Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue
Abstract
1. Introduction
2. Iron Deficiency
2.1. Etiology
- Inadequate DII, which is reviewed below, is related to prolonged exclusive breastfeeding in infants, or the insufficient intake of iron-rich foods considering the growth velocity at any age or menstruation in adolescent girls;
- Blood loss [19];
| Neonatal Period | Infancy | Childhood and Adolescence |
|---|---|---|
| Maternal iron deficiency anemia | Low neonatal iron stores | Low dietary iron supply and/or bioavailability |
| Fetal–maternal hemorrhage | Rapid growth | Vegetarian or vegan diet |
| Twin-to-twin transfusion | Frequent blood sampling | Pica; pagophagia |
| Premature birth | Prolonged exclusive breast feeding | Intense physical activity |
| Low birth weight | Cow’s milk feeding | Antacid therapy |
| Early umbilical cord clamping | Low-iron-content complementary diet | Esophagitis |
| Phlebotomy losses | Cow’s milk allergy | Helicobacter pylori infection |
| Esophagitis | Intestinal malabsorption (Celiac disease, short-bowel syndrome) | Intestinal malabsorption (Celiac disease, short-bowel syndrome) |
| Intestinal blood loss | Antacid therapy | Parasitosis (Giardia, hookworm) |
| Erythropoietin administration | Esophagitis | Use of nonsteroidal anti-inflammatory drugs |
| Lead exposure | Lead exposure | |
| Intestinal blood loss | Gastrointestinal blood loss (gastritis, varices, Meckel’s diverticulum, ulcerative colitis, vascular malformations, tumors, polyp) | |
| Obesity | ||
| Excessive menstrual losses | ||
| Adolescent pregnancy | ||
| Low socioeconomic status | Low socioeconomic status |
2.2. Development of Iron Deficiency
2.3. Epidemiology
2.4. Health Consequences of Iron Deficiency
3. Iron Requirements
3.1. Requirements According to Age Groups
3.1.1. Infancy (Birth through to 11 Completed Months of Age)
3.1.2. Toddlers (1–3 Years of Age) and Children
3.1.3. Adolescents (12–17 Years)
3.2. Dietary Reference Values for Iron Intake
4. Actual Dietary Iron Intakes in Children
5. Dietary Iron
5.1. Dietary Iron Bioavailability
5.2. Dietary Iron Sources
5.2.1. Dietary Recommendations and Usual Feeding Pattern in Children
5.2.2. Breastmilk
5.2.3. Formula
5.2.4. Cow’s Milk
5.2.5. Usual Solid Foods
Limitations in Iron-Rich Food Consumption
Sources of Heme Iron
Nonheme Iron
5.2.6. Fortified Foods
Addition of Iron to Staple Foods
Biofortification
6. Discussion and Strategies
6.1. Limitations
6.2. Strategies to Prevent Iron Deficiency
6.2.1. Dietary Strategies to Address Suboptimal Intake
In Infants and Toddlers
In Children over 3 Years Old and Adolescents
Cost/Benefit Ratio
6.2.2. Choice of a Health Policy
Implementation of Dietary Measures
Iron Supplementation
- WHO Recommendations
- 2.
- Concerns about ID Prevention in Malaria-Endemic Areas
- 3.
- Adverse Effects
- 4.
- Screening for Iron Deficiency
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Adequate intake |
| DII | Dietary iron intake |
| DRVs | Dietary reference values |
| EAR | Estimated average requirement |
| Hb | Hemoglobin |
| ID | Iron deficiency |
| IDA | Iron deficiency anemia |
| PRI | Population reference intake |
| UL | Tolerable upper intake level |
References
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.; Sharp, P. Iron. Adv. Food. Nutr. Res. 2021, 96, 219–250. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13, 4254. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2015.4254 (accessed on 3 May 2021). [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 121, 761. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Iron Review. J. Nutr. 2018, 148 (Suppl. S1), 1001S–1067S. [Google Scholar] [CrossRef]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71 (Suppl. S3), 8–14. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 36803. [Google Scholar] [CrossRef]
- Collings, R.; Harvey, L.J.; Hooper, L.; Hurst, R.; Brown, T.J.; Ansett, J.; King, M.; Fairweather-Tait, S.J. The absorption of iron from whole diets: A systematic review. Am. J. Clin. Nutr. 2013, 98, 65–81. [Google Scholar] [CrossRef]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Lönnerdal, B. Development of iron homeostasis in infants and young children. Am. J. Clin. Nutr. 2017, 10, 1575S–1580S. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ferdi, J.; Bardosono, S.; Medise, B.E. Iron intake and its correlation to ferritin and Hb level among children aged 24–36 months in Jakarta in 2020. World Nutr. J. 2021, 5, 106–112. [Google Scholar] [CrossRef]
- McDonald, S.J.; Middleton, P.; Dowswell, T.; Morris, P.S. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst. Rev. 2013, 7, CD004074. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Latunde-Dada, G.O.; Diaz-Castro, J. Iron Deficiency and Iron Homeostasis in Low Birth Weight Preterm Infants: A Systematic Review. Nutrients 2019, 11, 1090. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Santolaria-Piedrafita, S.; Cañamares-Orbis, P.; García-Erce, J.A. Iron Deficiency in Celiac Disease: Prevalence; Health Impact; and Clinical Management. Nutrients 2021, 13, 3437. [Google Scholar] [CrossRef]
- Belachew, A.; Tewabe, T. Under-five anemia and its associated factors with dietary diversity; food security; stunted; and deworming in Ethiopia: Systematic review and meta-analysis. Syst. Rev. 2020, 9, 31. [Google Scholar] [CrossRef]
- Mattiello, V.; Schmugge, M.; Hengartner, H.; von der Weid, N.; Renella, R.; SPOG Pediatric Hematology Working Group. Diagnosis and management of iron deficiency in children with or without anemia: Consensus recommendations of the SPOG Pediatric Hematology Working Group. Eur. J. Pediatr. 2020, 179, 527–545. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Anaemia Estimates. Global Anaemia Estimates in Women of Reproductive Age, by Pregnancy Status, and in Children Aged 6–59 Months. 2021. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 13 February 2022).
- Bayoumi, I.; Parkin, P.C.; Birken, C.S.; Maguire, J.L.; Borkhoff, C.M.; TARGet Kids! Collaboration. Association of Family Income and Risk of Food Insecurity with Iron Status in Young Children. JAMA Netw. Open 2020, 3, e208603. [Google Scholar] [CrossRef]
- Eussen, S.; Alles, M.; Uijterschout, L.; Brus, F.; van der Horst-Graat, J. Iron intake and status of children aged 6-36 months in Europe: A systematic review. Ann. Nutr. Metab. 2015, 66, 80–92. [Google Scholar] [CrossRef]
- Male, C.; Persson, L.A.; Freeman, V.; Guerra, A.; van’t Hof, M.A.; Haschke, F.; Euro-Growth Iron Study Group. Prevalence of iron deficiency in 12-mo-old infants from 11 European areas and influence of dietary factors on iron status (Euro-Growth study). Acta Paediatr. 2001, 90, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, R.; Ma, J.; Yan, X.; Hu, P.; Song, Y.; Jan, C.; Raat, H.; Patton, G.C. The associations of economic growth and anaemia for school-aged children in China. Matern. Child. Nutr. 2020, 16, e12936. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858, Erratum in Lancet 2019, 393, e44. [Google Scholar] [CrossRef]
- Turawa, E.; Awotiwon, O.; Dhansay, M.A.; Cois, A.; Labadarios, D.; Bradshaw, D.; Pillay-van Wyk, V. Prevalence of Anaemia; Iron Deficiency; and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12799. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Donahue Angel, M.; Rohner, F. The Proportion of Anemia Associated with Iron Deficiency in Low; Medium; and High Human Development Index Countries: A Systematic Analysis of National Surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of the Risk Factors Associated with Anaemia among Children Under Five Years in Sub-Saharan African Countries. Int. J Environ. Res. Public Health 2020, 17, 8829. [Google Scholar] [CrossRef]
- Muriuki, J.M.; Mentzer, A.J.; Webb, E.L.; Morovat, A.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Crane, R.J.; Berkley, J.A.; Lule, S.A.; et al. Estimating the burden of iron deficiency among African children. BMC Med. 2020, 18, 31. [Google Scholar] [CrossRef]
- Wong, A.Y.; Chan, E.W.; Chui, C.S.; Sutcliffe, A.G.; Wong, I.C. The phenomenon of micronutrient deficiency among children in China: A systematic review of the literature. Public Health Nutr. 2014, 17, 2605–2618. [Google Scholar] [CrossRef]
- Gupta, P.M.; Perrine, C.G.; Mei, Z.; Scanlon, K.S. Iron, Anemia, and Iron Deficiency Anemia among Young Children in the United States. Nutrients 2016, 8, 330, Erratum in Nutrients 2017, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Catellier, D.J.; Jun, S.; Dwyer, J.T.; Jacquier, E.F.; Anater, A.S.; Eldridge, A.L. Total Usual Nutrient Intakes of US Children (Under 48 Months): Findings from the Feeding Infants and Toddlers Study (FITS) 2016. J. Nutr. 2018, 148, 1557S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.D.L.; Fulgoni, V.L., III; Shah, N.; Patterson, A.C.; Gutierrez-Orozco, F.; Mathews, R.S.; Walsh, K.R. Nutrient Intake Adequacy from Food and Beverage Intake of US Children Aged 1–6 Years from NHANES 2001–2016. Nutrients 2021, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.; Thorpe, K.E.; Maguire, J.L.; Birken, C.S.; Fehlings, D.; Hanley, A.J.; Parkin, P.C. Risk factors, practice variation and hematological outcomes of children identified with non-anemic iron deficiency following screening in primary care setting. Paediatr. Child Health 2015, 20, 302–306. [Google Scholar] [CrossRef][Green Version]
- Akkermans, M.D.; van der Horst-Graat, J.M.; Eussen, S.R.; van Goudoever, J.B.; Brus, F. Iron and Vitamin D Deficiency in Healthy Young Children in Western Europe Despite Current Nutritional Recommendations. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 635–642. [Google Scholar] [CrossRef]
- Ferrari, M.; Mistura, L.; Patterson, E.; Sjöström, M.; Díaz, L.E.; Stehle, P.; Gonzalez-Gross, M.; Kersting, M.; Widhalm, K.; Molnár, D.; et al. Evaluation of iron status in European adolescents through biochemical iron indicators: The HELENA Study. Eur. J. Clin. Nutr. 2011, 65, 340–349. [Google Scholar] [CrossRef]
- Kang, W.; Barad, A.; Clark, A.G.; Wang, Y.; Lin, X.; Gu, Z.; O’Brien, K.O. Ethnic Differences in Iron Status. Adv. Nutr. 2021, 12, 1838–1853. [Google Scholar] [CrossRef]
- Bastian, T.W.; Rao, R.; Tran, P.V.; Georgieff, M.K. The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. Neurosci. Insights 2020, 15, 2633105520935104. [Google Scholar] [CrossRef]
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron Deficiency; Cognitive Functions; and Neurobehavioral Disorders in Children. J. Mol. Neurosci. 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.; Yang, W. Association of Iron-Deficiency Anemia and Non-Iron-Deficiency Anemia with Neurobehavioral Development in Children Aged 6–24 Months. Nutrients 2021, 13, 3423. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, L.; Qu, Y.; Mu, D. Iron Status in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169145. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Reich, S.; Lev, N.; Gafter-Gvili, A. Iron supplementation for restless legs syndrome—A systematic review and meta-analysis. Eur. J. Intern. Med. 2019, 63, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in immune cell function and host defense. Semin. Cell. Dev. Biol. 2021, 115, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Pasricha, S.R.; Cabantchik, I.; Hershko, C.; Weiss, G.; Girelli, D.; Stoffel, N.; Muckenthaler, M.U.; Nemeth, E.; Camaschella, C.; et al. Vaccine efficacy and iron deficiency: An intertwined pair? Lancet Haematol. 2021, 8, e666–e669. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Milman, N.; Samuelson, G. A longitudinal study of iron status in healthy Danish infants: Effects of early iron status; growth velocity and dietary factors. Acta Paediatr. 1995, 84, 1035–1044. [Google Scholar] [CrossRef]
- Roberts, J.L.; Stein, A.D. The Impact of Nutritional Interventions beyond the First 2 Years of Life on Linear Growth: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 323–336. [Google Scholar] [CrossRef]
- Andersen, A.T.N.; Husby, S.; Kyhl, H.B.; Sandberg, M.B.; Sander, S.D.; Mølgaard, C. Iron deficiency in healthy 18-month-old Danish children is associated with no oral iron supplementation in infancy and prolonged exclusive breast-feeding. Br. J. Nutr. 2019, 122, 1409–1416. [Google Scholar] [CrossRef]
- Frelut, M.L.; Girardet, J.P.; Bocquet, A.; Briend, A.; Chouraqui, J.P.; Darmaun, D.; Dupont, C.; Feillet, F.; Hankard, R.; Rozé, J.C.; et al. Committee on Nutrition of the French Society of Paediatrics. Impact of obesity on biomarkers of iron and vitamin D status in children and adolescents: The risk of misinterpretation. Arch. Pediatr. 2018, 25, 3–5. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 55529. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Daily Iron Supplementation in Infants and Children. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/204712/9789241549523_eng.pdf;jsessionid=751881E61978A57DB6A22232D443906F?sequence=1 (accessed on 15 July 2021).
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal. Neonatal. Med. 2007, 12, 54–63. [Google Scholar] [CrossRef]
- Siddappa, A.M.; Rao, R.; Long, J.D.; Widness, J.A.; Georgieff, M.K. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology 2007, 92, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Pinedo, H.G.; Cassel, F.; Duijts, L.; Muckenthaler, M.U.; Gassmann, M.; Jaddoe, V.W.V.; Reiss, I.K.M.; Vermeulen, M.J. Maternal Iron Status in Pregnancy and Child Health Outcomes after Birth: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2221. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.N.; Gupta, V.K.; Agarwal, K.N. Storage iron in human foetal organs. Acta Paediatr. Scand. 1985, 74, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Faa, G.; Sciot, R.; Farci, A.M.; Callea, F.; Ambu, R.; Congiu, T.; van Eyken, P.; Cappai, G.; Marras, A.; Costa, V.; et al. Iron concentration and distribution in the newborn liver. Liver 1994, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Ohls, R.K. The hematopoietic system. In Nelson Textbook of Pediatrics, 20th ed.; Kliegman, R.M., Stanton, B.F., St Geme, J.W., Schor, N.F., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 2304–2316. [Google Scholar]
- Mesías, M.; Seiquer, I.; Navarro, M.P. Iron nutrition in adolescence. Crit. Rev. Food. Sci. Nutr. 2013, 53, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; Available online: https://www.nap.edu/catalog/10026/dietary-reference-intakes-for-vitamin-a-vitamin-k-arsenic-boron-chromium-copper-iodine-iron-manganese-molybdenum-nickel-silicon-vanadium-and-zinc (accessed on 3 May 2021).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013, 11, 3408. Available online: https://www.efsa.europa.eu/fr/efsajournal/pub/3408 (accessed on 3 May 2021). [CrossRef]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/bitstream/handle/10665/42716/9241546123.pdf?ua=1 (accessed on 3 May 2021).
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000; Available online: https://www.nap.edu/catalog/9956/dietary-reference-intakes-applications-in-dietary-assessment (accessed on 6 June 2021).
- Gibson, S.; Sidnell, A. Nutrient adequacy and imbalance among young children aged 1–3 years in the UK. Nutr. Bull. 2014, 39, 172–180. [Google Scholar] [CrossRef]
- Chouraqui, J.P.; Tavoularis, G.; Turck, D.; Ferry, C.; Feillet, F. Mineral and vitamin intake of infants and young children: The Nutri-Bébé 2013 survey. Eur. J. Nutr. 2020, 59, 2463–2480. [Google Scholar] [CrossRef]
- ANSES. Report on the Third Individual and National Survey on Food Consumption (INCA3 survey) (Etude Individuelle Nationale des Consommations Alimentaires 3). (Saisine Request No 2014-SA-0234); ANSES: Maisons-Alfort, France, 2017; 535p, (In French). Available online: https://www.anses.fr/fr/system/files/NUT2014SA0234Ra.pdf (accessed on 15 July 2021).
- Eldridge, A.L.; Catellier, D.J.; Hampton, J.C.; Dwyer, J.T.; Bailey, R.L. Trends in Mean Nutrient Intakes of US Infants; Toddlers; and Young Children from 3 Feeding Infants and Toddlers Studies (FITS). J. Nutr. 2019, 149, 1230–1237. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hampton, J.C.; Finn, K.L. A Substantial Proportion of 6- to 12-Month-Old Infants Have Calculated Daily Absorbed Iron below Recommendations; Especially Those Who Are Breastfed. J. Pediatr. 2021, 231, 36–42.e2. [Google Scholar] [CrossRef]
- Atkins, L.A.; McNaughton, S.A.; Spence, A.C.; Szymlek-Gay, E.A. Adequacy of iron intakes and socio-demographic factors associated with iron intakes of Australian pre-schoolers. Eur. J. Nutr. 2020, 59, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Harika, R.; Faber, M.; Samuel, F.; Mulugeta, A.; Kimiywe, J.; Eilander, A. Are Low Intakes and Deficiencies in Iron, Vitamin A., Zinc, and Iodine of Public Health Concern in Ethiopian, Kenyan, Nigerian, and South African Children and Adolescents? Food Nutr. Bull. 2017, 38, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, O.H.; Thorsdottir, I.; Hibberd, P.L.; Fewtrell, M.S.; Wells, J.C.; Palsson, G.I.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Timing of the introduction of complementary foods in infancy: A randomized controlled trial. Pediatrics 2012, 130, 1038–1045. [Google Scholar] [CrossRef]
- Ohlund, I.; Lind, T.; Hörnell, A.; Hernell, O. Predictors of iron status in well-nourished 4-y-old children. Am. J. Clin. Nutr. 2008, 87, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Bramhagen, A.C.; Svahn, J.; Hallström, I.; Axelsson, I. Factors influencing iron nutrition among one-year-old healthy children in Sweden. J. Clin. Nurs. 2011, 20, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Counselling of Women to Improve Breastfeeding Practices. Licence: CC BY-NC-SA 3.0 IGO. 2018. Available online: https://www.who.int/publications/i/item/9789241550468 (accessed on 3 May 2021).
- Public Health Agency of Canada. Family-Centred Maternity and Newborn Care: National Guidelines. Chapter 6: Breastfeeding. 2021. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/maternity-newborn-care-guidelines-chapter-6.html#a1 (accessed on 8 March 2022).
- American Academy of Pediatrics. Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology; Hepatology; and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); et al. Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 7, 1423. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Lancet Breastfeeding Series Group; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- FAO-WHO-Codex Alimentarius. Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. CXS 72-1981. Formerly CAC/RS 72-1972. Adopted as a Worldwide Standard in 1981. Amended in 1983, 1985, 1987, 2011, 2015, 2016, 2020. Revised in 2007. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B72-1981%252FCXS_072e.pdf (accessed on 10 August 2020).
- Chiang, K.V.; Hamner, H.C.; Li, R.; Perrine, C.G. Timing of Introduction of Complementary Foods—United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Domínguez, G.; Neves, P.A.R.; Barros, A.J.D.; Victora, C.G. Complementary Feeding Practices in 80 Low- and Middle-Income Countries: Prevalence of and Socioeconomic Inequalities in Dietary Diversity, Meal Frequency, and Dietary Adequacy. J. Nutr. 2021, 151, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Greer, F.R.; Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Iron and copper in human milk. Nutrition 2000, 16, 209–220. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Georgieff, M.K.; Hernell, O. Developmental Physiology of Iron Absorption; Homeostasis; and Metabolism in the Healthy Term Infant. J. Pediatr. 2015, 167 (Suppl. S4), S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9 (Suppl. S1), 278S–294S. [Google Scholar] [CrossRef]
- Saarinen, U.M.; Siimes, M.A.; Dallman, P.R. Iron absorption in infants: High bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J. Pediatr. 1977, 91, 36–39. [Google Scholar] [CrossRef]
- Domellöf, M.; Lönnerdal, B.; Abrams, S.A.; Hernell, O. Iron absorption in breast-fed infants: Effects of age; iron status; iron supplements; and complementary foods. Am. J. Clin. Nutr. 2002, 76, 198–204. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Nelson, S.E.; Jeter, J.M. Iron stores of breastfed infants during the first year of life. Nutrients 2014, 6, 2023–2034. [Google Scholar] [CrossRef]
- Yang, Z.; Lönnerdal, B.; Adu-Afarwuah, S.; Brown, K.H.; Chaparro, C.M.; Cohen, R.J.; Domellöf, M.; Hernell, O.; Lartey, A.; Dewey, K.G. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: Comparison of data from 6 studies. Am. J. Clin. Nutr. 2009, 89, 1433–1440. [Google Scholar] [CrossRef]
- Pérez-Escamilla, R.; Buccini, G.S.; Segura-Pérez, S.; Piwoz, E. Perspective: Should Exclusive Breastfeeding Still Be Recommended for 6 Months? Adv. Nutr. 2019, 10, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R. Are Breastfed Infants Iron Deficient? The Question That Won’t Go Away. J. Pediatr. 2021, 231, 34–35. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Nelson, S.E.; Jeter, J.M. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am. J. Clin. Nutr. 2009, 90, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E.; Fomon, S.J.; Nelson, S.E.; Jeter, J.M.; Theuer, R.C. Dry cereals fortified with electrolytic iron or ferrous fumarate are equally effective in breast-fed infants. J. Nutr. 2011, 141, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Ansari, M.B.; Inayah, L.S.; Purnamasari, A.D.; Houghton, L.A. Differences in Micronutrient Intakes of Exclusive and Partially Breastfed Indonesian Infants from Resource-Poor Households are Not Accompanied by Differences in Micronutrient Status, Morbidity, or Growth. J. Nutr. 2021, 151, 705–715. [Google Scholar] [CrossRef]
- Maguire, J.L.; Salehi, L.; Birken, C.S.; Carsley, S.; Mamdani, M.; Thorpe, K.E.; Lebovic, G.; Khovratovich, M.; Parkin, P.C.; TARGet Kids! Collaboration. Association between total duration of breastfeeding and iron deficiency. Pediatrics 2013, 131, e1530–e1537. [Google Scholar] [CrossRef]
- Saunders, N.R.; Parkin, P.C.; Birken, C.S.; Maguire, J.L.; Borkhoff, C.M.; TARGet Kids! Collaboration. Iron status of young children from immigrant families. Arch. Dis. Child. 2016, 101, 1130–1136. [Google Scholar] [CrossRef]
- Uyoga, M.A.; Karanja, S.; Paganini, D.; Cercamondi, C.I.; Zimmermann, S.A.; Ngugi, B.; Holding, P.; Moretti, D.; Zimmermann, M.B. Duration of exclusive breastfeeding is a positive predictor of iron status in 6- to 10-month-old infants in rural Kenya. Matern. Child. Nutr. 2017, 13, e12386. [Google Scholar] [CrossRef]
- Scott, J.A.; Gee, G.; Devenish, G.; Ha, D.; Do, L. Determinants and Sources of Iron Intakes of Australian Toddlers: Findings from the SMILE Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 181. [Google Scholar] [CrossRef]
- Szymlek-Gay, E.A.; Ferguson, E.L.; Heath, A.L.; Gray, A.R.; Gibson, R.S. Food-based strategies improve iron status in toddlers: A randomized controlled trial12. Am. J. Clin. Nutr. 2009, 90, 1541–1551. [Google Scholar] [CrossRef]
- Ghisolfi, J.; Fantino, M.; Turck, D.; de Courcy, G.P.; Vidailhet, M. Nutrient intakes of children aged 1–2 years as a function of milk consumption; cows’ milk or growing-up milk. Public Health Nutr. 2013, 16, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.; Flynn, A. Nutritional adequacy of diets containing growing up milks or unfortified cow’s milk in Irish children (aged 12–24 months). Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Sidnell, A.; Pigat, S.; Gibson, S.; O’Connor, R.; Connolly, A.; Sterecka, S.; Stephen, A.M. Nutrient intakes and iron and vitamin D status differ depending on main milk consumed by UK children aged 12–18 months—Secondary analysis from the Diet and Nutrition Survey of Infants and Young Children. J. Nutr. Sci. 2016, 5, e32. [Google Scholar] [CrossRef] [PubMed]
- Lovell, A.L.; Milne, T.; Jiang, Y.; Chen, R.X.; Grant, C.C.; Wall, C.R. Evaluation of the Effect of a Growing up Milk Lite vs. Cow’s Milk on Diet Quality and Dietary Intakes in Early Childhood: The Growing up Milk Lite (GUMLi) Randomised Controlled Trial. Nutrients 2019, 11, 203.e1. [Google Scholar] [CrossRef]
- Chouraqui, J.P.; Turck, D.; Tavoularis, G.; Ferry, C.; Dupont, C. The Role of Young Child Formula in Ensuring a Balanced Diet in Young Children (1–3 Years Old). Nutrients 2019, 11, 2213. [Google Scholar] [CrossRef]
- Sacri, A.S.; Bocquet, A.; de Montalembert, M.; Hercberg, S.; Gouya, L.; Blondel, B.; Ganon, A.; Hebel, P.; Vincelet, C.; Thollot, F.; et al. Young children formula consumption and iron deficiency at 24 months in the general population: A national-level study. Clin. Nutr. 2021, 40, 166–173. [Google Scholar] [CrossRef]
- Haschke, F.; Ziegler, E.E.; Edwards, B.B.; Fomon, S.J. Effect of iron fortification of infant formula on trace mineral absorption. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 768–773. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Hernell, O. Iron; zinc; copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr. 1994, 83, 367–373. [Google Scholar] [CrossRef]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215. [Google Scholar] [CrossRef]
- Gahagan, S.; Delker, E.; Blanco, E.; Burrows, R.; Lozoff, B. Randomized Controlled Trial of Iron-Fortified versus Low-Iron Infant Formula: Developmental Outcomes at 16 Years. J. Pediatr. 2019, 212, 124–130.e1. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3760 (accessed on 3 May 2021). [CrossRef]
- Björmsjö, M.; Hernell, O.; Lönnerdal, B.; Berglund, S.K. Reducing Iron Content in Infant Formula from 8 to 2 mg/L Does Not Increase the Risk of Iron Deficiency at 4 or 6 Months of Age: A Randomized Controlled Trial. Nutrients 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Intestinal bioavailability of minerals and trace elements from milk and beverages in humans. Nahrung 2000, 44, 390–397. [Google Scholar] [CrossRef]
- Ziegler, E.E. Adverse effects of cow’s milk in infants. Nestle Nutr. Workshop Ser. Pediatr. Program. 2007, 60, 185–199. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachman, W.; Kraut, H. Food Composition and Nutrition Tables, 7th ed.; MedPharm Scientific Publishers; Taylor and Francis Group: Boca Raton, FL, USA, 2008; 1364p, Available online: https://www.sfk.online/#/home (accessed on 10 August 2021).
- EFSA. Food Composition Tables. Available online: https://www.efsa.europa.eu/en/microstrategy/food-composition-data (accessed on 10 August 2021).
- Peter Herman, C.; Polivy, J.; Pliner, P.; Vartanian, L.R. Mechanisms underlying the portion-size effect. Physiol. Behav. 2015, 144, 129–136. [Google Scholar] [CrossRef]
- More, J.A.; Lanigan, J.; Emmett, P. The development of food portion sizes suitable for 4-18-year-old children used in a theoretical meal plan meeting energy and nutrient requirements. J. Hum. Nutr. Diet. 2021, 34, 534–549. [Google Scholar] [CrossRef]
- Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 27 December 2021).
- Philippe, K.; Issanchou, S.; Roger, A.; Feyen, V.; Monnery-Patris, S. How Do French Parents Determine Portion Sizes for Their Pre-Schooler? A Qualitative Exploration of the Parent-Child Division of Responsibility and Influencing Factors. Nutrients 2021, 13, 2769. [Google Scholar] [CrossRef]
- Tuck, C. Complementary Feeding: A Research-Based Guide; Radcliffe Publishing: London, UK; New York, NY, USA, 2013; 197p. [Google Scholar]
- Hallberg, L.; Bjørn-Rasmussen, E.; Howard, L.; Rossander, L. Dietary heme iron absorption. A discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand. J. Gastroenterol. 1979, 14, 769–779. [Google Scholar] [CrossRef]
- Czerwonka, M.; Tokarz, A. Iron in red meat-friend or foe. Meat Sci. 2017, 123, 157–165. [Google Scholar] [CrossRef]
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Cox, K.A.; Parkin, P.C.; Anderson, L.N.; Chen, Y.; Birken, C.S.; Maguire, J.L.; Macarthur, C.; Borkhoff, C.M.; TARGet Kids! Collaboration. Association between Meat and Meat-Alternative Consumption and Iron Stores in Early Childhood. Acad. Pediatr. 2016, 16, 783–791. [Google Scholar] [CrossRef]
- Obbagy, J.E.; English, L.K.; Psota, T.L.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and micronutrient status: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. S7), 852S–871S. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.A.; McNaughton, S.A.; Spence, A.C.; Szymlek-Gay, E.A. Dietary patterns of Australian pre-schoolers and associations with haem and non-haem iron intakes. Eur. J. Nutr. 2021, 60, 3059–3070. [Google Scholar] [CrossRef]
- Moreno, L.A.; Gottrand, F.; Huybrechts, I.; Ruiz, J.R.; González-Gross, M.; De Henauw, S.; HELENA Study Group. Nutrition and lifestyle in European adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Adv. Nutr. 2014, 5, 615S–623S. [Google Scholar] [CrossRef] [PubMed]
- Milford, A.B.; Le Mouël, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Turck, D.; Briend, A.; Darmaun, D.; Bocquet, A.; Feillet, F.; Frelut, M.L.; Girardet, J.P.; Guimber, D.; Hankard, R.; et al. Religious dietary rules and their potential nutritional and health consequences. Int. J. Epidemiol. 2021, 50, 12–26. [Google Scholar] [CrossRef]
- Duchène, C.; Gandemer, G. Nutritional Values of Cooked Meats (Valeurs Nutritionnelles des Viandes Cuites); CIV: Paris, France, 2015; 93p. [Google Scholar]
- Alves, C.; Saleh, A.; Alaofè, H. Iron-containing cookware for the reduction of iron deficiency anemia among children and females of reproductive age in low- and middle-income countries: A systematic review. PLoS ONE 2019, 14, e0221094. [Google Scholar] [CrossRef]
- Sharma, S.; Khandelwal, R.; Yadav, K.; Ramaswamy, G.; Vohra, K. Effect of cooking food in iron-containing cookware on increase in blood Hb level and iron content of the food: A systematic review. Nepal J. Epidemiol. 2021, 11, 994–1005. [Google Scholar] [CrossRef]
- Charles, C.V.; Dewey, C.E.; Daniell, W.E.; Summerlee, A.J. Iron-deficiency anaemia in rural Cambodia: Community trial of a novel iron supplementation technique. Eur. J. Public Health 2010, 21, 43–48. [Google Scholar] [CrossRef]
- Charles, C.V.; Summerlee, A.J.; Dewey, C.E. Iron content of Cambodian foods when prepared in cooking pots containing an iron ingot. Trop. Med. Int. Health 2011, 16, 1518–1524. [Google Scholar] [CrossRef]
- Kröger-Ohlsen, M.; Trugvason, T.; Skibsted, L.H.; Michaelsen, K.F. Release of iron into foods cooked in an iron pot: Effect of pH; salt; and organic acids. J. Food Sci. 2002, 67, 3301–3303. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Perfecto, A.; Fairweather-Tait, S.J. Dietary Factors Modulate Iron Uptake in Caco-2 Cells from an Iron Ingot Used as a Home Fortificant to Prevent Iron Deficiency. Nutrients 2017, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.M. The effects of meat consumption on global health. Rev. Sci. Tech. 2018, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- The Scientific Advisory Committee on Nutrition. Recommendations on Iron and Health and Consumption of Red and Processed Meat; Public Health England: London, UK, 2011; 374p. Available online: https://www.gov.uk/government/publications/sacn-iron-and-health-report (accessed on 3 May 2021).
- Platel, K.; Srinivasan, K. Bioavailability of Micronutrients from Plant Foods: An Update. Crit. Rev. Food. Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- World Health Organisation. Nutritional Anaemias: Tools for Effective Prevention and Control. Licence: CC BY-NC-SA 3.0 IGO. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259425/9789241513067-eng.pdf?sequence=1 (accessed on 27 December 2021).
- Eichler, K.; Hess, S.; Twerenbold, C.; Sabatier, M.; Meier, F.; Wieser, S. Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: A systematic review. PLoS ONE 2019, 14, e0210899. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Mahmood, S.B.; Moin, A.; Kumar, R.; Mukhtar, K.; Lass, Z.S.; Bhutta, Z.A. Food fortification with multiple micronutrients: Impact on health outcomes in general population. Cochrane Database Syst. Rev. 2019, 12, CD011400. [Google Scholar] [CrossRef]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food Fortification: The Advantages; Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef]
- Field, M.S.; Mithra, P.; Peña-Rosas, J.P. Wheat flour fortification with iron and other micronutrients for reducing anaemia and improving iron status in populations. Cochrane Database Syst. Rev. 2021, 1, CD011302. [Google Scholar] [CrossRef]
- Da Silva Lopes, K.; Yamaji, N.; Rahman, M.O.; Suto, M.; Takemoto, Y.; Garcia-Casal. M.N.; Ota, E. Nutrition-specific interventions for preventing and controlling anaemia throughout the life cycle: An overview of systematic reviews. Cochrane Database Syst. Rev. 2021, 9, CD013092. [Google Scholar] [CrossRef]
- Eichler, K.; Wieser, S.; Rüthemann, I.; Brügger, U. Effects of micronutrient fortified milk and cereal food for infants and children: A systematic review. BMC Public Health 2012, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Gupta, P.M.; Cogswell, M.E.; Hamner, H.C.; Perrine, C.G. Iron Content of Commercially Available Infant and Toddler Foods in the United States; 2015. Nutrients 2020, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Sadighi, J.; Nedjat, S.; Rostami, R. Systematic review and meta-analysis of the effect of iron-fortified flour on iron status of populations worldwide. Public Health Nutr. 2019, 22, 3465–3484. [Google Scholar] [CrossRef] [PubMed]
- Biemi, F.D.; Ganji, V. Temporal Relation between Double Fortification of Wheat Flour with Iron and Folic Acid; and Markers and Prevalence of Anemia in Children. Nutrients 2021, 13, 2013. [Google Scholar] [CrossRef]
- Garcia-Casal, M.N.; Peña-Rosas, J.P.; De-Regil, L.M.; Gwirtz, J.A.; Pasricha, S.R. Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations. Cochrane Database Syst. Rev. 2018, 12, CD010187. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; Mithra, P.; Unnikrishnan, B.; Kumar, N.; De-Regil, L.M.; Nair, N.S.; Garcia-Casal, M.N.; Solon, J.A. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database Syst. Rev. 2019, 25, CD009902. [Google Scholar] [CrossRef]
- Mahapatra, S.; Parker, M.E.; Dave, N.; Zobrist, S.C.; Shajie Arul, D.; King, A.; Betigeri, A.; Sachdeva, R. Micronutrient-fortified rice improves haemoglobin; anaemia prevalence and cognitive performance among schoolchildren in Gujarat; India: A case-control study. Int. J. Food. Sci. Nutr. 2021, 72, 690–703. [Google Scholar] [CrossRef]
- Huo, J.S.; Yin, J.Y.; Sun, J.; Huang, J.; Lu, Z.X.; Regina, M.P.; Chen, J.S.; Chen, C.M. Effect of NaFeEDTA-Fortified Soy Sauce on Anemia Prevalence in China: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biomed. Environ. Sci. 2015, 28, 788–798. [Google Scholar] [CrossRef]
- Garcia-Casal, M.N.; Peña-Rosas, J.P.; Mclean, M.; De-Regil, L.M.; Zamora, G.; Consultation Working Groups. Fortification of condiments with micronutrients in public health: From proof of concept to scaling up. Ann. N. Y. Acad. Sci. 2016, 1379, 38–47. [Google Scholar] [CrossRef]
- Jalal, C.; Wuehler, S.; Osendarp, S.; De-Regil, L.M. Estimating nutrient fortification levels in condiments and seasonings for public health programs: Considerations and adaptations. Ann. N. Y. Acad. Sci. 2016, 1379, 28–37. [Google Scholar] [CrossRef][Green Version]
- Vinod Kumar, M.; Erhardt, J. Improving Micronutrient Status of Children and Women in Rural Communities in India Using Crystal Salt Enriched with Multiple Micronutrients. J. Nutr. Sci. Vitaminol. 2021, 67, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Cyriac, S.; Djimeu, E.W.; Mbuya, M.N.N.; Neufeld, L.M. Can Double Fortification of Salt with Iron and Iodine Reduce Anemia; Iron Deficiency Anemia; Iron Deficiency; Iodine Deficiency; and Functional Outcomes? Evidence of Efficacy, Effectiveness, and Safety. J. Nutr. 2021, 151 (Suppl. S1), 15S–28S. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.U.; Mahmudiono, T. Effectiveness of Food Fortification in Improving Nutritional Status of Mothers and Children in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 2133. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.; Dewey, K.G. Perspective: Putting the youngest among us into the nutrition “call for action” for food fortification strategies. Am. J. Clin. Nutr. 2021, 114, 1257–1260. [Google Scholar] [CrossRef]
- Global Fortification Data Exchange. Available online: https://fortificationdata.org/map-number-of-nutrients/ (accessed on 26 December 2021).
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R.; World Health Organization/Food and Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. 2006. Available online: https://www.who.int/publications/i/item/9241594012 (accessed on 16 August 2021).
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn; Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Osendarp, S.J.M.; Martinez, H.; Garrett, G.S.; Neufeld, L.M.; De-Regil, L.M.; Vossenaar, M.; Darnton-Hill, I. Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food Nutr. Bull. 2018, 39, 315–331. [Google Scholar] [CrossRef]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified crops for tackling micronutrient deficiencies—What impact are these having in developing countries and could they be of relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Fothergill, A.; Hackl, L.S.; Haas, J.D.; Mehta, S. Iron biofortification interventions to improve iron status and functional outcomes. Proc. Nutr. Soc. 2019, 78, 197–207. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Mehta, S.; Villalpando, S.; Mundo-Rosas, V.; Luna, S.V.; Rahn, M.; Shamah-Levy, T.; Beebe, S.E.; Haas, J.D. A Randomized Feeding Trial of Iron-Biofortified Beans on School Children in Mexico. Nutrients 2019, 11, 381. [Google Scholar] [CrossRef]
- Anderson, G.J. Iron Biofortification: Who Gives a Bean? J. Nutr. 2020, 150, 2841–2842. [Google Scholar] [CrossRef]
- Kodkany, B.S.; Bellad, R.M.; Mahantshetti, N.S.; Westcott, J.E.; Krebs, N.F.; Kemp, J.F.; Hambidge, K.M. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J. Nutr. 2013, 143, 1489–1493, Erratum in J. Nutr. 2013, 143, 2055. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.C.; Barsotti, R.C.F.; Maltez, H.F.; Lopes Júnior, C.A.; Barbosa, H.S. Expanding information on the bioaccessibility and bioavailability of iron and zinc in biofortified cowpea seeds. Food Chem. 2021, 347, 129027. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.M.; Costa, N.M.B.; Nutti, M.R.; Tako, E.; Martino, H.S.D. Advantages and limitations of in vitro and in vivo methods of iron and zinc bioavailability evaluation in the assessment of biofortification program effectiveness. Crit. Rev. Food. Sci. Nutr. 2018, 58, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.J.; Griffin, I. Iron Balance and Iron Nutritional Status in Preterm Infants during the First Four Months of Life. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 403–407. [Google Scholar] [CrossRef]
- Kleinman, R.E. Expert Recommendations on Iron Fortification in Infants. J. Pediatr. 2015, 167 (Suppl. S4), S48–S49. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Conlon, C.A.; Kruger, R.; Coad, J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: A review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef]
- Ahmad Fuzi, S.F.; Koller, D.; Bruggraber, S.; Pereira, D.I.; Dainty, J.R.; Mushtaq, S. A 1-h time interval between a meal containing iron and consumption of tea attenuates the inhibitory effects on iron absorption: A controlled trial in a cohort of healthy UK women using a stable iron isotope. Am. J. Clin. Nutr. 2017, 106, 1413–1421. [Google Scholar] [CrossRef]
- World Health Organization. Delayed Clamping of the Umbilical Cord to Reduce Infant Anaemia. 2014. Available online: http://apps.who.int/iris/bitstream/handle/10665/120074/WHO_RHR_14.19_eng.pdf;jsessionid=35643E5ED8760F892DD23B5164321A96?sequence=1 (accessed on 19 June 2021).
- Sundararajan, S.; Rabe, H. Prevention of iron deficiency anemia in infants and toddlers. Pediatr. Res. 2021, 89, 63–73. [Google Scholar] [CrossRef]
- Cofnas, N. Is vegetarianism healthy for children? Crit. Rev. Food. Sci. Nutr. 2019, 59, 2052–2060. [Google Scholar] [CrossRef]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Yip, R. Prevention and control of iron deficiency: Policy and strategy issues. J. Nutr. 2002, 132 (Suppl. S4), 802S–805S. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Jefferds, M.E.D.; Ota, E.; da Silva Lopes, K.; De-Regil, L.M. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst. Rev. 2020, 2, CD008959. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K.; Rao, R. Approaches for Reducing the Risk of Early-Life Iron Deficiency-Induced Brain Dysfunction in Children. Nutrients 2018, 10, 227. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Mzembe, G.; Moya, E.; Verhoef, H. Iron deficiency anaemia in sub-Saharan Africa: A review of current evidence and primary care recommendations for high-risk groups. Lancet Haematol. 2021, 8, e732–e743. [Google Scholar] [CrossRef]
- Neuberger, A.; Okebe, J.; Yahav, D.; Paul, M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev. 2016, 2, CD006589. [Google Scholar] [CrossRef]
- Spottiswoode, N.; Duffy, P.E.; Drakesmith, H. Iron; anemia and hepcidin in malaria. Front. Pharmacol. 2014, 5, 125. [Google Scholar] [CrossRef]
- Clark, M.A.; Goheen, M.M.; Fulford, A.; Prentice, A.M.; Elnagheeb, M.A.; Patel, J.; Fisher, N.; Taylor, S.M.; Kasthuri, R.S.; Cerami, C. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat. Commun. 2014, 5, 4446. [Google Scholar] [CrossRef]
- Gera, T.; Sachdev, H.P.; Nestel, P.; Sachdev, S.S. Effect of iron supplementation on haemoglobin response in children: Systematic review of randomised controlled trials. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 468–486. [Google Scholar] [CrossRef]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. S1), 359S–371S. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1688S–1693S. [Google Scholar] [CrossRef]
- Lönnerdal, B. Excess iron intake as a factor in growth; infections; and development of infants and young children. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1681S–1687S. [Google Scholar] [CrossRef] [PubMed]
- Christian, P. Iron in infancy and long-term development. Arch. Pediatr. Adolesc. Med. 2012, 166, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Jullien, S. Screening of iron deficiency anaemia in early childhood. BMC Pediatr. 2021, 21 (Suppl. S1), 337. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Pasricha, S.R.; Martinez, R.X.; Lopez-Perez, L.; Peña-Rosas, J.P. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst. Rev. 2021, 5, CD011817. [Google Scholar] [CrossRef]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- World Health Organization. Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. 2020. Available online: https://www.who.int/docs/default-source/micronutrients/ferritin-guideline/ferritin-guidelines-brochure.pdf?sfvrsn=76a71b5a_4 (accessed on 3 August 2021).
- Pérez-Acosta, A.; Duque, X.; Trejo-Valdivia, B.; Flores-Huerta, S.; Flores-Hernández, S.; Martínez-Andrade, G.; González-Unzaga, M.; Turnbull, B.; Escalante-Izeta, E.; Klünder-Klünder, M.; et al. Cut-off points for serum ferritin to identify low iron stores during the first year of life in a cohort of Mexican infants. Matern. Child. Nutr. 2021, 17, e13205. [Google Scholar] [CrossRef]
- Galetti, V.; Stoffel, N.U.; Sieber, C.; Zeder, C.; Moretti, D.; Zimmermann, M.B. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine 2021, 39, 101052. [Google Scholar] [CrossRef]
- Sezgin, G.; Li, L.; Westbrook, J.; Wearne, E.; Azar, D.; McLeod, A.; Pearce, C.; Ignjatovic, V.; Monagle, P.; Georgiou, A. Influence of serum iron test results on the diagnosis of iron deficiency in children: A retrospective observational study. BMJ Open 2021, 11, e046865. [Google Scholar] [CrossRef]
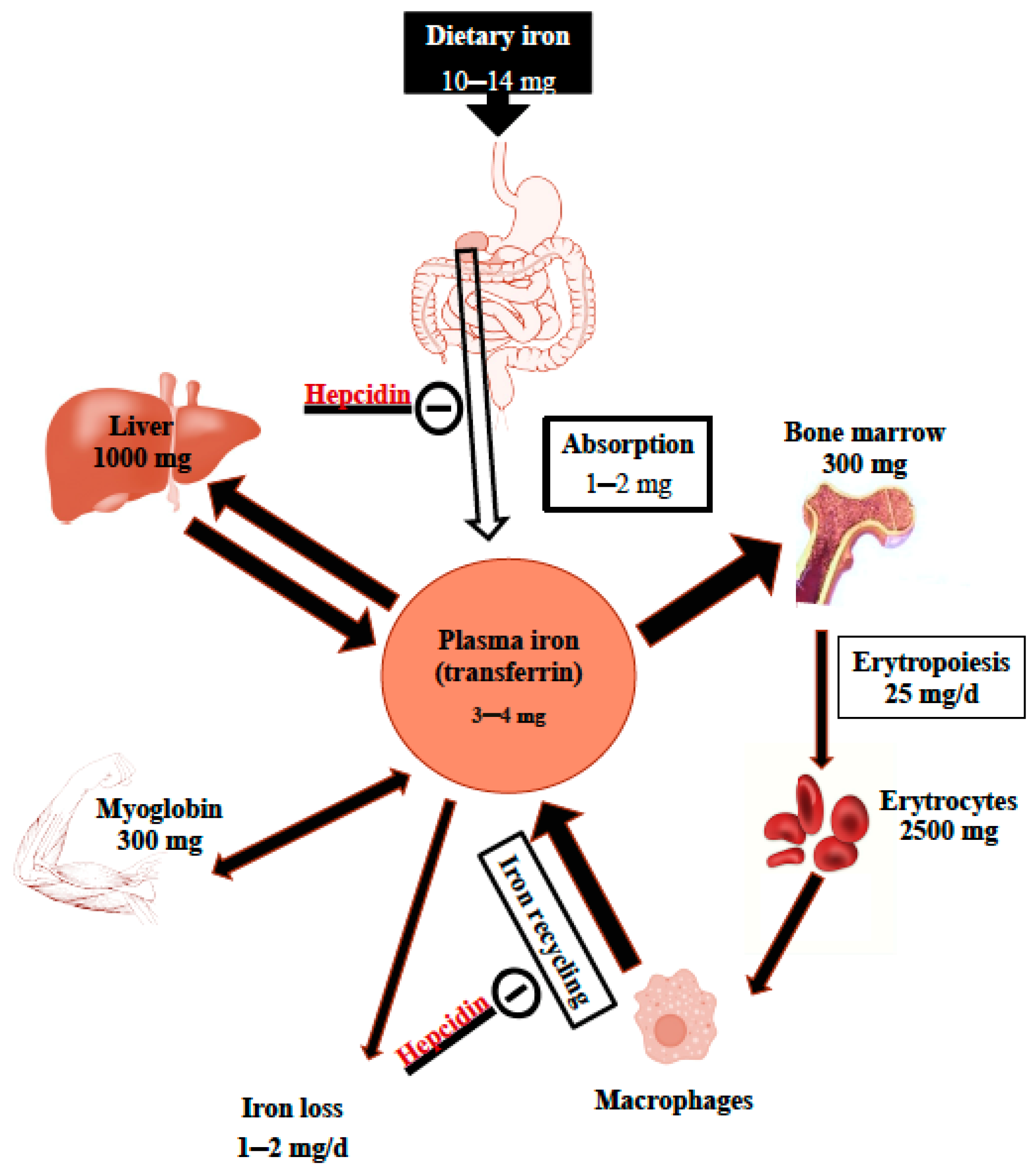
| Stage 1 Iron Depletion | Stage 2 Iron Deficiency Erythropoiesis without Anemia | Stage 3 Iron Deficiency Anemia | |
|---|---|---|---|
| Body iron stores | Reduced | ↓↓ | ↓↓↓ |
| Symptoms | Nil or mild (fatigue, poor concentration) | Nil or mild (fatigue, poor concentration) | Pallor, anorexia, irritability, systolic flow murmur, tachycardia, lethargy |
| Ferritin | ↓ | ↓↓ | ↓↓↓ |
| Transferrin saturation | → | ↓ | ↓ |
| Soluble transferrin receptors | → | ↑ | ↑ |
| Zinc protoporphyrin | → | ↑ | ↑ |
| Concentration of hemoglobin in reticulocytes | → | ↓ | ↓ |
| Hepcidin | ±↓ | ↓ | ↓↓ |
| Mean corpuscular volume | → | → | ↓ |
| Hemoglobin | → | → or ±↓ | ↓ |
| Bone marrow stainable iron | ± | 0 | 0 |
| IOM [57] | WHO/FAO [59] | EFSA [6,58] | |||||
|---|---|---|---|---|---|---|---|
| Age | DRV | Age | DRV | Age | DRV | ||
| 0–6 months | AI: 0.27 | - | 0–6 months | AI: 0.30 | |||
| EAR a | PRI | PRI b | EAR b | PRI b | |||
| 7–11 months | 6.9 | 11 | 7–11 months | 9.3 | 7–11 months | 8 | 11 |
| 1–3 years | 3 | 7 | 1–3 years | 5.8 | 1–6 years | 5 | 7 |
| 4–8 years | 4.1 | 10 | 4–6 years | 6.3 | |||
| 9–13 years Boys Girls | 5.9 5.7 | 8 8 | 7–10 years | 8.9 | |||
| 7–11 years | 8 | 11 | |||||
| 11–14 years | |||||||
| Boys | 14.6 | ||||||
| 14–18 years Boys Girls | 7.7 7.9 | 11 15 | Girls | 14 c, 32.7 | 12–17 years | ||
| 15–17 years | Boys | 8 | 11 | ||||
| Boys | 18.8 | Girls | 7 | 13 | |||
| Girls | 31 | ||||||
| ≥18 years | ≥18 years | ||||||
| Boys | 13.7 | Boys | 6 | 11 | |||
| Girls | 29.4 | Girls | 7 | 16 | |||
| Author [Reference] (Year) | Country (Study) | Age (Months, Years) | Average DII (mg/d) | Source of DRV | % DII < DRV * | ID/IDA Prevalence * |
|---|---|---|---|---|---|---|
| EFSA [3] (Review 2015) | Finland, France, Germany, Italy, Ireland, Latvia, Netherlands, Spain, Sweden, UK | 0–11 m | 2.6 to 6.0 | EFSA-EAR | >50% | -/- |
| 12–35 m | 5.0 to 7.0 | <50% | ||||
| 3–10 y | 7.5 to 11.5 | |||||
| 10–18 y | 9.2 to 14.7 | |||||
| Eussen et al. [22] (Review 2015) | Albania, Austria, Belgium, Denmark, Estonia, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Netherlands, Norway, Poland, Spain, Sweden, Turkey, UK | 6–11 m | 5.0 to 9.7 | EFSA-EAR | 6 to 60% | 0% to 21%/- |
| 12–35 m | 1.6 to 8.5 | 4 to 64% | 0% to 48% (85% if breastfed)/0 to 42% | |||
| Gibson and Sidnell [63] (2014) | UK | 12–17 m | 6.4 | British Lower Reference Nutrient Intake | 13% | -/- |
| 18–35 m | 6.4 | 7% | ||||
| Chouraqui et al. [64] 2020 | France (Nutri-Bébé study) | 0.5–5.9 m | 6.4 | EFSA-EAR | 0% | -/- |
| 6–11 m | 8.1 | 52% | ||||
| 12–35 m | 7.1 | 30% | ||||
| ANSES [65] 2017 | France (INCA 3) | 0–11 m | 6.6 | EFSA-EAR | <50% ** | -/- |
| 1–3 y | 8.5 | <50% ** | ||||
| 4–6 y | 7.3 | >50% ** | ||||
| 7–10 y | 9.0 | <50% ** | ||||
| 11–14 y | 10.1 | <50% ** | ||||
| 15–17 y | 9.4 | <50% ** | ||||
| Eldridge et al. [66] (2019) | USA (FITS 2016 study) | 0.5–5.9 m | 7.6 | - | - | -/- |
| 6–11.9 m | 13.4 | |||||
| 12–23.9 m | 8.6 | |||||
| 24–47.9 m | 9.7 | |||||
| Abrams et al. [67] | USA (daily absorbed iron calculated on the basis of data from the FITS 2016 study) | 6–12 m | 0.7 | - | - | -/- |
| Breastfed | 0.3 | |||||
| Formula fed | 0.9 | |||||
| Atkins et al. [68] (2020) | Australia | 2–5 y | 7.7 | IOM *** | 10.1% | -/- |
| Harika et al. [69] (review 2017) | Ethiopia, Kenya, Nigeria, South Africa | 0–6 y | 3.5 to 28 | WHO-EAR | 13 to 100% | 12% to 29%/- |
| Mesias et al. [58] (Review 2013) | Austria, Bolivia, Brazil, Canada, Denmark, England, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Norway, Perou, Scotland, Spain, Sweden, Turkey, USA | 10–19 y Boys | 9.0 to 24.5 | - | - | -/- |
| Girls | 8.7 to 17.2 |
| Meat * and Eggs | Vegetables | ||
|---|---|---|---|
| Calf’s kidney | 12.0 (7.9–15) | Lentil | 8.0 (5.0–13.0) |
| Eggs | 8.8 | Soya bean (dry) | 6.6 (6.6–8.7) |
| Calf’s liver | 7.9 (5.7–9.3) | Dry beans | 6.5 |
| Chicken’s liver | 7.4 | Chickpea | 6.1 (4.9–7.2) |
| Black pudding | 6.4 (6.4–6.5) | Topinambour a | 3.7 (3.4–4.0) |
| Sheep heart | 6.1 | Tofu | 3.7 (2.0–5.4) |
| Sheep brain | 3.8 (2.0-6.7) | Spinach | 3.4 (1.3–7.7) |
| Rabbit’s meat | 2.7 (1.8–6.0) | Water cress | 3.1 (2.0–7.2) |
| Duck | 2.7 | Fennel | 2.7 |
| Ham | 2.3 (1.7–2.9) | Lamb’s lettuce | 2.0 |
| Beef | 2.1 (1.7–2.4) | Kale | 1.9 |
| Veal | 2.1 (1.5–3.0) | Pea | 1.6 (1.3–2.0) |
| Goose | 1.9 (1.8–2.0) | Endive b | 1.4 (1.0–1.7) |
| Mutton | 1.8 (1.5–2.7) | Mushroom | 1.2 (0.7–2.0) |
| Pork | 1.8 (0.9–2.3) | Cassava c | 1.2 |
| Lamb | 1.6 (1.2–1.9) | Zucchini | 1.0 (0.5–2.4) |
| Turkey | 1.0 (0.8–2.0) | Broccoli | 0.8 (0.7–1.1) |
| Chicken | 0.7 (0.6–2.0) | Leek | 0.8 (0.6–1.1) |
| Seafood | Fruits | ||
| Clams | 7.5 [115] | Dried apricot | 4.4 (3.5–5.5) |
| Anchovy | 4.9 | Dried fig | 3.3 (3.0–4.0) |
| Mussel | 4.2 (3.6–6) | Prune | 2.3 (1.0–3.9) |
| Oyster | 3.1 (2.6–7.5) | Grape (dried) | 2.3 |
| Sardine | 2.4 (1.3–3.0) | Date (dried) | 1.9 (1.5–2.1) |
| Shrimp | 2.3 [115] | Green olive (marinated) | 1.8 (1.6–2.0) |
| Herring | 1.1 (0.9–1.3) | Black currant | 1.3 (0.9–1.2) |
| Tuna | 1.0 | Durian d | 1.0 (0.8–1.1) |
| Salmon | 0.6 (0.4–1.5) | Raspberry | 1.0 (0.9–1.0) |
| Cod | 0.3 (0.2–0.5) | Kiwi fruit | 0.8 (0.3–1.6) |
| Strawberry | 0.7 (0.6–1.3) | ||
| Bread and Cereals | Nuts | ||
| Wheat germ | 8.6 (7.9–8.9) | Pistachio | 7.3 |
| Quinoa | 8.0 (7.0–11.0) | Almond | 4.1 (4.0–4.4) |
| Rolled oats | 5.8 (4.6–6.3) | Hazelnut | 3.8 (3.0–4.5) |
| Sorghum | 5.7 | Cashew nut | 2.8 (1.8–3.8) |
| Rice (unpolished) | 3.2 (2.0–3.6) | Walnut | 2.5 (2.0–3.1) |
| Pasta made with eggs | 3.0 (1.0–4.4) | Pecan nut | 2.4 |
| Wheat flours | 2.2 (0.9–5.2) | Coconut | 2.3 (2.0–2.7) |
| Whole wheat bread | 2.0 (1.9–2.0) | Peanut roasted | 2.3 (2.1–2.7) |
| Corn flakes | 2.0 (1.3–2.7) | Peanut | 1.8 (1.8–5.9) |
| Rice (polished) | 0.9 (0.6–12.0) | Chestnut | 1.3 (0.9–1.7) |
| Miscellaneous | |||
| Honey | 1.3 (0.9–2.0) | ||
| Cane sugar (unrefined) | (1.0–8.0) | ||
| Chocolate >40% cocoa | 3.2 (2.5–4.4) | ||
| Baker yeast | 3.5 (2.1–4.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouraqui, J.-P. Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue. Nutrients 2022, 14, 1604. https://doi.org/10.3390/nu14081604
Chouraqui J-P. Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue. Nutrients. 2022; 14(8):1604. https://doi.org/10.3390/nu14081604
Chicago/Turabian StyleChouraqui, Jean-Pierre. 2022. "Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue" Nutrients 14, no. 8: 1604. https://doi.org/10.3390/nu14081604
APA StyleChouraqui, J.-P. (2022). Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue. Nutrients, 14(8), 1604. https://doi.org/10.3390/nu14081604





