Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection Methods
2.3. Statistical Methods
3. Results
3.1. Description of the Sample Characteristics
3.2. VA and Blood Lipid Concentrations
3.3. Distribution of Blood Lipids in Different Vitamin A Status
3.4. Description of Dyslipidemias and Risk Factors
3.5. Description of Multi-Variable Associations of Vitamin A with Blood Lipids
3.6. Description of Serum VA and Dyslipidemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, C.J. Vitamin A. Lancet 1995, 345, 31–35. [Google Scholar] [CrossRef]
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. In WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; Brown, E., Arke, J., Eds.; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Pan, L.; Du, J.; Liu, F.; Jin, Y.; Ma, J.; Wang, L.; Jia, P.; Hu, Z.; Shan, G. Prevalence of, and biochemical and anthropometric risk factors for, dyslipidemia in children and adolescents aged 7 to 18 years in China: A cross-sectional study. Am. J. Hum. Biol. 2019, 31, e23286. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Dong, H.; Mi, J. Prevalence of dyslipidemia in Chinese children and adolescents: A meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi 2015, 36, 71–77. (In Chinese) [Google Scholar]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.-X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. J. Atheroscler. 2016, 248, 2–9. [Google Scholar] [CrossRef]
- Kit, B.K.; Kuklina, E.; Carroll, M.D.; Ostchega, Y.; Freedman, D.S.; Ogden, C.L. Prevalence of and Trends in Dyslipidemia and Blood Pressure Among US Children and Adolescents, 1999–2012. JAMA Pediatr. 2015, 169, 272–279. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 177–189. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G. The Roles of Vitamin A in the Regulation of Carbohydrate, Lipid, and Protein Metabolism. J. Clin. Med. 2014, 3, 453–479. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Yang, F.-C.; Xu, F.; Wang, T.-N.; Chen, G.-X. Roles of vitamin A in the regulation of fatty acid synthesis. World J. Clin. Cases 2021, 9, 4506–4519. [Google Scholar] [CrossRef]
- Miller, A.P.; Coronel, J.; Amengual, J. The role of β-carotene and vitamin A in atherogenesis: Evidences from preclinical and clinical studies. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158635. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Kaplan, A.G.; Salonen, J.T. Serum lycopene concentrations and carotid atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2003, 77, 133–138. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Paul-Labrador, M.J.; Fan, J.; Shircore, A.M.; Merz, C.N.B.; Dwyer, K.M. Progression of Carotid Intima-Media Thickness and Plasma Antioxidants: The Los Angeles Atherosclerosis Study. Arter. Thromb. Vasc. Biol. 2004, 24, 313–319. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Mannisto, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Yehya, A.; Baer, J.T.; Smiley, W.; Dollar, A.; Sperling, L. Hypervitaminosis A altering the lipid profile in a hypercholesterolemic patient. J. Clin. Lipidol. 2009, 3, 205–207. [Google Scholar] [CrossRef]
- Bershad, S.; Rubinstein, A.; Paterniti, J.R.; Le, N.A.; Poliak, S.C.; Heller, B.; Ginsberg, H.N.; Fleischmajer, R.; Brown, W.V. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N. Engl. J. Med. 1985, 313, 981–985. [Google Scholar] [CrossRef]
- Tallman, M.S.; Kwaan, H.C. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood 1992, 79, 543–553. [Google Scholar] [CrossRef]
- Miller, V.A.; Rigas, J.R.; Muindi, J.R.; Tong, W.P.; Venkatraman, E.; Kris, M.G.; Warrell, R.P. Modulation of all-trans retinoic acid pharmacokinetics by liarozole. Cancer Chemother. Pharmacol. 1994, 34, 522–526. [Google Scholar] [CrossRef]
- Prashanth, A.; Jeyakumar, S.M.; Giridharan, N.V.; Vajreswari, A. Vitamin A-enriched Diet Modulates Reverse Cholesterol Transport in Hypercholesterolemic Obese Rats of the WNIN/Ob Strain. J. Atheroscler. Thromb. 2014, 21, 1197–1207. [Google Scholar] [CrossRef][Green Version]
- Ribot, J.; Felipe, F.; Bonet, M.L.; Palou, A. Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes. Res. 2001, 9, 500–509. [Google Scholar] [CrossRef]
- Relevy, N.Z.; Harats, D.; Harari, A.; Ben-Amotz, A.; Bitzur, R.; Ruhl, R.; Shaish, A. Vitamin A-deficient diet accelerated atherogenesis in apolipoprotein E(−/−) mice and dietary beta-carotene prevents this consequence. Biomed. Res. Int. 2015, 9, 758723. [Google Scholar]
- Zhang, Y.; Li, R.; Li, Y.; Chen, W.; Zhao, S.; Chen, G. Vitamin A status affects obesity development and hepatic expression of key genes for fuel metabolism in Zucker fatty rats. Biochem. Cell Biol. 2012, 90, 548–557. [Google Scholar] [CrossRef]
- Reddy, M.R.G.; Kumar, C.P.; Mahesh, M.; Kumar, M.S.; Venkata, S.M.; Putcha, U.K.; Vajreswari, A.; Jeyakumar, S.M. Vitamin A deficiency suppresses high fructose-induced triglyceride synthesis and elevates resolvin D1 levels. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 156–165. [Google Scholar] [CrossRef]
- Maguire, M.; Bushkofsky, J.R.; Larsen, M.C.; Foong, Y.H.; Tanumihardjo, S.A.; Jefcoate, C.R. Diet-dependent retinoid effects on liver gene expression include stellate and inflammation markers and parallel effects of the nuclear repressor Shp. J. Nutr. Biochem. 2017, 47, 63–74. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Li, Y.; Zhang, Q.; Zhu, L.; Ding, N.; Zhang, B.; Zhang, J.; Liu, W.; Li, S.; Zhang, J. Association between dietary essential amino acids intake and metabolic biomarkers: Infuence of obesity among Chinese children and adolescents. Amino Acids 2021, 53, 635–644. [Google Scholar] [CrossRef]
- Li, T.Y. Diagnosis, treatment and prevention of vitamin A deficiency. Chin. J. Pract. Clin. Pediatrics 2013, 19, 1519–1520. (In Chinese) [Google Scholar]
- The Children Health Committee of the Chinese Physician Association and the Atherosclerosis Group of the Chinese Association of Cardiovascular Disease. The Expert Consensus on the prevention and treatment of dyslipidemia among Chinese children and adolescents. Chin. Trop. Med. 2008, 8, 124–127. (In Chinese) [Google Scholar]
- Zhang, Y.; Liu, X.B.; Yang, L.C.; Chen, J.; Wang, R.; Yang, X.G.; Piao, J.H.; Mao, D.Q. Vitamin A nutrition status of Urban children aged 6 to 17 years in China from 2010 to 2012. Chin. J. Prev. Med. 2017, 51, 117–120. [Google Scholar]
- Hu, Y.; Chen, J.; Wang, R.; Li, M.; Yun, C.; Li, W.; Yang, Y.; Piao, J.; Yang, X.; Yang, L. Vitamin D Nutritional Status and its Related Factors for Chinese Children and Adolescents in 2010–2012. Nutrients 2017, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Yeasmin, S.; Abdulla, F.; Rahman, A. Rural-urban determinants of vitamin a deficiency among under 5 children in Bangladesh: Evidence from National Survey 2017–18. BMC Public Health 2021, 21, 1569. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sapkota, M. Knowledge on vitamin a rich foods among mothersof preschool children in Nepal: Impacts on public health and policyconcerns. Sci. J. Public Health 2014, 2, 316–322. [Google Scholar] [CrossRef][Green Version]
- Kassu, A.; Andualem, B.; Van Nhien, N.; Nakamori, M.; Nishikawa, T.; Yamamoto, S.; Ota, F. Vitamin a deficiency in patients with diarrhea and HIV infection in Ethiopia. Asia Pac. J. Clin. Nutr. 2007, 16, 323–328. [Google Scholar]
- Imdad, A.; Mayo-Wilson, E.; Haykal, M.R.; Regan, A.; Sidhu, J.; Smith, A.; Bhutta, A.Z. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. 2022, 16, CD008524. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Xerophthalmia and Night Blindness for the Assessment of Clinical Vitamin A Deficiency in Individuals and Populations; World Health Organization (WHO): Geneve, Switzerland, 2014. [Google Scholar]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin a deficiency in children in 138 lowincome and middle-income countries between 1991 and 2013: A pooledanalysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Wirth, J.P.; Petry, N.; Tanumihardjo, S.A.; Rogers, L.M.; McLean, E.; Greig, A.; Garrett, G.S.; Klemm, R.D.W.; Rohner, F. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients 2017, 9, 190. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Gieng, S.H.; Green, M.H.; Green, J.B.; Rosales, F.J. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 2007, 48, 904–913. [Google Scholar] [CrossRef]
- Aklamati, E.K.; Mulenga, M.; Dueker, S.R.; Buchholz, B.A.; Peerson, J.M.; Kafwembe, E.; Brown, K.H.; Haskell, M.J. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J. Nutr. 2010, 140, 1588–1594. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.; Li, Y.; Zhang, Y.; Chen, G. Retinoids synergized with insulin to induce Srebp-1cexpression and activated its promoter via the two liver X receptor binding sites that mediate insulin action. Biochem. Biophys. Res. Commun. 2011, 406, 268–272. [Google Scholar] [CrossRef]
- Tepasse, P.-R.; Vollenberg, R.; Fobker, M.; Kabar, I.; Schmidt, H.; Meier, J.A.; Nowacki, T.; Hüsing-Kabar, A. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients 2021, 13, 2173. [Google Scholar] [CrossRef]
- Oliveros, L.; Domeniconi, M.; Vega, V.; Gatica, L.; Brigada, A.; Gimenez, M. Vitamin A deficiency modifies lipid metabolism in rat liver. Br. J. Nutr. 2007, 97, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Klör, H.-U.; Weizel, A.; Augustin, M.; Diepgen, T.L.; Elsner, P.; Homey, B.; Luger, T. The impact of oral vitamin A derivatives on lipid metabolism—What recommendations can be derived for dealing with this issue in the daily dermatological practice. JDDG J. Dtsch. Dermatol. Ges. 2011, 9, 600–606. [Google Scholar] [CrossRef]
- Vahlquist, A. Retinoid-Induced Hyperlipidaemia and the Risk of Atherosclerosis in Dermatology; CRC Press: Boca Raton, FL, USA, 2007; pp. 249–259. [Google Scholar]
- Chen, G. The Interactions of Insulin and Vitamin a Signaling Systems for the Regulation of Hepatic Glucose and Lipid Metabolism. Cells 2021, 10, 2160. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Howell, M.L.; Li, Y.; Li, R.; Chen, G. Vitamin A and feeding statuses modulate the insulin-regulated gene expression in Zucker lean and fatty primary rat hepatocytes. PLoS ONE 2014, 9, e100868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Li, R.; Li, Y.; Ge, Y.; Chen, G. Insulin-regulated Srebp-1c and Pck1 mRNA expression in primary hepatocytes from zucker fatty but not lean rats is affected by feeding conditions. PLoS ONE 2011, 6, e21342. [Google Scholar] [CrossRef] [PubMed]
- Vu-Dac, N.; Gervois, P.; Torra, I.P.; Fruchart, J.C.; Kosykh, V.; Kooistra, T.; Princen, H.M.; Dallongeville, J.; Staels, B. Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J. Clin. Investig. 1998, 102, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Petrov, P.; Bonet, M.L.; Ribot, J.; Palou, A. Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cells. Int. J. Biochem. Cell Biol. 2012, 44, 2019–2027. [Google Scholar] [CrossRef]
- Vega, V.A.; Anzulovich, A.C.; Varas, S.M.; Bonomi, M.R.; Giménez, M.S.; Oliveros, L.B. Effect of nutritional vitamin A deficiency on lipid metabolism in the rat heart: Its relation to PPAR gene expression. Nutrients 2009, 25, 828–838. [Google Scholar] [CrossRef]
- Akhtar, S.; Ahmed, A.; Atukorala, S.; Arlappa, N.; Ismail, T.; Ali, Z. Prevalence of vitamin A deficiency in South Asia: Causes, outcomes, and possible remedies. J. Health Popul. Nutr. 2013, 31, 413–423. [Google Scholar] [CrossRef]
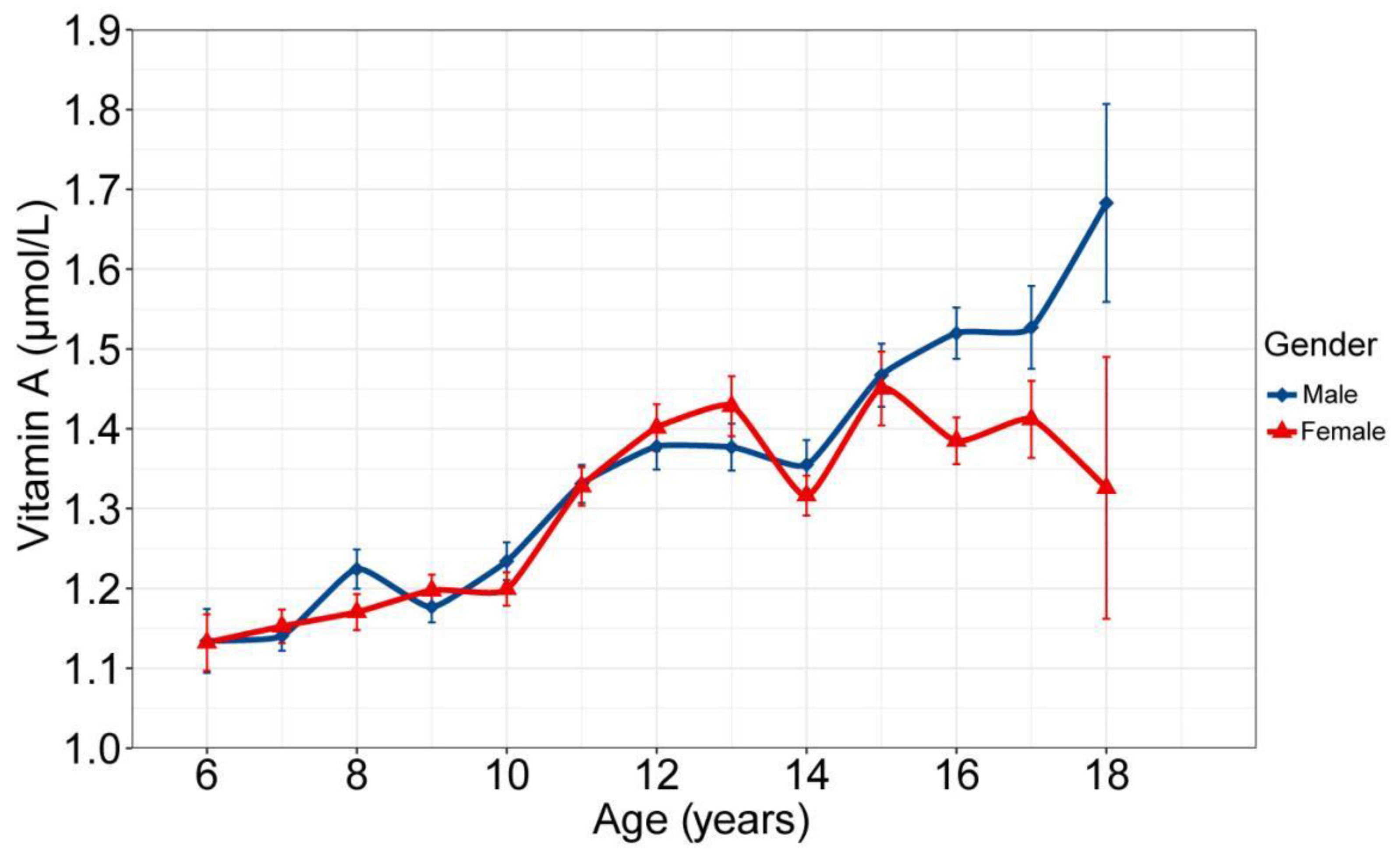
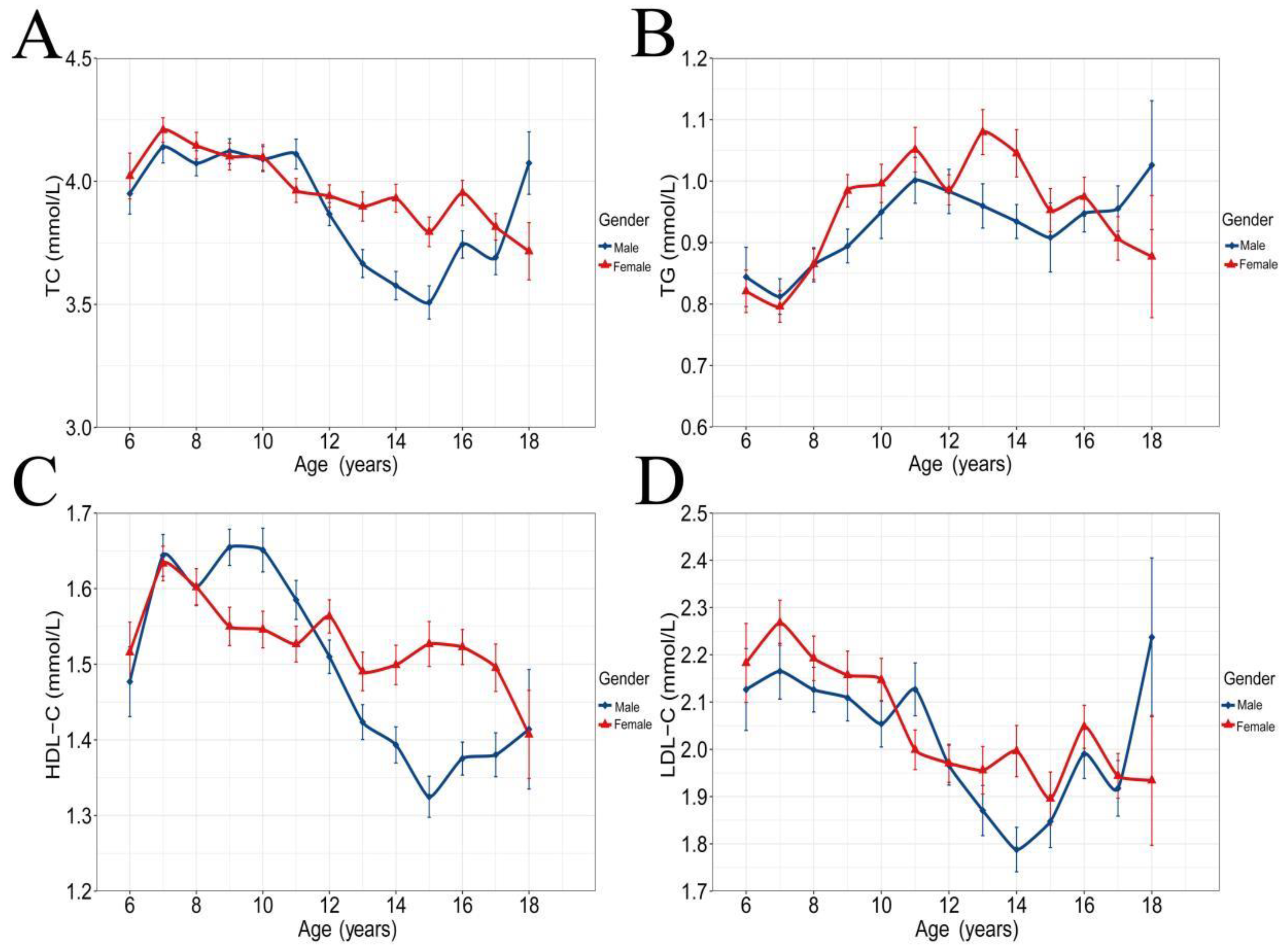
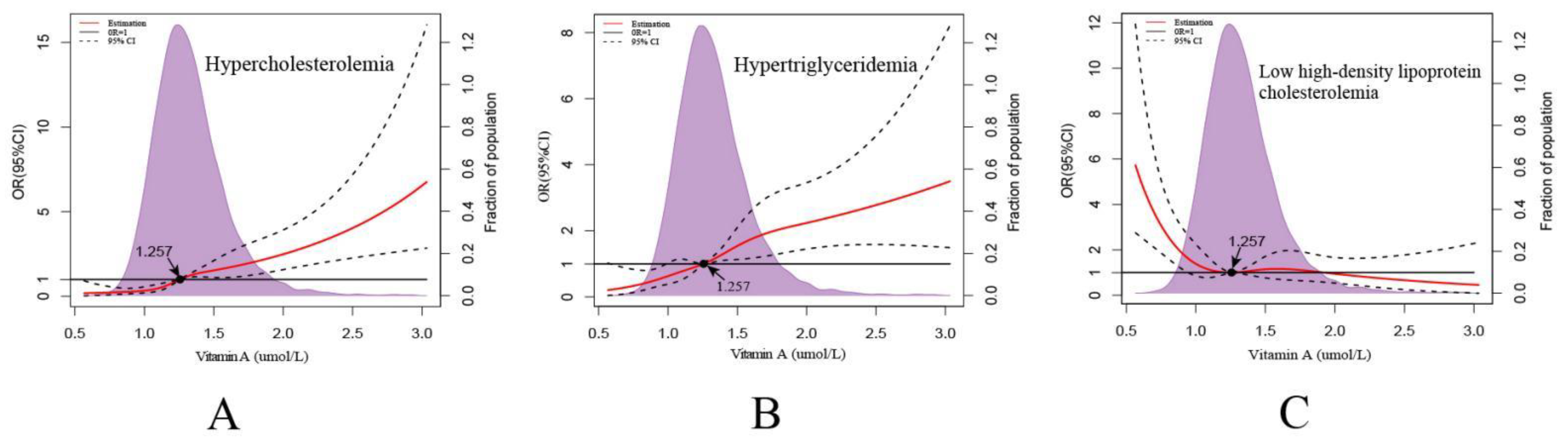
| Variables | Total (N = 3551, %) | Vitamin A Deficiency (<0.7 μmol/L) | Edge Vitamin A Deficiency (0.7–1.05 μmol/L) | Normal (≥1.05 μmol/L) | χ2/F | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Total (n, %) | 3551 (100.0) | 58 (1.6) | 786 (22.2) | 2707 (76.2) | ||||||
| Male (n, %) | 1766 (49.7) | 32 (0.9) | 361 (10.3) | 1373 (38.6) | 6.3 | 0.043 | ||||
| Female (n, %) | 1785 (50.2) | 26 (0.7) | 425 (11.9) | 1334 (37.6) | ||||||
| Age (years) | 11.4 | 3.2 | 10.1 | 3 | 10.4 | 3.1 | 11.7 | 3.1 | 59.1 | <0.001 |
| 6–12 years (n, %) | 2265 (63.8) | 47 (1.3) | 585 (16.5) | 1633 (46.0) | 60.0 | <0.001 | ||||
| 13–18 years (n, %) | 1286 (36.2) | 11 (0.3) | 201 (5.7) | 1074 (30.2) | ||||||
| Dietary factors | ||||||||||
| Protein intake (g/day) | 139.5 | 131.6 | 139.1 | 141.4 | 140.8 | 185 | 139.2 | 111.3 | 0.04 | 0.959 |
| Fat intake (g/day) | 52.1 | 76.3 | 50.5 | 55.9 | 50.4 | 74.8 | 52.7 | 77.1 | 0.3 | 0.751 |
| Energy intake (g/day) | 2388.9 | 2104.4 | 2301.9 | 2052.8 | 2348.7 | 2329.3 | 2402.5 | 2036.2 | 0.2 | 0.780 |
| Anthropometrics | ||||||||||
| Systolic pressure (mmHg) | 114.1 | 12.4 | 110.5 | 11.4 | 111 | 11.9 | 115.1 | 12.4 | 35.1 | <0.001 |
| Diastolic pressure (mmHg) | 67.9 | 9.2 | 67.7 | 7.6 | 66.9 | 9.1 | 68.2 | 9.1 | 6.2 | 0.002 |
| Weight (kg) | 45.2 | 16.5 | 37.8 | 14.5 | 38.5 | 14 | 47.2 | 16.7 | 95.0 | <0.001 |
| Height (cm) | 150.5 | 16.7 | 142.9 | 16.6 | 144.8 | 16.6 | 152.4 | 16.3 | 72.9 | <0.001 |
| BMI (kg/m2) | 19.4 | 5.9 | 17.9 | 3.4 | 17.8 | 3.4 | 19.9 | 6.4 | 40.4 | <0.001 |
| Biochemistry | ||||||||||
| Hemoglobin (g/L) | 136.8 | 13.3 | 130.8 | 13.6 | 131.6 | 11.7 | 138.5 | 13.3 | 92.7 | <0.001 |
| Blood glucose (mmol/L) | 5.2 | 0.4 | 5.1 | 0.6 | 5.2 | 0.4 | 5.3 | 0.4 | 15.6 | <0.001 |
| Total cholesterol (mmol/L) | 3.9 | 0.7 | 3.7 | 0.8 | 3.8 | 0.6 | 4.0 | 0.7 | 46.0 | <0.001 |
| Triglyceride (mmol/L) | 0.9 | 0.4 | 0.8 | 0.3 | 0.8 | 0.3 | 1 | 0.4 | 38.3 | <0.001 |
| HDL-C (mmol/L) | 1.5 | 0.3 | 1.5 | 0.4 | 1.5 | 0.3 | 1.5 | 0.3 | 6.4 | 0.002 |
| LDL-C (mmol/L) | 2.0 | 0.6 | 1.9 | 0.8 | 1.9 | 0.5 | 2.1 | 0.6 | 18.3 | <0.001 |
| Blood uric acid (μmol/L) | 316.6 | 82.6 | 279.7 | 76.7 | 284.6 | 69.1 | 326.6 | 83.7 | 89.0 | <0.001 |
| Serum creatinine (μmol/L) | 52.7 | 13.2 | 47.3 | 11.2 | 47.4 | 10.7 | 54.3 | 13.5 | 94.3 | <0.001 |
| Ferritin (ng/mL) | 62.0 | 39.2 | 69.0 | 48.6 | 55.7 | 31.5 | 63.7 | 40.8 | 13.5 | <0.001 |
| Transferrin receptor (mg/L) | 3.2 | 1.1 | 3.3 | 1.0 | 3.3 | 1.2 | 3.2 | 1.1 | 3.5 | <0.001 |
| hs-CRP (mg/L) | 1.0 | 3.2 | 2.7 | 5.3 | 1.4 | 4.3 | 0.8 | 2.6 | 18.3 | <0.001 |
| Albumin (g/L) | 49.5 | 2.9 | 47.5 | 2.7 | 48.3 | 2.6 | 49.9 | 2.9 | 107.9 | <0.001 |
| Total protein (g/L) | 76.5 | 4.5 | 75.4 | 4.6 | 75.0 | 4.4 | 76.9 | 4.5 | 57.5 | <0.001 |
| Serum Zn (μmol/L) | 87.5 | 18.1 | 87.4 | 24.7 | 84.9 | 18.3 | 88.2 | 17.8 | 9.9 | <0.001 |
| Vitamin D (ng/mL) | 17.6 | 6.6 | 13.9 | 5.8 | 16.7 | 6.2 | 17.9 | 6.7 | 17.8 | <0.001 |
| Variables | Vitamin A Deficiency (<0.7 μmol/L | Edge Vitamin A Deficiency (0.7–1.05 μmol/L) | Normal (≥1.05 μmol/L) | F | p | |||
|---|---|---|---|---|---|---|---|---|
| 6–12 Years | Mean | SD | Mean | SD | Mean | SD | ||
| TC (mmol/L) | 3.7 | 0.9 | 3.8 | 0.6 | 4.1 | 0.7 | 58.9 | <0.001 |
| TG (mmol/L) | 0.7 | 0.2 | 0.8 | 0.3 | 0.9 | 0.4 | 30.3 | <0.001 |
| HDL-C (mmol/L) | 1.5 | 0.4 | 1.5 | 0.3 | 1.6 | 0.3 | 9.2 | <0.001 |
| LDL-C (mmol/L) | 1.9 | 0.8 | 1.9 | 0.6 | 2.2 | 0.7 | 24.4 | <0.001 |
| 13–18 years | ||||||||
| TC (mmol/L) | 3.5 | 0.5 | 3.6 | 0.6 | 3.8 | 0.7 | 9.4 | <0.001 |
| TG (mmol/L) | 0.8 | 0.4 | 0.9 | 0.4 | 0.9 | 0.4 | 5.9 | 0.003 |
| HDL-C (mmol/L) | 1.3 | 0.3 | 1.4 | 0.3 | 1.5 | 0.3 | 5.2 | 0.005 |
| LDL-C (mmol/L) | 1.9 | 0.4 | 1.9 | 0.5 | 2 | 0.6 | 2.5 | 0.083 |
| Hypercholesterolemia | Hypertriglyceridemia | Mixed Hyperlipidemia | Low High-Density Lipoprotein Cholesterolemia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |||||
| Age (years) | 0.8 | 0.7 | 0.9 | <0.001 | 1.0 | 0.9 | 1.1 | 0.908 | 1.0 | 0.8 | 1.2 | 0.778 | 1.1 | 1.0 | 1.2 | 0.193 |
| Sex | 1.1 | 0.8 | 1.5 | 0.605 | 0.9 | 0.7 | 1.3 | 0.599 | 1.1 | 0.5 | 2.4 | 0.871 | 0.9 | 0.6 | 1.4 | 0.782 |
| BMI (kg/m2) | 1.0 | 1.0 | 1.0 | 0.810 | 1.0 | 1.0 | 1.0 | <0.001 | 1.0 | 1.0 | 1.1 | 0.032 | 1.0 | 1.0 | 1.0 | 0.001 |
| Vitamin A (μmol/L) | 3.3 | 2.2 | 5.0 | <0.001 | 3.2 | 2.1 | 4.9 | <0.001 | 5.2 | 2.2 | 12.4 | 0.000 | 0.3 | 0.2 | 0.6 | 0.000 |
| Vitamin D (ng/mL) | 1.0 | 1.0 | 1.1 | 0.005 | 1.0 | 0.9 | 1.0 | 0.008 | 1.0 | 0.9 | 1.1 | 0.735 | 1.0 | 0.9 | 1.0 | 0.101 |
| Blood glucose (mmol/L) | 1.0 | 0.7 | 1.5 | 0.850 | 1.0 | 0.7 | 1.5 | 0.948 | 1.5 | 0.8 | 2.6 | 0.166 | 0.7 | 0.5 | 1.1 | 0.156 |
| Blood uric acid (μmol/L) | 1.0 | 1.0 | 1.0 | 0.919 | 1.0 | 1.0 | 1.0 | <0.001 | 1.0 | 1.0 | 1.0 | 0.054 | 1.0 | 1.0 | 1.0 | 0.000 |
| Serum creatinine (μmol/L) | 1.0 | 1.0 | 1.0 | 0.411 | 1.0 | 1.0 | 1.0 | 0.001 | 0.9 | 0.9 | 1.0 | 0.021 | 1.0 | 1.0 | 1.0 | 0.284 |
| Ferritin (ng/mL) | 1.0 | 1.0 | 1.0 | 0.042 | 1.0 | 1.0 | 1.0 | 0.249 | 1.0 | 1.0 | 1.0 | 0.504 | 1.0 | 1.0 | 1.0 | 0.000 |
| Transferrin receptor (mg/L) | 1.1 | 1.0 | 1.3 | 0.051 | 1.2 | 1.1 | 1.3 | 0.000 | 1.1 | 0.9 | 1.4 | 0.237 | 1.2 | 1.1 | 1.3 | 0.004 |
| hs-CRP (mg/L) | 1.0 | 0.9 | 1.0 | 0.534 | 1.0 | 1.0 | 1.1 | 0.849 | 1.0 | 0.9 | 1.1 | 0.572 | 1.0 | 1.0 | 1.0 | 0.990 |
| Albumin (g/L) | 1.0 | 0.9 | 1.1 | 0.928 | 1.1 | 1.0 | 1.2 | 0.015 | 1.1 | 1.0 | 1.4 | 0.128 | 0.9 | 0.8 | 0.9 | 0.000 |
| Total protein (g/L) | 1.1 | 1.0 | 1.1 | <0.001 | 1.0 | 0.9 | 1.0 | 0.087 | 1.0 | 0.9 | 1.1 | 0.482 | 1.0 | 1.0 | 1.1 | 0.540 |
| Dietary Factors | ||||||||||||||||
| Dietary energy (kcal/day) | 1.0 | 1.0 | 1.0 | 0.100 | 1.0 | 1.0 | 1.0 | 0.935 | 1.0 | 0.9 | 1.1 | 0.650 | 1.0 | 1.0 | 1.0 | 0.935 |
| Dietary carbohydrate (g/day) | 1.0 | 1.0 | 1.0 | 0.668 | 1.0 | 0.9 | 1.1 | 0.838 | 1.1 | 0.7 | 1.7 | 0.670 | 1.0 | 0.9 | 1.1 | 0.838 |
| Dietary fat (g/day) | 1.0 | 1.0 | 1.0 | 0.286 | 1.0 | 0.8 | 1.3 | 0.956 | 1.3 | 0.4 | 4.6 | 0.668 | 1.0 | 0.8 | 1.3 | 0.956 |
| Dietary protein (g/day) | 1.0 | 1.0 | 1.0 | 0.091 | 1.0 | 0.9 | 1.0 | 0.288 | 1.1 | 0.9 | 1.4 | 0.447 | 1.0 | 0.9 | 1.0 | 0.288 |
| Dietary total carotene (μg/day) | 1.0 | 1.0 | 1.0 | 0.904 | 1.0 | 1.0 | 1.0 | 0.623 | 1.0 | 0.9 | 1.1 | 0.745 | 1.0 | 1.0 | 1.0 | 0.623 |
| Dietary retinol (μg/day) | 1.0 | 1.0 | 1.0 | 0.242 | 1.0 | 1.0 | 1.0 | 0.618 | 1.0 | 0.7 | 1.6 | 0.862 | 1.0 | 1.0 | 1.0 | 0.618 |
| Dietary vitamin A (μg/day) | 1.0 | 1.0 | 1.0 | 0.774 | 1.0 | 1.0 | 1.0 | 0.626 | 0.9 | 0.6 | 1.4 | 0.745 | 1.0 | 1.0 | 1.0 | 0.626 |
| Dietary vitamin E (mg/day) | 1.0 | 1.0 | 1.0 | 0.927 | 1.0 | 0.9 | 1.0 | 0.321 | 0.9 | 0.5 | 1.9 | 0.823 | 1.0 | 0.9 | 1.0 | 0.321 |
| Model 1 | Model 2 | Model 3 | Model 4 | p-Value Interaction for Age | p-Value Interaction for BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood lipids | Coefficient | 95%CI | p | Coefficient | 95%CI | p | Coefficient | 95%CI | p | Coefficient | 95%CI | p | ||
| TC (mmol/L) | 0.319 | 0.257 0.381 | <0.001 | 0.315 | 0.245 0.385 | <0.001 | 0.473 | 0.410 0.536 | <0.001 | 0.347 | 0.278 0.418 | <0.001 | <0.001 | 0.462 |
| TG (mmol/L) | 0.242 | 0.206 0.279 | <0.001 | 0.229 | 0.188 0.272 | <0.001 | 0.232 | 0.194 0.269 | <0.001 | 0.229 | 0.187 0.272 | <0.001 | 0.001 | 0.478 |
| HDL-C (mmol/L) | −0.0003 | (−0.029 0.029) | 0.998 | 0.075 | 0.043 0.108 | <0.001 | 0.0572 | 0.027 0.087 | <0.001 | 0.083 | 0.051 0.116 | <0.001 | 0.425 | 0.946 |
| LDL-C (mmol/L) | 0.21 | 0.154 0.267 | <0.001 | 0.134 | 0.070 0.198 | <0.001 | 0.311 | 0.254 0.369 | <0.001 | 0.158 | 0.094 0.222 | <0.001 | 0.016 | 0.747 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Wang, Y.; Yu, D.; Zhang, S.; Zheng, F.; Ding, N.; Zhu, L.; Zhu, Q.; Sun, W.; Li, S.; et al. Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents. Nutrients 2022, 14, 1444. https://doi.org/10.3390/nu14071444
Yu L, Wang Y, Yu D, Zhang S, Zheng F, Ding N, Zhu L, Zhu Q, Sun W, Li S, et al. Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents. Nutrients. 2022; 14(7):1444. https://doi.org/10.3390/nu14071444
Chicago/Turabian StyleYu, Lianlong, Yongjun Wang, Dongmei Yu, Shixiu Zhang, Fengjia Zheng, Ning Ding, Lichao Zhu, Qianrang Zhu, Wenkui Sun, Suyun Li, and et al. 2022. "Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents" Nutrients 14, no. 7: 1444. https://doi.org/10.3390/nu14071444
APA StyleYu, L., Wang, Y., Yu, D., Zhang, S., Zheng, F., Ding, N., Zhu, L., Zhu, Q., Sun, W., Li, S., Zhang, G., Chen, L., Liu, Y., Yang, L., & Feng, J. (2022). Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents. Nutrients, 14(7), 1444. https://doi.org/10.3390/nu14071444






