2003, 42, 1200–1211. [Google Scholar] [CrossRef]
- Kurita, N.; Hayashino, Y.; Yamazaki, S.; Akizawa, T.; Akiba, T.; Saito, A.; Fukuhara, S. Revisiting Interdialytic Weight Gain and Mortality Association With Serum Albumin Interactions: The Japanese Dialysis Outcomes and Practice Pattern Study. J. Ren. Nutr. 2017, 27, 421–429. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Zhou, Z.; Liu, J.; Li, C.; Liu, C. Impact of serum albumin level and variability on short-term cardiovascular-related and all-cause mortality in patients on maintenance hemodialysis. Medicine 2021, 100, e27666. [Google Scholar] [CrossRef]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Yasur, H.; Shapiro, G.; Feldman, L.; Averbukh, Z.; Weissgarten, J. IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2253–2263. [Google Scholar] [CrossRef][Green Version]
- Honda, H.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Wang, K.; Pecoits-Filho, R.; Stenvinkel, P.; Lindholm, B. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 2006, 47, 139–148. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Kubrusly, M.; Mota, R.S.; Silva, C.A.; Choukroun, G.; Oliveira, V.N. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J. Ren. Nutr. 2010, 20, 314–320. [Google Scholar] [CrossRef]
- Chertow, G.M.; Jacobs, C.; Lazarus, J.M.; Lew, N.; Lowrie, E.G. Phase angle predicts survival in hemodialysis patients. J. Ren. Nutr. 1997, 7, 204–207. [Google Scholar] [CrossRef]
- Bansal, N.; McCulloch, C.E.; Lin, F.; Alper, A.; Anderson, A.H.; Cuevas, M.; Go, A.S.; Kallem, R.; Kusek, J.W.; Lora, C.M.; et al. Blood Pressure and Risk of Cardiovascular Events in Patients on Chronic Hemodialysis: The CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 2017, 70, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.; Konings, C.; Canaud, B.; Carioni, P.; Guinsburg, A.; Madero, M.; van der Net, J.; Raimann, J.; van der Sande, F.; Stuard, S.; et al. Pre-dialysis fluid status, pre-dialysis systolic blood pressure and outcome in prevalent haemodialysis patients: Results of an international cohort study on behalf of the MONDO initiative. Nephrol. Dial. Transpl. 2018, 33, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 90) | Survivors (n = 70) | Non-Survivors (n = 20) | p-Value |
|---|---|---|---|---|
| Gender (Male) n (%) | 47 (52.20) | 35 (50.0) | 12 (60.0) | 0.43 |
| Age (years) | 75.91 ± 3.97 | 75.77 ± 3.89 | 76.40 ± 4.28 | 0.53 |
| Time on HD (months) | 36.57 ± 38.70 | 37.19 ± 36.70 | 34.40 ± 41.20 | 0.77 |
| DM n; (%) | 23 (25.60) | 14 (20.0) | 9 (45.0) | 0.20 |
| SBP (mmHg) | 125.74 ± 20.02 | 128.11 ± 19.07 | 118.40 ± 21.61 | 0.059 |
| DBP (mmHg) | 71.46 ± 12.47 | 73.55 ± 11.68 | 65.00 ± 12.92 | 0.007 |
| Catheter n (%) | 48 (55.80) | 30 (45.50) | 18 (90.0) | <0.001 |
| Kt/V urea (sp) | 1.33 ± 0.44 | 1.32 ± 0.46 | 1.43 ± 0.31 | 0.50 |
| URR (%) | 67.12 ± 12.05 | 66.04 ± 12.40 | 72.17 ± 9.07 | 0.11 |
| Hospital admissions (number/per year) | 1.07 ± 1.32 | 0.89 ± 1.01 | 1.70 ± 1.97 | 0.014 |
| Charlson index (points) | 7.54 ± 2.68 | 7.07 ± 2.60 | 9.20 ± 2.31 | 0.001 |
| Variable | Total (n = 90) | Survivors (n = 70) | Non-Survivors (n = 20) | p-Value |
|---|---|---|---|---|
| BMI (kg/m2) | 25.18 ± 5.14 | 24.89 ± 4.76 | 26.19 ± 6.32 | 0.32 |
| Exchange Na/K | 1.36 ± 0.39 | 1.30 ± 0.35 | 1.57 ± 0.47 | 0.007 |
| TBW (L) | 35.88 ± 7.17 | 35.17 ± 6.72 | 38.38 ± 8.25 | 0.078 |
| ECW (L) | 19.13 ± 4.06 | 18.12 ± 3.26 | 22.70 ± 4.61 | <0.001 |
| ICW (L) | 16.84 ± 4.48 | 17.14 ± 4.56 | 15.80 ± 4.15 | 0.24 |
| ECM (kg) | 28.26 ± 6.62 | 26.85 ± 5.74 | 33.19 ± 7.27 | 0.002 |
| FFM (kg) | 46.34 ± 9.87 | 45.65 ± 9.68 | 48.74 ± 10.41 | 0.22 |
| BCM (kg) | 18.07 ± 4.83 | 18.80 ± 4.96 | 15.55 ± 3.38 | 0.007 |
| MM (kg) | 23.42 ± 5.68 | 24.10 ± 5.85 | 21.10 ± 4.41 | 0.037 |
| ECM/BCM ratio (points) | 1.61 ± 0.41 | 1.46 ± 0.32 | 2.14 ± 0.23 | <0.001 |
| PA (°) | 4.33 ± 0.97 | 4.56 ± 0.93 | 3.53 ± 0.66 | <0.001 |
| s-Albumin (g/dL) | 3.76 ± 0.42 | 3.80 ± 0.43 | 3.50 ± 0.32 | 0.033 |
| s-Transferrin (mg/dL) | 169.40 ± 37.90 | 169.86 ± 35.29 | 167.90 ± 46.49 | 0.20 |
| s-CRP (mg/dL) | 0.95 ± 0.83 | 0.84 ± 0.81 | 1.32 ± 0.81 | 0.022 |
| MIS (points) | 8.46 ± 5.01 | 7.74 ± 4.99 | 10.95 ± 4.33 | 0.011 |
| IL-6 (pg/mL) | 4.19 ± 4.67 | 3.43 ± 4.38 | 6.84 ± 4.80 | 0.003 |
| Predictor Variable | Hazard Ratio (95%CI) | p-Value |
|---|---|---|
| Age (years) | 1.046 (0.947–1.154) | 0.377 |
| Time on HD (months) | 0.996 (0.985–1.008) | 0.511 |
| SBP (mmHg) | 0.980 (0.958–1.003) | 0.086 |
| DBP (mmHg) | 0.953 (0.920–0.987) | 0.007 |
| Vascular access (AVF/catheter) | 0.223 (0.076–0.652) | 0.006 |
| URR (%) | 1.046 (0.991–1.105) | 0.105 |
| Kt/V urea (sp) | 1.443 (0.357–5.834) | 0.607 |
| BMI (kg/m2) | 1.039 (0.967–1.117) | 0.300 |
| Exchange Na/K | 2.859 (1.349–6.061) | 0.006 |
| TBW(L) | 1.035 (0.981–1.092) | 0.213 |
| ECW (L) | 1.220 (1.114–1.336) | <0.001 |
| ICW(L) | 0.917 (0.826–1.018) | 0.105 |
| ECM (kg) | 1.090 (1.031–1.151) | 0.002 |
| FFM (kg) | 1.013 (0.975–1.053) | 0.500 |
| BCM (kg) | 0.836 (0.738–0.947) | 0.005 |
| MM (kg) | 0.900 (0.820–0.987) | 0.025 |
| ECM/BCM ratio < 1.20 points | 0.175 (0.051–0.606) | 0.006 |
| PA (°) | 0.319 (0.199–0.512) | <0.001 |
| s-Albumin (g/dL) | 0.227 (0.085–0.610) | 0.003 |
| s-Transferrin (mg/dL) | 0.990 (0.988 to 1.010) | 0.790 |
| MIS (points) | 1.125 (1.036–1.221) | 0.005 |
| s-CRP (mg/dL) | 1.607 (0.980–2.634) | 0.06 |
| IL-6 (median 3.1) (pg/mL) | 0.206 (0.048 to 0.880) | 0.033 |
| Charlson index (points) | 1.379 (1.161–1.638) | <0.001 |
| Hospital admissions (number/per year) | 1.405 (1.131–1.746) | 0.002 |
| Predictor Variable | Hazard Ratio (95%CI) | p-Value |
|---|---|---|
| ECM/BCM ratio >1.20 points | 5.078 (1.509 to 17.090) | 0.009 |
| IL-6 (median 3.1) (pg/mL) ‡ | 2.853 (1.076 to 7.565) | 0.035 |
| s-Albumin > 3.8 g/dL ⁌ | 0.225 (0.102 to 0.494) | <0.001 |
| s-CRP (mg/dL) | 1.107 (1.017 to 1.205) | 0.019 |
| Charlson index (points) | 1.339 (1.130 to 1.587) | <0.001 |
| Hospital admissions (number/per year) | 0.858 (0.653 to 1.127) | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruperto, M.; Barril, G. The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients. Nutrients 2022, 14, 1659. https://doi.org/10.3390/nu14081659
Ruperto M, Barril G. The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients. Nutrients. 2022; 14(8):1659. https://doi.org/10.3390/nu14081659
Chicago/Turabian StyleRuperto, Mar, and Guillermina Barril. 2022. "The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients" Nutrients 14, no. 8: 1659. https://doi.org/10.3390/nu14081659
APA StyleRuperto, M., & Barril, G. (2022). The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients. Nutrients, 14(8), 1659. https://doi.org/10.3390/nu14081659







