Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
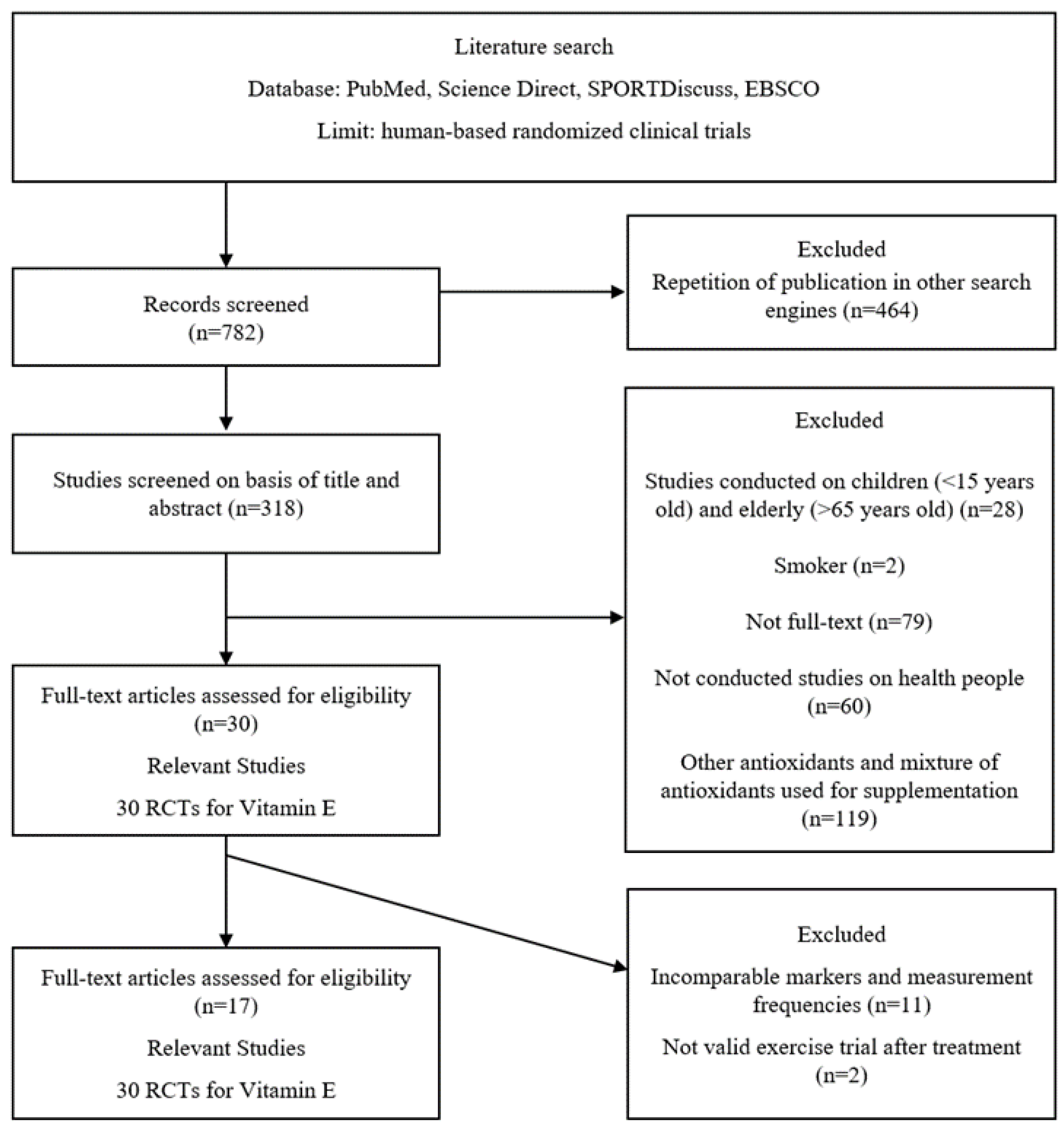
3.1. Literature Research
3.2. Characteristics of the Included Studies
3.3. Quality Analysis of RCT Included in the Meta-Analysis
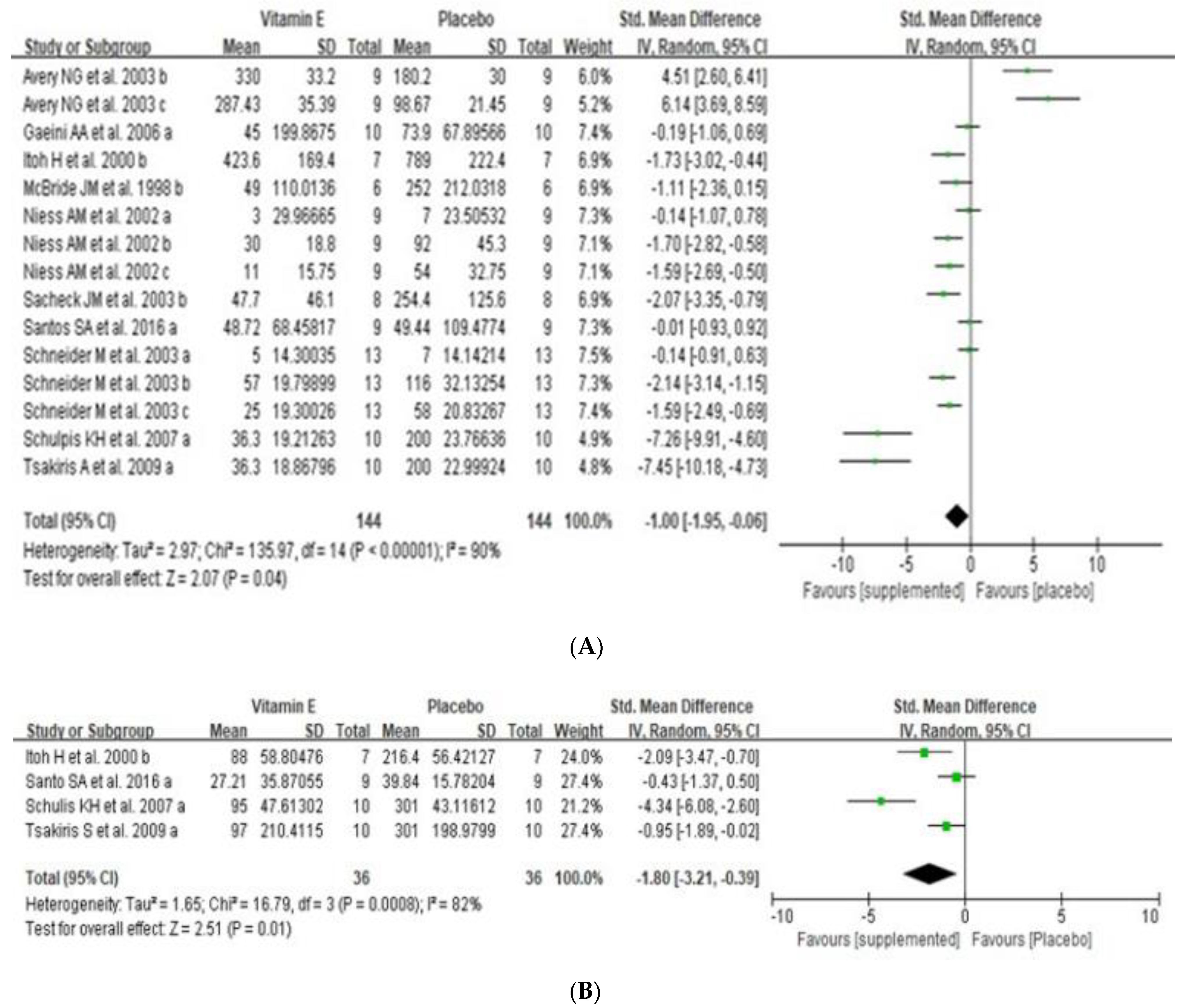
3.4. Effects of Dietary Vitamin E Supplementation on Exercise-Induced Muscle Damage
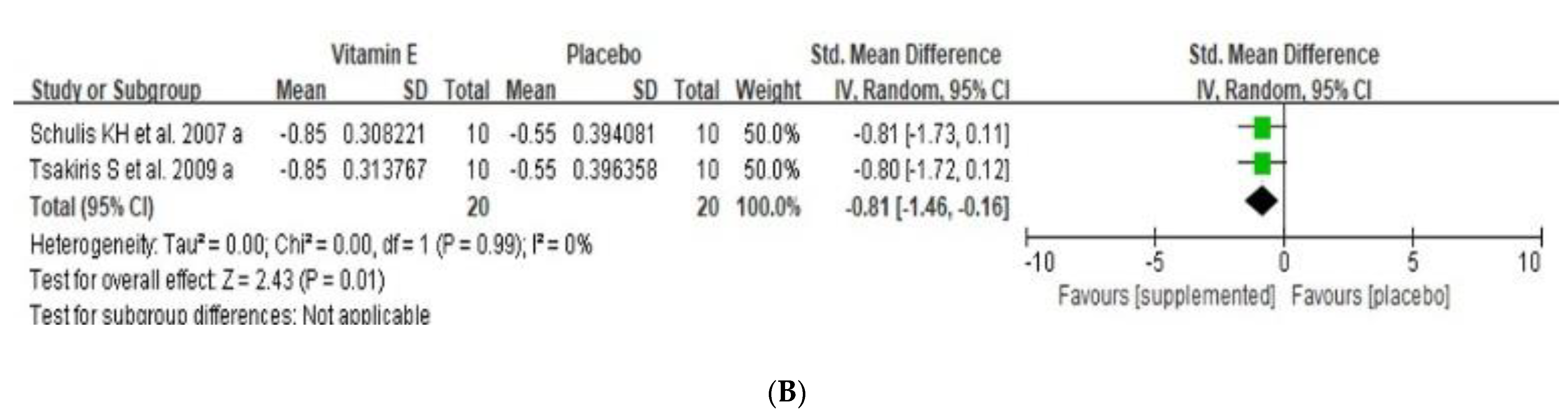
3.5. Effects of Dietary Vitamin E Supplementation on Exercise Induced Oxidative Stress
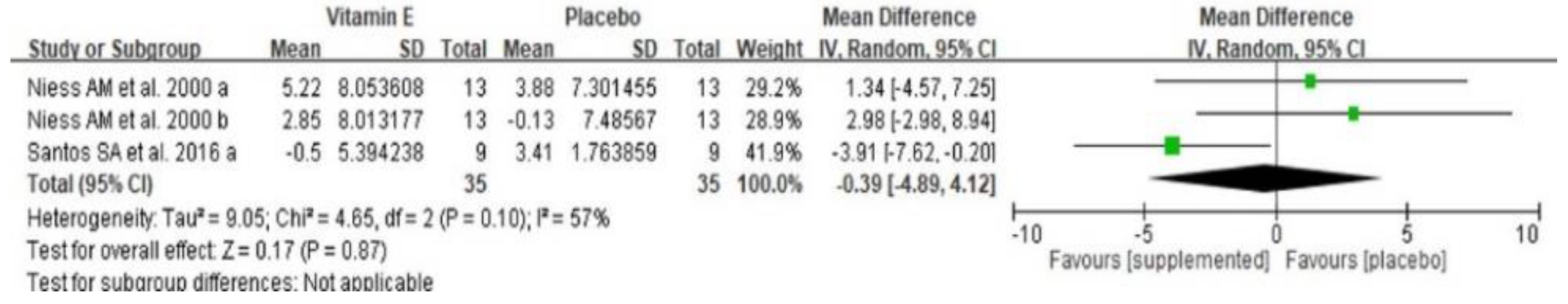
3.6. Effect of Dietary Vitamin E Supplementation on Exercise-Induced Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiney, H.; Machado, L. Benefits of Regular Aerobic Exercise for Executive Functioning in Healthy Populations. Psychon. Bull. Rev. 2013, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Iavicoli, I.; Calabrese, V. Hormesis. Hum. Exp. Toxicol. 2013, 32, 120–152. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Pingitore, A.; de Giuseppe, R.; Vigna, L.; Bamonti, F. Biomarkers Part II: Biomarkers to Estimate Bioefficacy of Dietary/Supplemental Antioxidants in Sport; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466567573. [Google Scholar]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Oxidative Stress and Delayed-Onset Muscle Damage after Exercise. Free Radic. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate Exercise Is an Antioxidant: Upregulation of Antioxidant Genes by Training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Peterson, J.M.; Bakkar, N.; Guttridge, D.C. NF-ΚB Signaling in Skeletal Muscle Health and Disease. Curr. Top. Dev. Biol. 2011, 96, 85–119. [Google Scholar]
- Pyne, D.B.; Smith, J.A.; Baker, M.S.; Telford, R.D.; Weidemann, M.J. Neutrophil Oxidative Activity Is Differentially Affected by Exercise Intensity and Type. J. Sci. Med. Sport 2000, 3, 44–54. [Google Scholar] [CrossRef]
- Jackson, N.D.; Gutierrez, G.M.; Kaminski, T. The Effect of Fatigue and Habituation on the Stretch Reflex of the Ankle Musculature. J. Electromyogr. Kinesiol. 2009, 19, 75–84. [Google Scholar] [CrossRef]
- Jackson, B.; Knapp, P.; Beauchamp, M.R. Origins and Consequences of Tripartite Efficacy Beliefs within Elite Athlete Dyads. J. Sport Exerc. Psychol. 2008, 30, 512–540. [Google Scholar] [CrossRef][Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Hanninen, O.; Sen, C.K. (Eds.) Physiological Antioxidants and Exercise Training. In Handbook of Oxidants and Antioxidants in Exercise; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.-M.; Azzi, A. The European Perspective on Vitamin E: Current Knowledge and Future Research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, S.; Karikas, G.A.; Parthimos, T.; Tsakiris, T.; Bakogiannis, C.; Schulpis, K.H. Alpha-Tocopherol Supplementation Prevents the Exercise-Induced Reduction of Serum Paraoxonase 1/Arylesterase Activities in Healthy Individuals. Eur. J. Clin. Nutr. 2009, 63, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Fehrenbach, E.; Schlotz, E.; Sommer, M.; Angres, C.; Tschositsch, K.; Battenfeld, N.; Golly, I.C.; Biesalski, H.K.; Northoff, H.; et al. Effects of RRR-α-Tocopherol on Leukocyte Expression of HSP72 in Response to Exhaustive Treadmill Exercise. Int. J. Sports Med. 2002, 23, 445–452. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; Kraemer, W.J.; Triplett-McBride, T.; Sebastianelli, W. Effect of Resistance Exercise on Free Radical Production. Med. Sci. Sports Exerc. 1998, 30, 67–72. [Google Scholar] [CrossRef]
- Bryant, R.J.; Ryder, J.; Martino, P.; Kim, J.; Craig, B.W. Effects of Vitamin E and C Supplementation Either Alone or in Combination on Exercise-Induced Lipid Peroxidation in Trained Cyclists. J. Strength Cond. Res. 2003, 17, 792–800. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Silveira, P.C.L.; Tuon, T.; de Souza, C.T.; Dal-Pizzol, F.; Pinho, R.A. Vitamin E Supplementation Decreases Muscular and Oxidative Damage but Not Inflammatory Response Induced by Eccentric Contraction. J. Physiol. Sci. 2010, 60, 51. [Google Scholar] [CrossRef]
- Satoshi, S.; Kiyoji, T.; Hiroyo, K.; Fumio, N. Exercise-Induced Lipid Peroxidation and Leakage of Enzymes before and after Vitamin E Supplementation. Int. J. Biochem. 1989, 21, 835–838. [Google Scholar] [CrossRef]
- Gaeini, A.A.; Rahnama, N.; Hamedinia, M.R. Effects of Vitamin E Supplementation on Oxidative Stress at Rest and after Exercise to Exhaustion in Athletic Students. J. Sports Med. Phys. Fit. 2006, 46, 458–461. [Google Scholar]
- Avery, N.G.; Kaiser, J.L.; Sharman, M.J.; Scheett, T.P.; Barnes, D.M.; Gómez, A.L.; Kraemer, W.J.; Volek, J.S. Effects of Vitamin E Supplementation on Recovery from Repeated Bouts of Resistance Exercise. J. Strength Cond. Res. 2003, 17, 801–809. [Google Scholar] [CrossRef]
- Santos, S.A.; Silva, E.T.; Caris, A.V.; Lira, F.S.; Tufik, S.; dos Santos, R.V.T. Vitamin E Supplementation Inhibits Muscle Damage and Inflammation after Moderate Exercise in Hypoxia. J. Hum. Nutr. Diet. 2016, 29, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Sommer, M.; Schneider, M.; Angres, C.; Tschositsch, K.; Golly, I.C.; Battenfeld, N.; Northoff, H.; Biesalski, H.K.; Dickhuth, H.-H.; et al. Physical Exercise-Induced Expression of Inducible Nitric Oxide Synthase and Heme Oxygenase-1 in Human Leukocytes: Effects of α -Tocopherol Supplementation. Antioxid. Redox Signal. 2000, 2, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Stepanyan, V.; Crowe, M.; Haleagrahara, N.; Bowden, B. Effects of Vitamin E Supplementation on Exercise-Induced Oxidative Stress: A Meta-Analysis. Appl. Physiol. Nutr. Metab. 2014, 39, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Schulpis, K.H.; Moukas, M.; Parthimos, T.; Tsakiris, T.; Parthimos, N.; Tsakiris, S. The Effect of α-Tocopherol Supplementation on Training-Induced Elevation of S100B Protein in Sera of Basketball Players. Clin. Biochem. 2007, 40, 900–906. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Parker, R.I.; Hagan-Burke, S. Useful Effect Size Interpretations for Single Case Research. Behav. Ther. 2007, 38, 95–105. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of Vitamin E and Eccentric Exercise on Selected Biomarkers of Oxidative Stress in Young and Elderly Men. Free Radic. Biol. Med. 2003, 34, 1575–1588. [Google Scholar] [CrossRef]
- Itoh, H.; Ohkuwa, T.; Yamazaki, Y.; Shimoda, T.; Wakayama, A.; Tamura, S.; Yamamoto, T.; Sato, Y.; Miyamura, M. Vitamin E Supplementation Attenuates Leakage of Enzymes Following 6 Successive Days of Running Training. Int. J. Sports Med. 2000, 21, 369–374. [Google Scholar] [CrossRef]
- Viitala, P.E.; Newhouse, I.J.; LaVoie, N.; Gottardo, C. The Effects of Antioxidant Vitamin Supplementation on Resistance Exercise Induced Lipid Peroxidation in Trained and Untrained Participants. Lipids Health Dis. 2004, 3, 14. [Google Scholar] [CrossRef][Green Version]
- Schneider, M.; Niess, A.M.; Rozario, F.; Angres, C.; Tschositsch, K.; Battenfeld, N.; Schäffer, M.; Northoff, H.; Dickhuth, H.-H.; Fehrenbach, E.; et al. Vitamin E Supplementation Does Not Increase the Vitamin C Radical Concentration at Rest and after Exhaustive Exercise in Healthy Male Subjects. Eur. J. Nutr. 2003, 42, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Nieβ, A.M.; Grünert-Fuchs, M.; Poch, B.; Speit, G. Vitamin E Prevents Exercise-Induced DNA Damage. Mutat. Res. Lett. 1995, 346, 195–202. [Google Scholar] [CrossRef]
- Akova, B.; Sürmen-Gür, E.; Gür, H.; Dirican, M.; Sarandöl, E.; Küçükoglu, S. Exercise-Induced Oxidative Stress and Muscle Performance in Healthy Women: Role of Vitamin E Supplementation and Endogenous Oestradiol. Eur. J. Appl. Physiol. 2001, 84, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses After Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Mirabilio, A.; Pennelli, A.; Cacchio, M.; di Baldassarre, A.; Gallina, S.; Passerini, A.; Amicarelli, F. Differential Impact of Acute Bout of Exercise on Redox- and Oxidative Damage-Related Profiles between Untrained Subjects and Amateur Runners. Physiol. Res. 2010, 59, 953–961. [Google Scholar] [CrossRef]
- Maughan, R.J.; Donnelly, A.E.; Gleeson, M.; Whiting, P.H.; Walker, K.A.; Clough, P.J. Delayed-Onset Muscle Damage and Lipid Peroxidation in Man after a Downhill Run. Muscle Nerve 1989, 12, 332–336. [Google Scholar] [CrossRef]
- Pedersen, B.K. Effects of Exercise on Lymphocytes and Cytokines. Br. J. Sports Med. 2000, 34, 246–251. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37. [Google Scholar] [CrossRef]


| Study | Year | Study | Supplementation | Subjects | Exercise Protocol | Measurement | Muscle Damage | Oxidative Stress | Inflammation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (First Author) | Design | Duration (day) | Daily Dosage (IU) | (Number, Age) | Marker | Effect | Marker | Effect | Marker | Effect | |||
| Santos S.A. [23] | 2016 | RCT, double-blind design | Single dose (1) | 302.5 | 9, healthy males, 24.2 ± 2.2 years | 60 min at an intensity of 70% VT I in normoxia and hypoxia simulating an altitude of 4200 m | before and immediately, at 1 h after exercise | CK LDH | ↓ ↓ | IL-6 | ↓ | ||
| Schulpis K.H. [26] | 2007 | RCT | 30 | 300 | 10, male basketball players, 18.5 ± 0.6 years | Stretching, technical-tactical part, a heavy training load part | before, immediately after the training | CK LDH | ↓ ↓ | TAS | ↓ | ||
| Gaeini A.A. [21] | 2006 | RCT, double-blind design | 42 | 672 | 20, male students, 23.1 ± 2.0 years | incremental exercise test | before and immediately after exercise | CK | ↔ | MDA | ↔ | ||
| Avery N.G. [22] | 2003 | RCT, double-blind design | 31 | 1200 | 18, healthy men, 22.7 ± 4.1 years | repeated bouts of whole-body resistance exercise | each day beginning with the first exercise session at, 24 h and 48 h after exercise | CK | ↑ | MDA | ↔ | ||
| Sacheck J.M. [30] | 2003 | RCT, double-blind design | 84 | 1000 | 16, healthy men, 26.4 ± 3.3 years | ran downhill for 45 min at 75% VO2 max | before (baseline) and immediately after exercise (0 h), and at 6, 24, and 72 h after exercise | CK | ↓ | MDA | ↔ | ||
| Itoh H. [31] | 2000 | RCT, double-blind design | 28 | 1200 | 14, male students, 21.1 ± 2.3 years | 6-day running training session (48.3 ± 5.7 km × day–1) | baseline, the day immediately before, the next day after, and three weeks after the 6 day running training | CK | ↓ | MDA | ↓ | ||
| Mcbride J.M. [17] | 1998 | RCT | 14 | 1200 | 12, weight-trained males VE: 22.2 ± 0.7 years P: 22.0 ± 0.9 years | heavy resistance exercise | before and at 24 h and 48 h after exercise | CK | ↓ | MDA | ↑ | ||
| Bryant R.J. [18] | 2003 | RCT, | 21 | 400 | 7, male cyclists, 22.3 ± 2 years | 60 min steady state ride and a 30-min performance ride | before and immediately after exercise | MDA | ↓ | ||||
| Viitala P.E. [32] | 2004 | RCT, double-blind, crossover design | 14 | 1318 | 27, males and females, ages of 19 and 30 years | resistance exercise test | before and immediately and 6 h after exercise | MDA | ↔ | ||||
| Niess A.M. [16] | 2002 | RCT, double-blind, crossover design | 8 | 500 | 9, healthy males, 25.3 ± 1.0 years | incremental exercise test + continuous run | before the beginning of supplementation and 3, 24 and 48 h after the end of the continuous run | CK | ↓ | ||||
| Schneider M. [33] | 2003 | RCT, crossover design | 8 | 500 | 13, males, 26.5 ± 0.9 years | incremental exercise test + continuous run | at rest, 0, 0.25, 1, 3, 24 and 48 h after exercise. | CK | ↔ | ||||
| Tsakiris S. [15] | 2009 | RCT | 30 | 300 | 10, male basketball players, 18.5 ± 0.6 years | training program two or three times a week | before, immediately after the training | CK LDH | ↓ ↓ | TAS | ↓ | ||
| Hartmann A. [34] | 1995 | RCT | 14 | 1200 | 8, men, 29–34 years | a single bout of exhaustive exercise | before and at 15 min and 24 h after exercise | MDA | ↓ | ||||
| Sumida [20] | 1989 | RCT | 28 | 447 | 21, healthy male college students, 20.3 ± 0.3 years | incremental exercise test | before, immediately after exhaustion, and at 1 and 3 h in the recovery period. | MDA | ↓ | ||||
| Silva L.A. [19] | 2010 | RCT, double-blind design | 14 | 800 | 21, male volunteers, 22.5 ± 4 years | EE | days 0, 2, 4, and 7 after EE | MDA | ↓ | ||||
| Akova B. [35] | 2001 | RCT | 56 | 300 | 18, sedentary women, 19–35 years | fatigue test | before and after the exercise | MDA | ↔ | ||||
| Niess A.M. [24] | 2000 | RCT, double-blind design | 56 | 500 | 38, triathletes, VE: 35.2 ± 1.6 years P: 39.2 ± 1.4 years | the race included a 3.9-km ocean swim, 180-km bike race and 42-km run | before the race, at 0 h, 3 h, 24 h, and 48 h postrace | IL-6 | ↑ | ||||
| Group | No. of Subject | Std, Mean Difference in CK, U/L (95%CI) | p | Pheterogeneity | I2 (%) |
|---|---|---|---|---|---|
| Total | 288 | −1.00, (−1.95 to −0.06) | 0.04 | <0.00001 | 90 |
| Measurement timepoint | |||||
| Immediately after exercise | 122 | −1.89, (−3.39 to −0.39) | 0.01 | <0.00001 | 91 |
| at 24 h after exercise | 104 | −0.084, (−2.31 to 0.63) | 0.26 | <0.00001 | 88 |
| at 48 h after exercise | 61 | 0.71, (−2.43 to 3.86) | 0.66 | <0.00001 | 94 |
| Daily dosage | |||||
| ≤500 | 190 | −1.94, (−2.99 to −0.89) | 0.0003 | <0.00001 | 88 |
| >500 | 98 | 0.73, (−1.27 to 2.73) | 0.48 | <0.00001 | 92 |
| Subject | |||||
| Athlete | 52 | −5.15, (−9.92 to −0.39) | 0.03 | <0.00001 | 93 |
| Non-athlete | 236 | −0.31, (−1.21 to 0.58) | 0.49 | <0.00001 | 88 |
| Group | No. of Subject | Std, Mean Difference in CK, U/L (95%CI) | p | Pheterogeneity | I2 (%) |
|---|---|---|---|---|---|
| Total | 249 | −0.17, (−0.52 to 0.18) | 0.35 | 0.04 | 42 |
| Measurement timepoint | |||||
| Immediately after exercise | 126 | −0.21, (−0.57 to 0.14) | 0.24 | 0.71 | 0 |
| at 24 h after exercise | 72 | 0.20, (−0.41 to 0.81) | 0.52 | 0.36 | 37 |
| at 48 h after exercise | 51 | −0.92, (−2.23 to 0.39) | 0.17 | 0.04 | 76 |
| Daily dosage | |||||
| ≤500 | 74 | −0.48, (−0.95 to −0.01) | 0.04 | 0.53 | 0 |
| >500 | 175 | −0.06, (−0.49 to 0.38) | 0.62 | 0.04 | 47 |
| Subject | |||||
| Athlete | 38 | −0.55, (−2.00 to 0.90) | 0.46 | 0.002 | 80 |
| Non-athlete | 211 | −0.15, (−0.43 to 0.13) | 0.30 | 0.68 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Eo, H.; Lim, J.G.; Lim, H.; Lim, Y. Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 1599. https://doi.org/10.3390/nu14081599
Kim M, Eo H, Lim JG, Lim H, Lim Y. Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(8):1599. https://doi.org/10.3390/nu14081599
Chicago/Turabian StyleKim, Myunghee, Hyeyoon Eo, Josephine Gahyun Lim, Hyunjung Lim, and Yunsook Lim. 2022. "Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 8: 1599. https://doi.org/10.3390/nu14081599
APA StyleKim, M., Eo, H., Lim, J. G., Lim, H., & Lim, Y. (2022). Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(8), 1599. https://doi.org/10.3390/nu14081599






