Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
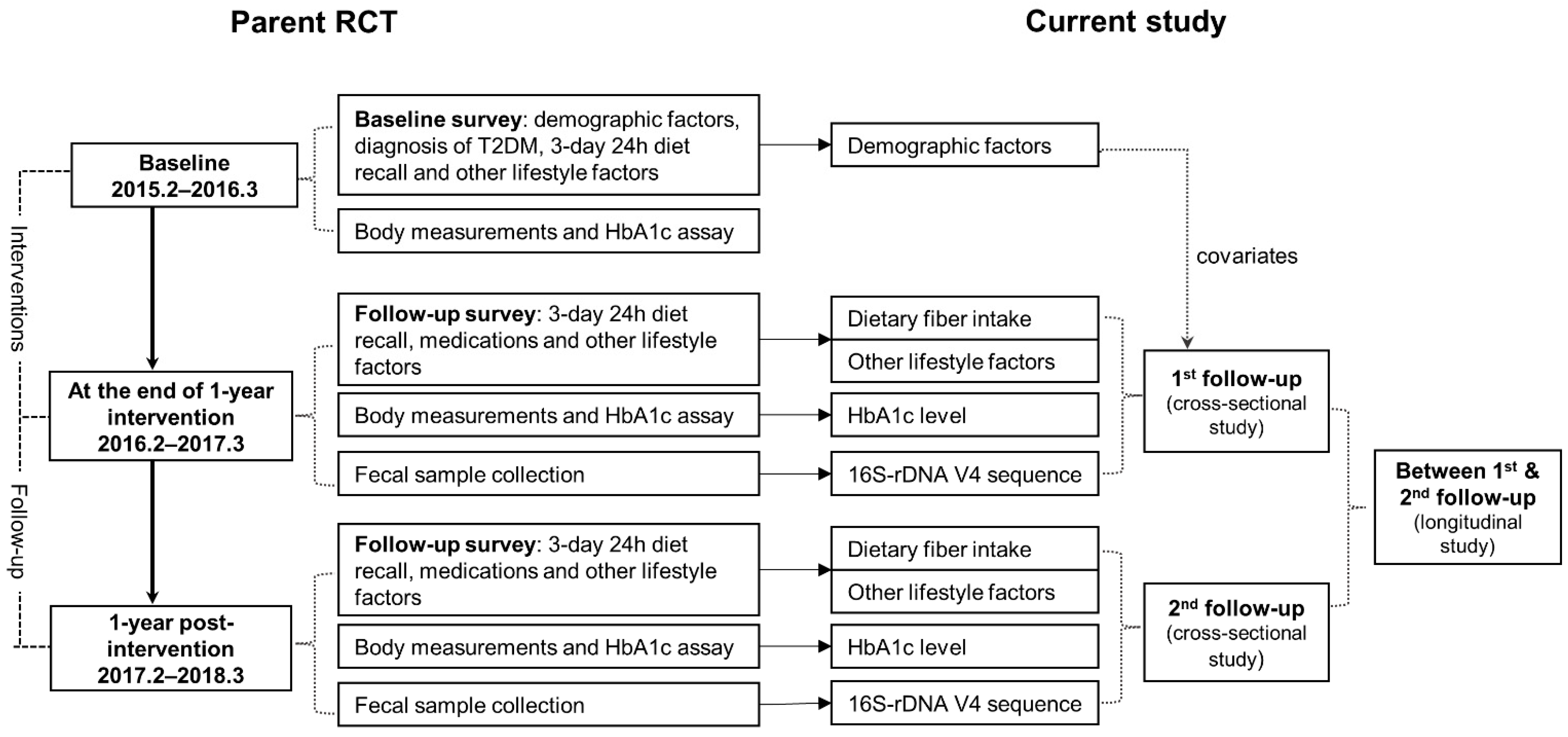
2.1. Study Design and Participants
2.2. Data Collection
2.3. Stool Sample Collection
2.4. Bacterial DNA Sequencing and Bio-Informatics Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants of the Baseline, First and Second Follow-Up Surveys
3.2. Associations of Habitual Dietary Fiber Intake with HbA1c Level
3.3. Diversity of Fecal Microbiota by Habitual Dietary Fiber Intake and HbA1c Level
3.4. Differential Taxa by Glycemic Control Status and Habitual Dietary Fiber Intake
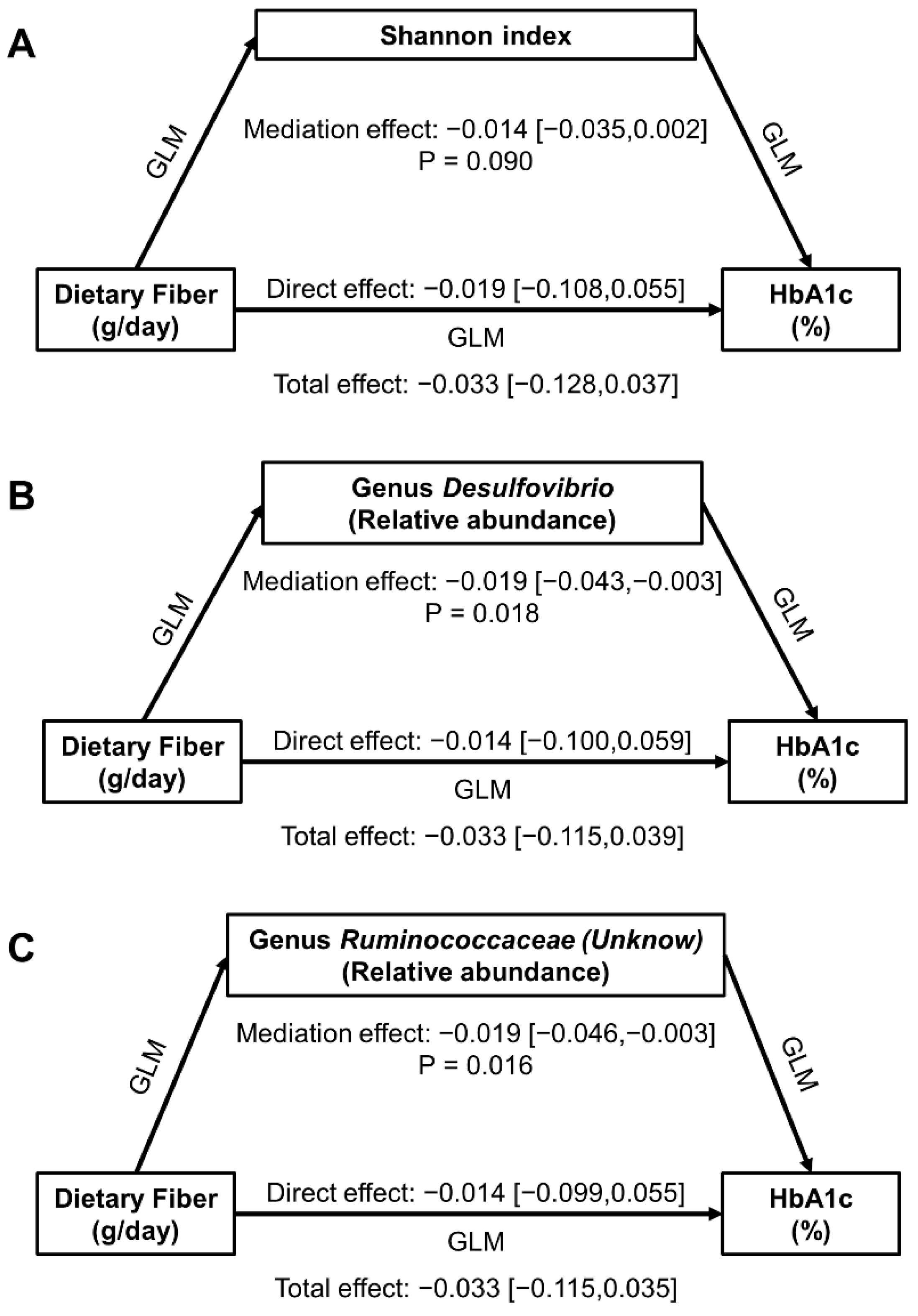
3.5. Mediation Effect of Gut Microbiota in Dietary Fiber-HbA1c Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Sawhney, M.; Shi, L.; Duan, S.; Yu, Y.; Wu, Z.; Qiu, G.; Dong, H. A systematic review of the direct economic burden of type 2 diabetes in china. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2015, 6, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Shao, J.; Bian, Y.; Wu, H.; Shi, L.; Zeng, L.; Li, W.; Dong, J. Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): A meta-analysis. J. Diabetes Investig. 2016, 7, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. (Encinitas) 2014, 13, 17–22. [Google Scholar]
- Ursell, L.K.; Clemente, J.C.; Rideout, J.R.; Gevers, D.; Caporaso, J.G.; Knight, R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J. Allergy Clin. Immunol. 2012, 129, 1204–1208. [Google Scholar] [CrossRef] [Green Version]
- Brunkwall, L.; Orho-Melander, M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Fischer, F.; Romero, R.; Hellhund, A.; Linne, U.; Bertrams, W.; Pinkenburg, O.; Eldin, H.S.; Binder, K.; Jacob, R.; Walker, A.; et al. Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota. Gut Microbes 2020, 12, 1–17. [Google Scholar] [CrossRef]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol. Spectr. 2017, 5, BAD-0019-2017. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.Z.; et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.; Feng, Q.Q.; Ojo, O.O.; Wang, X.H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef]
- Shu, L.; Shen, X.M.; Li, C.; Zhang, X.Y.; Zheng, P.F. Dietary patterns are associated with type 2 diabetes mellitus among middle-aged adults in Zhejiang Province, China. Nutr. J. 2017, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.B. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr. Rev. 2020, 78, 29–36. [Google Scholar] [CrossRef]
- Wang, L.; Fang, H.; Xia, Q.; Liu, X.; Chen, Y.; Zhou, P.; Yan, Y.; Yao, B.; Wei, Y.; Jiang, Y.; et al. Health literacy and exercise-focused interventions on clinical measurements in Chinese diabetes patients: A cluster randomized controlled trial. EClinicalMedicine 2019, 17, 100211. [Google Scholar] [CrossRef]
- National Institute for Nutrition and Health of Chinese Center for Disease Control and Prevention. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-scale Meta-omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.; Teppei, H.; Mit, Y.; Keele, L.; State, P.; Imai, K. Mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin. J. Diabetes Mellit. 2018, 10, 4–67. [Google Scholar]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [Green Version]
- Segata, N. Gut Microbiome: Westernization and the Disappearance of Intestinal Diversity. Curr. Biol. 2015, 25, R611–R613. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Su, C.; Zhang, J.; Zhai, F.Y.; Zhang, B. Trends in dietary fiber intake in Chinese aged 45 years and above, 1991–2011. Eur. J. Clin. Nutr. 2014, 68, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, C.; Liu, P.; Li, D.; Li, Y.; Ma, X. Dietary Fiber Gap and Host Gut Microbiota. Protein Pept. Lett. 2017, 24, 388–396. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Huang, J.; Yuan, X.; Wang, Q.; Ma, L. Dietary fiber extracted from pomelo fruitlets promotes intestinal functions, both in vitro and in vivo. Carbohydr. Polym. 2021, 252, 117186. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Tomsett, K.I.; Barrett, H.L.; Dekker, E.E.; Callaway, L.K.; McIntyre, D.H.; Dekker Nitert, M. Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy. Nutrients 2020, 12, 3862. [Google Scholar] [CrossRef]
- Moon, J.Y.; Zolnik, C.P.; Wang, Z.; Qiu, Y.; Usyk, M.; Wang, T.; Kizer, J.R.; Landay, A.L.; Kurland, I.J.; Anastos, K.; et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine 2018, 37, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Chen, X.; Chu, X.; Fan, M.; Ye, Y.; Wang, Y.; Han, M.; Yang, X.; Yuan, J.; Zha, L.; et al. Association of Gut Microbiota during Early Pregnancy with Risk of Incident Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2021, 106, e4128–e4141. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, S.L.; Kwok, M.K.; Leung, G.M.; Schooling, C.M. The Roles of 27 Genera of Human Gut Microbiota in Ischemic Heart Disease, Type 2 Diabetes Mellitus, and Their Risk Factors: A Mendelian Randomization Study. Am. J. Epidemiol. 2018, 187, 1916–1922. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Menni, C.; Louca, P.; Berry, S.E.; Vijay, A.; Astbury, S.; Leeming, E.R.; Gibson, R.; Asnicar, F.; Piccinno, G.; Wolf, J.; et al. High intake of vegetables is linked to lower white blood cell profile and the effect is mediated by the gut microbiome. BMC Med. 2021, 19, 37. [Google Scholar] [CrossRef]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes Complicat. 2018, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | At Baseline (n = 400) | At the First Follow-Up (n = 356) | At the Second Follow-Up (n = 310) |
|---|---|---|---|
| Age at survey (years) | 65.0 (58.0, 70.0) | 66.5 (60.0, 71.5) | 68.0 (60.0, 73.0) |
| Sex | |||
| Men | 174 (43.5) | 155 (43.5) | 132 (42.6) |
| Women | 226 (56.5) | 201 (56.5) | 178 (57.4) |
| Educational level | |||
| Primary school or below | 96 (24.0) | 88 (24.7) | 77 (24.8) |
| Junior high school | 146 (36.5) | 130 (36.5) | 114 (36.8) |
| Senior high or vocational school | 97 (24.2) | 81 (22.8) | 71 (22.9) |
| College or above | 61 (15.3) | 57 (16.0) | 48 (15.5) |
| Duration of diabetes (year) | 9 (5, 14) | 10 (6, 15) | 11 (7, 17) |
| Diagnosis of hypertension | 272 (68.0) | 244 (68.5) | 213 (68.7) |
| Diagnosis of hyperlipidemia | 126 (31.5) | 117 (32.9) | 97 (31.3) |
| Intervention status in parent trial | |||
| Health literacy group | 100 (25.0) | 84 (23.6) | 75 (24.2) |
| Exercise Group | 100 (25.0) | 97 (27.3) | 77 (24.8) |
| Comprehensive group | 100 (25.0) | 92 (25.8) | 88 (28.4) |
| Control group | 100 (25.0) | 83 (23.3) | 70 (22.6) |
| Body mass index (kg/m2) | 24.9 (23.1, 26.8) | 24.5 (22.9, 26.5) | 24.9 (23.0, 26.6) |
| Waist-to-hip ratio | 0.90 (0.87, 0.93) | 0.90 (0.87, 0.92) | 0.90 (0.87, 0.93) |
| Physical activity (METs-hours/week) | 61 (37, 91) | 60 (34, 88) | 56 (29, 98) |
| Use of anti-diabetes medicines | |||
| Never | 12 (3.0) | 11 (3.1) | 11 (3.6) |
| Insulin shot only | 41 (10.3) | 33 (9.3) | 33 (10.7) |
| Oral agents only | 309 (77.3) | 251 (70.5) | 215 (69.4) |
| Both | 38 (9.5) | 61 (17.1) | 51 (16.5) |
| Total energy intake (kcal/day) | 1557 (1211, 1864) | 1570 (1261, 1899) | 1643 (1310, 2033) |
| Sources of calorie (%) | |||
| Carbohydrate | 39 (33, 44) | 36 (30, 41) | 33 (29, 39) |
| Fat | 45 (40, 51) | 48 (41, 53) | 50 (44, 55) |
| Protein | 15 (13, 17) | 16 (14, 18) | 16 (13, 17) |
| Dietary fiber intake (g/d) | 6.9 (4.5, 10.0) | 7.4 (5.5, 9.7) | 7.1 (5.1, 11.1) |
| HbA1c level (%) | 7.8 (7.2, 8.6) | 7.8 (6.9, 9.0) | 8.0 (7.0, 9.2) |
| Glycemic status: HbA1c < 7.0% | 63 (15.8) | 94 (26.4) | 74 (23.9) |
| Glycemic status: HbA1c < 8.0% | 220 (55.0) | 189 (53.1) | 153(49.4) |
| Medications at sample collection | |||
| Use of antibiotics | -- | 31 (8.7) | 9 (2.9) |
| Use of NSAIDs | -- | 69 (19.4) | 46 (14.8) |
| No. of Subjects | Chao1 | Shannon | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | β [95%CI] | p-Value | Median (IQR) | β [95%CI] | p-Value | ||
| At the first follow-up | |||||||
| All subjects | 325 | 425 (379, 488) | 0.63 [−2.12, 3.37] | 0.654 | 4.36 (3.68, 4.95) | −0.02 [−0.05, 0.01] | 0.146 |
| By dietary fiber intake (g/day) | |||||||
| Lower (<7.2) | 153 | 425 (379, 480) | −0.83 [−11.46, 9.81] | 0.878 | 4.35 (3.68, 5.00) | −0.07 [−0.19, 0.06] | 0.299 |
| Higher (≥7.2) | 172 | 425 (379, 491) | −4.11 [−8.68, 0.47] | 0.078 | 4.37 (3.68, 4.89) | −0.06 [−0.11, −0.01] | 0.011 |
| Higher versus lower | 14.98 [−4.16, 34.11] | 0.125 | 0.00 [−0.20, 0.21] | 0.964 | |||
| At the second follow-up | |||||||
| All subjects | 301 | 406 (358, 462) | −2.06 [−3.66, −0.46] | 0.012 | 4.20 (3.74,4.77) | −0.01 [−0.02, 0.01] | 0.427 |
| By dietary fiber intake (g/day) | |||||||
| Lower (<7.2) | 152 | 424 (362, 491) | −1.68 [−12.65, 9.29] | 0.763 | 4.27 (3.65, 4.89) | −0.05 [−0.15, 0.06] | 0.352 |
| Higher (≥7.2) | 149 | 390 (358, 433) | −0.96 [−2.77, 0.85] | 0.297 | 4.17 (3.80, 4.57) | −0.01 [−0.03, 0.01] | 0.419 |
| Higher versus lower | −28.78 [−47.58, −9.97] | 0.003 | 0.00 [−0.19, 0.19] | 0.979 | |||
| Longitudinal analysis | |||||||
| Lower-lower | 80 | 424 (364, 490) | ref | 4.31 (3.66, 4.89) | ref | ||
| Higher-lower | 62 | 428 (381, 544) | 14.92 [−11.26, 41.11] | 0.263 | 4.28 (3.70, 4.95) | −0.04 [−0.30, 0.23] | 0.773 |
| Lower-higher | 60 | 390 (358, 429) | −24.50 [−51.06, 2.07] | 0.071 | 4.09 (3.63, 4.58) | −0.10 [−0.37, 0.17] | 0.450 |
| Higher-higher | 82 | 391 (358, 435) | −33.63 [−59.03, −8.23] | 0.010 | 4.15 (3.85, 4.57) | −0.15 [−0.41, 0.11] | 0.252 |
| Phylum | Class | Order | Family | Genus/Species | Relative Abundance (%) | Transformed Abundance (%) | β [95%CI] for HbA1c (%) | p-Value | Q-Value |
|---|---|---|---|---|---|---|---|---|---|
| All subjects at the first follow-up (n = 325) | |||||||||
| Euryarchaeota | 0.000 (0.000, 0.003) | 0.000 (0.000, 0.506) | 0.19 [0.08, 0.31] | 0.001 | 0.013 | ||||
| Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter | 0.000 (0.000, 0.002) | 0.000 (0.000, 0.438) | 0.24 [0.10, 0.38] | 0.001 | 0.053 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | (Unknown) | 0.000 (0.000, 0.001) | 0.000 (0.000, 0.386) | 0.30 [0.09, 0.52] | 0.006 | 0.131 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | (Unknown) | 0.743 (0.243, 2.283) | 8.630 (4.928, 15.168) | 0.03 [0.01, 0.06] | 0.006 | 0.131 |
| Firmicutes | Erysipelotrichi | Erysipelotrichales | Erysipelotrichaceae | Holdemania | 0.006 (0.002,0.014) | 0.754 (0.390, 1.184) | 0.33 [0.11, 0.55] | 0.004 | 0.120 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Methylobacteriaceae | (Unknown) | 0.000 (0.000, 0.000) | 0.000 (0.000,0.000) | 1.66 [0.88, 2.44] | 0.000 | 0.006 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | 0.036 (0.022, 0.191) | 1.909 (1.472, 4.367) | 0.05 [0.02, 0.09] | 0.001 | 0.053 |
| All subjects at the second follow-up (n = 301) | |||||||||
| -- | |||||||||
| Phylum | Class | Order | Family | Genus | Relative Abundance (%) | Transformed Abundance (%) | β [95%CI] with Dietary Fiber Intake | p-Value | Q-Value |
|---|---|---|---|---|---|---|---|---|---|
| At the first follow-up | |||||||||
| All subjects (n = 325) | |||||||||
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Megamonas | 0.341 (0.229, 1.295) | 5.847 (4.782, 11.405) | −0.46 [−0.89, −0.03] | 0.038 | 0.745 |
| Subjects taking dietary fiber ≥ 7.2 g/day (n = 172) | |||||||||
| Firmicutes | 35.830 (24.757, 43.854) | 64.173 (52.077, 72.378) | −0.98 [−1.78, −0.18] | 0.017 | 0.110 | ||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 19.104 (9.551, 35.675) | 45.235 (31.419, 64.011) | 1.36 [0.26, 2.46] | 0.017 | 0.393 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | (Unknown) | 0.738 (0.279, 2.318) | 8.599 (5.281, 15.283) | −0.43 [−0.79, −0.06] | 0.023 | 0.441 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | 0.037 (0.021, 0.202) | 1.928 (1.433, 4.492) | −0.32 [−0.59, −0.04] | 0.024 | 0.441 |
| Dietary fiber intake: ≥7.2 g/day (n = 172) versus <7.2 g/day (n = 153) | |||||||||
| Fusobacteria | 0.162 (0.097, 0.405) | 4.026 (3.111, 6.368) | −3.27 [−5.68, −0.87] | 0.008 | 0.103 | ||||
| At the second follow-up | |||||||||
| All subjects (n = 301) | |||||||||
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | (Unknown) | 0.644 (0.276, 1.780) | 8.035 (5.257, 13.381) | −0.15 [−0.27, −0.03] | 0.014 | 0.448 |
| Dietary fiber intake: ≥7.2 g/day (n = 149) versus <7.2 g/day (n = 152) | |||||||||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Adlercreutzia | 0.001 (0.000, 0.006) | 0.354 (0.000, 0.774) | −0.23 [−0.39, −0.08] | 0.003 | 0.139 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 25.017 (8.147, 41.863) | 52.380 (28.945, 70.367) | 7.19 [1.43, 12.94] | 0.015 | 0.249 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | 2.865 (1.857, 34.708) | 17.008 (13.671, 62.999) | −10.95 [−18.40, −3.50] | 0.004 | 0.139 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | 0.272 (0.108, 0.905) | 5.215 (3.287, 9.525) | −2.05 [−3.32, −0.78] | 0.002 | 0.139 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | (Unknown) | 0.644 (0.276, 1.780) | 8.035 (5.257, 13.381) | −1.43 [−2.86, −0.01] | 0.050 | 0.330 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | 0.041 (0.021, 0.188) | 2.019 (1.441, 4.341) | −1.81 [−3.07, −0.54] | 0.005 | 0.139 |
| Longitudinal analysis | |||||||||
| Higher-higher (n = 82) versus Lower-lower (n = 80) | |||||||||
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Megamonas | 0.354 (0.237, 0.870) | 5.953 (4.874, 9.340) | −4.42 [−8.33, −0.51] | 0.027 | 0.347 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | 0.041 (0.021, 0.198) | 2.037 (1.454, 4.455) | −2.10 [−3.71, −0.49] | 0.011 | 0.319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Xu, K.; Ni, X.; Li, X.; Zhu, X.; Xu, W. Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients 2022, 14, 1003. https://doi.org/10.3390/nu14051003
Fu J, Xu K, Ni X, Li X, Zhu X, Xu W. Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients. 2022; 14(5):1003. https://doi.org/10.3390/nu14051003
Chicago/Turabian StyleFu, Jiongxing, Kelin Xu, Xumin Ni, Xiaoqiang Li, Xiaofeng Zhu, and Wanghong Xu. 2022. "Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes" Nutrients 14, no. 5: 1003. https://doi.org/10.3390/nu14051003
APA StyleFu, J., Xu, K., Ni, X., Li, X., Zhu, X., & Xu, W. (2022). Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients, 14(5), 1003. https://doi.org/10.3390/nu14051003






