The Prevention Role of Theaflavin-3,3′-digallate in Angiotensin II Induced Pathological Cardiac Hypertrophy via CaN-NFAT Signal Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Treatments
2.3. Cell Viability Determination
2.4. F-actin and Nucleus Staining
2.5. Total RNA Extraction and Real-Time PCR Analysis for Fetal Genes
2.6. Western Blot Analysis
2.7. Reactive Oxygen Species Level Determination
2.8. Superoxide Dismutase Activity, Catalase Activity, and Nitric Oxide Level Determination
2.9. Intracellular Ca2+ Level Determination
2.10. Statistical Analysis
3. Results
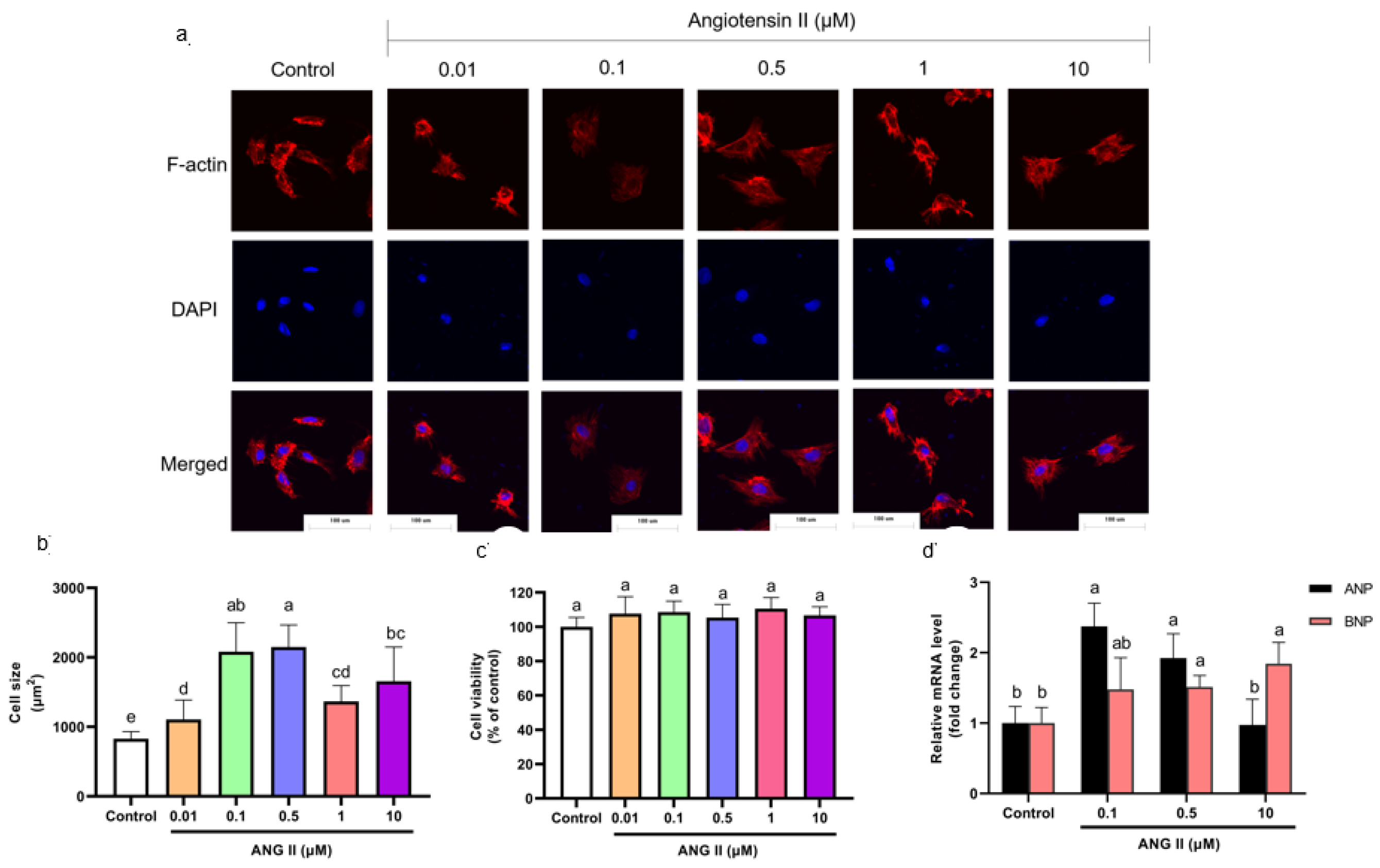
3.1. H9c2 Cell PCH Model Induction
3.2. Effect of TF3 on Cell Viability
3.3. TF3 Pretreatment Alleviated Cell Hypertrophy and Fetal Gene Expression
3.4. TF3 Pretreatment Rebalance of Redox System in PCH Model
3.5. TF3 Pretreatment Inhibited the Increase of Ca2+ Level in the PCH Model
3.6. TF3 Pretreatment Prevented PCH through the CaN-NFAT Signal Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, S.Q.; Shi, W.; Ma, H.Y.; Yan, D.; Luo, P.C.; Guo, J.Y.; Li, C.L.; Lin, J.Y.; Zhang, C.T.; Li, S.; et al. Alleviation of inflammation and oxidative stress in pressure overload-induced cardiac remodeling and heart failure via IL-6/STAT3 inhibition by raloxifene. Oxid. Med. Cell. Longev. 2021, 2021, 6699054. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Badrealam, K.F.; Kuo, C.H.; Daddam, J.; Shibu, M.A.; Lin, K.H.; Ho, T.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Small molecule compound nerolidol attenuates hypertension induced hypertrophy in spontaneously hypertensive rats through modulation of Mel-18-IGF-IIR signaling. Phytomedicine 2021, 84, 153450. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Zhu, Z.M.; Zhu, S.J.; Wang, H.Y. Effects of antihypertensive agent on cardiac hypertrophy in hypertensive rats induced by high salt diet combined with cold stress. Acta Acad. Med. Mil. Tertiae 2003, 25, 1240–1242. [Google Scholar]
- Mineharu, Y.; Koizumi, A.; Wada, Y.; Iso, H.; Watanabe, Y.; Date, C.; Yamamoto, A.; Kikuchi, S.; Inaba, Y.; Toyoshima, H.; et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J. Epidemiol. Community Health 2011, 65, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.M.D.; Uiterwaal, C.S.P.M.; van der Schouw, Y.T.; Boer, J.M.A.; Grobbee, D.E.; Verschuren, W.M.M.; Beulens, J.W.J. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Li, W.; Xu, Y.; Jin, E.-H.; Tu, Y.-Y. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J. Zhejiang Univ. Sci. B 2011, 12, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vik, S.B.; Tu, Y. Theaflavins inhibit the ATP synthase and the respiratory chain without increasing superoxide production. J. Nutr. Biochem. 2012, 23, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, Z.Y.; Jie, G.L.; Gao, Y.; Zhang, X.H.; Li, W.; Li, B.; Tu, Y. Evaluation of antioxidant activity of tea polyphenols by a quantum chemistry calculation method - PM6. J. Food Nutr. Res. 2014, 2, 965–972. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.; Zhao, L.; Qin, Y.; Wu, X.Q. EGCG Blocked Phenylephrin-induced hypertrophy in H9C2 cardiomyocytes, by activating AMPK-dependent pathway. Korean J. Physiol. Pharmacol. 2015, 19, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Cao, J.L.; Zhang, G.Y.; Wang, Y.G. Kaempferol attenuates cardiac hypertrophy via regulation of ASK1/MAPK signaling pathway and oxidative stress. Planta Med. 2017, 83, 837–845. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, Y.J.; Wu, J.W.; Wen, J.R.; Li, S.; Zhang, L.J.; Zhang, J.; Li, Y.F.; Li, J. Epigallocatechin-3-gallate inhibits angiotensin II-induced cardiomyocyte hypertrophy via regulating Hippo signaling pathway in H9c2 rat cardiomyocytes. Acta Biochim. Biophys. Sin. 2019, 51, 422–430. [Google Scholar] [CrossRef]
- Lin, J.; Piao, Z.H.; Sun, S.M.; Liu, B.; Kim, G.R.; Seok, Y.M.; Lin, M.Q.; Ryu, Y.; Choi, S.Y.; Kee, H.J.; et al. Gallic acid reduces blood pressure and attenuates oxidative stress and cardiac hypertrophy in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 15607. [Google Scholar]
- Cheang, W.S.; Ngai, C.Y.; Tam, Y.Y.; Tian, X.Y.; Wong, W.T.; Zhang, Y.; Lau, C.W.; Chen, Z.Y.; Bian, Z.X.; Huang, Y.; et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015, 5, 10340. [Google Scholar] [CrossRef]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. Vitr. Cell. Dev. Biol.-Anim. 2011, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, Y.X.; Wu, Y.Y.; Tu, Y.Y. Isolation and purification of four individual theaflavins using semi-preparative high performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1791–1801. [Google Scholar] [CrossRef]
- Gao, Y.; Rankin, G.O.; Tu, Y.Y.; Chen, Y.C. Theaflavin-3, 3’-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int. J. Oncol. 2016, 48, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Shibu, M.A.; Peramaiyan, R.; Chen, Y.F.; Shen, C.Y.; Hsieh, Y.L.; Cheng, R.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Bioactive flavone fisetin attenuates hypertension associated cardiac hypertrophy in H9c2 cells and in spontaneously hypertension rats. J. Funct. Foods 2019, 52, 212–228. [Google Scholar] [CrossRef]
- Akhondzadeh, F.; Astani, A.; Najjari, R.; Samadi, M.; Rezvani, M.E.; Zare, F.; Ranjbar, A.M.; Safari, F. Resveratrol suppresses interleukin-6 expression through activation of sirtuin 1 in hypertrophied H9c2 cardiomyoblasts. J. Cell. Physiol. 2020, 235, 6969–6977. [Google Scholar] [CrossRef]
- SLC7A11/xCT Prevents Cardiac Hypertrophy by Inhibiting Ferroptosis. 2021. Available online: https://link.springer.com/article/10.1007/s10557-021-07220-z#citeas (accessed on 25 March 2022).
- Manivassagam, S.; Velusamy, T.; Sowndharajan, B.; Chandrasekar, N.; Dhanusu, S.; Vellaichamy, E. Valporic acid enhances the Atrial Natriuretic Peptide (ANP) mediated anti-hypertrophic activity by modulating the Npr1 gene transcription in H9c2 cells In Vitro. Eur. J. Pharmacol. 2017, 813, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Zhou, D.L.; Jing, Y.Y.; Liu, S.X. Hydroxysafflor yellow A protects against angiotensin II-induced hypertrophy. Mol. Med. Rep. 2018, 18, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.G.; Zhang, M.; Meng, Z.J.; Yu, Y.; Yao, F.; Hatch, G.M.; Chen, L. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5 ’-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur. J. Pharmacol. 2015, 769, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.C.; Yu, D.S.; Zhang, J.; Hu, Q.Y.; Tang, C.F.; Liu, P.Y.; Ye, P.; Wang, X.L.; Lv, Q.; Chen, M.L.; et al. Wogonin attenuates isoprenaline-induced myocardial hypertrophy in mice by suppressing the PI3K/Akt pathway. Front. Pharmacol. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.J.; Liu, S.B.; Qian, W.C.; Wang, J.C.; Chu, C.Y.; Wang, J.J.; Li, K.; Yu, Y.J.; Xu, G.H.; Mao, Z.S.; et al. Swietenine extracted from Swietenia relieves myocardial hypertrophy induced by isoprenaline in mice. Environ. Toxicol. 2020, 35, 1343–1351. [Google Scholar] [CrossRef]
- Ismael, S.; Nair, R.R. Reactivation of fatty acid oxidation by medium chain fatty acid prevents myocyte hypertrophy in H9c2 cell line. Mol. Cell. Biochem. 2021, 476, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Fan, W.Y.; Xiong, X.Q. FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3-associated inflammation and oxidative stress. J. Transl. Med. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Wang, Z.X.; Shan, P.R.; Huang, S.J.; Lin, S.; Huang, W.J.; Huang, Z.Q. Suppression of Netrin-1 attenuates angiotension II-induced cardiac remodeling through the PKC/MAPK signaling pathway. Biomed. Pharmacother. 2020, 130, 110495. [Google Scholar] [CrossRef]
- Lin, F.F.; Zhang, N.; Wu, Q.Q.; Yuan, Y.; Yang, Z.; Zhou, M.Q.; Zhu, J.X.; Tang, Q.Z. Syringin prevents cardiac hypertrophy induced by pressure overload through the attenuation of autophagy. Int. J. Mol. Med. 2017, 39, 199–207. [Google Scholar]
- Gu, X.; Shi, Y.; Chen, X.; Sun, Z.; Luo, W.; Hu, X.; Jin, G.; You, S.; Qian, Y.; Wu, W.; et al. Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine 2020, 78, 153319. [Google Scholar] [CrossRef]
- Chen, S.J.; Yang, B.S.; Xu, Y.F.; Rong, Y.Q.; Qiu, Y.G. Protection of Luteolin-7-O-glucoside against apoptosis induced by hypoxia/reoxygenation through the MAPK pathways in H9c2 cells. Mol. Med. Rep. 2018, 17, 7156–7162. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Lorzen, M.; Urban, J.; Engelhardt, U.; Baumann, G.; Stangl, K.; Stangl, V. Green and black tea are equally potent stimuli of NO production and vasodilation: New insights into tea ingredients involved. Basic Res. Cardiol. 2009, 104, 100–110. [Google Scholar]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.M.; Soltys, C.L.M.; Young, M.E.; Proud, C.G.; Dyck, J.R.B. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004, 279, 32771–32779. [Google Scholar] [CrossRef] [PubMed]
- Shioi, T.; McMullen, J.R.; Tarnavski, O.; Converso, K.; Sherwood, M.C.; Manning, W.J.; Izumo, S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 2003, 107, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.A.; Force, T.; Wang, Y.B. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol. Rev. 2010, 90, 1507–1546. [Google Scholar] [CrossRef]
- Hong, M.H.; Na, S.W.; Jang, Y.J.; Yoon, J.J.; Lee, Y.J.; Lee, H.S.; Kim, H.Y.; Kang, D.G. Betulinic acid improves cardiac-renal dysfunction caused by hypertrophy through Calcineurin-NFATc3 signaling. Nutrients 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Cheng, Y.C.; Lee, K.W.; Hsu, H.H.; Chu, C.H.; Tsai, F.J.; Tsai, C.H.; Chu, C.Y.; Liu, J.Y.; Kuo, W.W.; et al. Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells. Mol. Cell. Biochem. 2008, 313, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Okada, M.; Yamawaki, H. Canstatin suppresses isoproterenol-induced cardiac hypertrophy through inhibition of calcineurin/nuclear factor of activated T-cells pathway in rats. Eur. J. Pharmacol. 2020, 871, 172849. [Google Scholar] [CrossRef]
- Nieves-Cintron, M.; Amberg, G.C.; Nichols, C.B.; Molkentin, J.D.; Santana, L.F. Activation of NFATc3 down-regulates the beta 1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J. Biol. Chem. 2007, 282, 3231–3240. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Fox, D.; Bobay, B.G.; Hoy, M.J.; Gobeil, S.M.C.; Venters, R.A.; Chang, Z.; Lin, J.J.; Averette, A.F.; Cole, D.C.; et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat. Commun. 2019, 10, 4275. [Google Scholar] [CrossRef] [PubMed]






| Gene | Primer Sequences (5′-3′) | Annealing Temperature (°C) |
|---|---|---|
| ANPc 1 | Forward: AAAGCAAACTGAGGGCTCTGCTCG Reverse: TTCGGTACCGGAAGCTGTTGCA | 65.66 64.69 |
| BNP | Forward: GTCAGTCGCTTGGGCTGT Reverse: CCAGAGCTGGGGAAAGAAG | 59.97 57.43 |
| GAPDH | Forward: CTCATGACCACAGTCCATGC Reverse: TTCAGCTCTGGGATGACCTT | 58.62 58.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Xia, C.; Yang, Y.; Warusawitharana, H.K.; Liu, X.; Tu, Y. The Prevention Role of Theaflavin-3,3′-digallate in Angiotensin II Induced Pathological Cardiac Hypertrophy via CaN-NFAT Signal Pathway. Nutrients 2022, 14, 1391. https://doi.org/10.3390/nu14071391
Zhou H, Xia C, Yang Y, Warusawitharana HK, Liu X, Tu Y. The Prevention Role of Theaflavin-3,3′-digallate in Angiotensin II Induced Pathological Cardiac Hypertrophy via CaN-NFAT Signal Pathway. Nutrients. 2022; 14(7):1391. https://doi.org/10.3390/nu14071391
Chicago/Turabian StyleZhou, Hui, Chen Xia, Yaqing Yang, Hasitha Kalhari Warusawitharana, Xiaohui Liu, and Youying Tu. 2022. "The Prevention Role of Theaflavin-3,3′-digallate in Angiotensin II Induced Pathological Cardiac Hypertrophy via CaN-NFAT Signal Pathway" Nutrients 14, no. 7: 1391. https://doi.org/10.3390/nu14071391
APA StyleZhou, H., Xia, C., Yang, Y., Warusawitharana, H. K., Liu, X., & Tu, Y. (2022). The Prevention Role of Theaflavin-3,3′-digallate in Angiotensin II Induced Pathological Cardiac Hypertrophy via CaN-NFAT Signal Pathway. Nutrients, 14(7), 1391. https://doi.org/10.3390/nu14071391






