Effect of Dietary Glycemic Index on β-Cell Function in Prediabetes: A Randomized Controlled Feeding Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
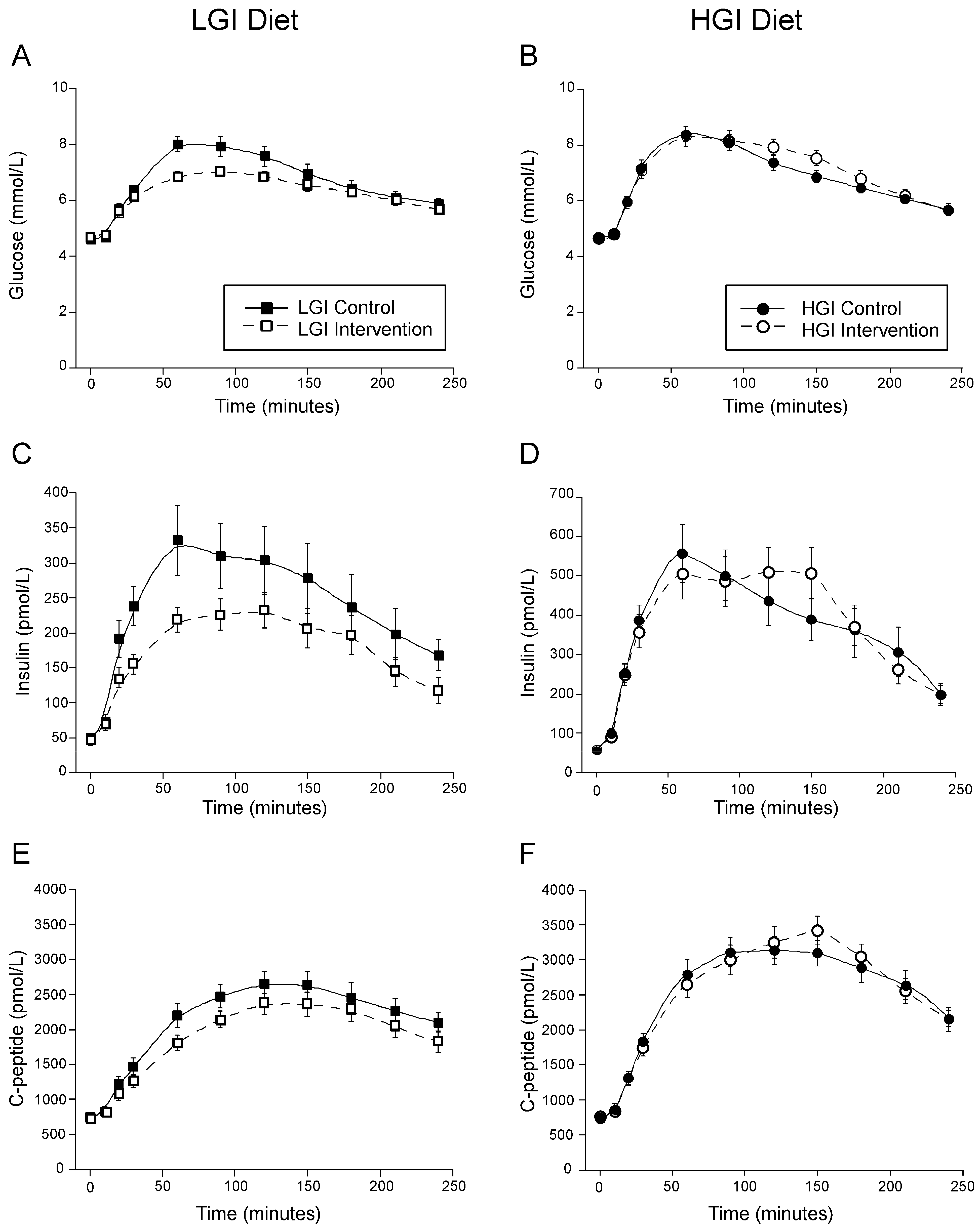
3.2. Effect of Dietary Glycemic Index on MTT Glucose, Insulin and C-Peptide
3.3. Effect of Dietary Glycemic Index on Insulin Secretion
3.4. Effect of Dietary Glycemic Index on Insulin Sensitivity, β-Cell Function and Insulin Clearance by Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. National Diabetes Statistics Report 2020 Estimates of Diabetes and Its Burden in the United States 2020. Available online: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html (accessed on 10 February 2022).
- Kahn, S.E. Clinical review 135: The importance of ß-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058. [Google Scholar] [PubMed] [Green Version]
- Utzschneider, K.M.; Prigeon, R.L.; Carr, D.B.; Hull, R.L.; Tong, J.; Shofer, J.B.; Retzlaff, B.M.; Knopp, R.H.; Kahn, S.E. Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 2006, 29, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53 (Suppl. 3), S16–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.G.; Shaw, J.A.; Taylor, R. Type 2 Diabetes: The Pathologic Basis of Reversible beta-Cell Dysfunction. Diabetes Care 2016, 39, 2080–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes care 2021, 44 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 1, CD006296. [Google Scholar] [CrossRef]
- Venn, B.J.; Green, T.J. Glycemic index and glycemic load: Measurement issues and their effect on diet-disease relationships. Eur. J. Clin. Nutr. 2007, 61 (Suppl. 1), S122–S131. [Google Scholar] [CrossRef]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.; Mehling, C.; Chiasson, J.L.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W.; Ryan, E.A. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of carbohydrates in diabetes): A randomised controlled trial. Diabetologia 2008, 51, 1607–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmerón, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Wolever, T.M.; Mehling, C. High-carbohydrate-low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br. J. Nutr. 2002, 87, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Cook, M.D.; Filion, J.; Rocco, M.; Kashyap, S.R.; Watanabe, R.M.; Barkoukis, H.; Kirwan, J.P. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am. J. Clin. Nutr. 2010, 92, 1359–1368. [Google Scholar] [CrossRef]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R.; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Johnson, T.N.; Breymeyer, K.L.; Bettcher, L.; Raftery, D.; Newton, K.M.; Neuhouser, M.L. Small changes in glucose variability induced by low and high glycemic index diets are not associated with changes in beta-cell function in adults with pre-diabetes. J. Diabetes Complicat. 2020, 34, 107586. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Schwarz, Y.; Wang, C.; Breymeyer, K.; Coronado, G.; Wang, C.Y.; Noar, K.; Song, X.; Lampe, J.W. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J. Nutr. 2012, 142, 369–374. [Google Scholar] [CrossRef]
- Van Cauter, E.; Mestrez, F.; Sturis, J.; Polonsky, K.S. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992, 41, 368–377. [Google Scholar] [CrossRef]
- Mari, A.; Tura, A.; Gastaldelli, A.; Ferrannini, E. Assessing insulin secretion by modeling in multiple-meal tests: Role of potentiation. Diabetes 2002, 51 (Suppl. 1), S221–S226. [Google Scholar] [CrossRef] [Green Version]
- Mari, A.; Schmitz, O.; Gastaldelli, A.; Oestergaard, T.; Nyholm, B.; Ferrannini, E. Meal and oral glucose tests for assessment of b-cell function: Modeling analysis in normal subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1159–E1166. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Ferrannini, E. Beta-cell function assessment from modelling of oral tests: An effective approach. Diabetes Obes. Metab. 2008, 10 (Suppl. 4), 77–87. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001, 24, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Trico, D.; Natali, A.; Arslanian, S.; Mari, A.; Ferrannini, E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight 2018, 3, e124912. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Polidori, D.C.; Yoshino, M.; Kearney, M.L.; Patterson, B.W.; Mittendorfer, B.; Klein, S. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J. Clin. Investig. 2020, 130, 3305–3314. [Google Scholar] [CrossRef] [Green Version]
- Utzschneider, K.M.; Kahn, S.E.; Polidori, D.C. Hepatic Insulin Extraction in NAFLD Is Related to Insulin Resistance Rather Than Liver Fat Content. J. Clin. Endocrinol. Metab. 2019, 104, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Abdul Ghani, M.; DeFronzo, R.A. Adaptation of Insulin Clearance to Metabolic Demand Is a Key Determinant of Glucose Tolerance. Diabetes 2021, 70, 377–385. [Google Scholar] [CrossRef]
- Bizzotto, R.; Tricò, D.; Natali, A.; Gastaldelli, A.; Muscelli, E.; De Fronzo, R.A.; Arslanian, S.; Ferrannini, E.; Mari, A. New Insights on the Interactions Between Insulin Clearance and the Main Glucose Homeostasis Mechanisms. Diabetes Care 2021, 44, 2115–2123. [Google Scholar] [CrossRef]

| All N = 35 | LGI N = 17 | HGI N = 18 | p Value | |
|---|---|---|---|---|
| Age (years) | 54.20 ± 1.57 | 58.06 ± 1.52 | 50.56 ± 2.44 | 0.01 |
| Gender (M/F) | 18/17 | 9/8 | 9/9 | 0.86 |
| BMI (kg/m2) | 32.44 ± 0.94 | 32.45 ± 1.16 | 32.42 ± 1.50 | 0.99 |
| HbA1c (% and mmol/mol) | 5.76 ± 0.06 39.48 ± 0.63 | 5.76 ± 0.06 39.44 ± 0.68 | 5.77 ± 0.10 39.53 ± 1.07 | 0.95 |
| Screening fasting glucose (mmol/L) | 5.82 ± 0.05 | 5.93 ± 0.06 | 5.73 ± 0.08 | 0.07 |
| Screening 2 h glucose (mmol/L) | 7.92 ± 0.22 | 7.71 ± 0.36 | 8.12 ± 0.27 | 0.36 |
| IFG/IGT/IFG + IGT | 14/5/16 | 9/2/6 | 5/4/10 | 0.31 |
| Race (n) | 0.38 | |||
| White | 24 | 13 | 11 | |
| African American | 5 | 1 | 4 | |
| Asian | 6 | 3 | 3 | |
| Ethnicity (n) | 0.95 | |||
| Hispanic | 4 | 2 | 2 | |
| Non-Hispanic | 31 | 15 | 16 |
| LGI Diet (n = 17) | HGI Diet (n = 18) | GEE Analysis LGI vs. HGI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LGI | Change | p Value | Control | HGI | Change | p Value | p Value | |
| OGIS [mL/min·m2] | 440.27 ± 12.06 | 446.26 ± 9.57 | 5.99 ± 2.49 | 0.37 | 407.63 ± 12.16 | 407.67 ± 11.25 | 0.04 ± 0.91 | 0.99 | 0.08 |
| Matsuda Index | 7.10 (5.39) | 8.85 (6.25) | 0.57 (2.62) | 0.01 | 5.72 (3.60) | 4.53 (3.22) | −0.24 (1.52) | 0.41 | 0.001 |
| Basal glucose [mmol/L] | 4.62 ± 0.12 | 4.70 ± 0.11 | 0.08 ± 0.01 | 0.07 | 4.70 ± 0.11 | 4.65 ± 0.12 | −0.05 ± 0.01 | 0.39 | 0.06 |
| incAUCglu during MTT [mmol/L] | 526.87 ± 49.72 | 393.31 ± 37.28 | −133.56 ± 12.44 | <0.001 | 532.06 ± 37.73 | 591.53 ± 38.05 | 59.47 ± 0.32 | 0.14 | <0.001 |
| Early Insulin Response (0–30 min) [pmol/mmol] | 102.66 (55.3) | 68.98 (59.73) | −24.25 (55.71) | 0.02 | 166.96 (129.13) | 107.55 (149.09) | −3.54 (60.3) | 0.24 | 0.10 * |
| incAUCins/incAUCglu (0–240 min) [pmol/mmol] | 85.46 (67.54) | 103.03 (53.42) | −7.48 (47.44) | 0.48 | 140.23 (262.29) | 141.40 (111.53) | −7.18 (50.21) | 0.13 | 0.50 * |
| Early C-peptide Response (0–30 min) [nmol/mmol] | 0.41 (0.25) | 0.33 (0.24) | −0.008 (0.03) | 0.75 | 0.48 (0.26) | 0.49 (0.33) | −0.005 (0.03) | 0.13 | 0.28 * |
| incAUCcp/incAUCglu (0–240 min) [nmol/mmol] | 0.71 (0.28) | 0.78 (0.38) | 0.06 (0.32) | 0.03 | 0.88 (0.49) | 0.81 (0.48) | −0.08 (0.24)) | 0.03 | 0.003 * |
| Glucose Sensitivity [pmol/min·m2·mM] | 65.23 (48.56) | 91.23 (26.98) | −6.85 (51.91) | 0.69 | 95.75(50.04) | 106.52 (75.55) | 7.59 (35.06) | 0.13 | 0.29 |
| Rate Sensitivity [pmol/m2·mM] | 1017.97 (963.20) | 813.77 (632.38) | −287.73 (639.05) | 0.82 | 898.35 (987.52) | 916.39 (1165.96) | 32.73 (793.58) | 0.65 | 0.35 |
| Potentiation Factor Ratio | 1.18 (0.65) | 1.08 (0.45) | −0.20 (0.56) | 0.13 | 1.165 (0.30) | 1.28 (0.48) | 0.02 (0.49) | 0.84 | 0.25 |
| ISR @ 5mmol glucose [pmol/min·m2] | 135.85 ± 12.13 | 134.54 ± 10.54 | −1.31 ± 1.59 | 0.82 | 171.46 ± 13.30 | 148.85 ± 15.95 | −22.61 ± 2.65 | 0.02 | 0.20 * |
| Total Insulin Secretion [nmol/m2] | 70.18 (32.70) | 60.45 (29.17) | −8.57 (19.50) | 0.02 | 82.25 (435.67) | 82.23 (36.64) | −1.88 (12.03) | 0.87 | 0.002 * |
| Total IS/incAUCglu [nmol·L/mmol·m2] | 162.27 (71.89) | 189.10 (100.29) | 29.00 (52.25) | 0.002 | 170.49 (114.65) | 151.51 (80.38) | −11.48 (38.50) | 0.10 | 0.001 * |
| Basal Insulin Clearance [pmol/L] | 1.86 (1.12) | 2.21 (0.76) | −0.063 (0.51) | 0.24 | 1.73 (0.59) | 1.65 (1.09) | −0.11(0.67) | 0.99 | 0.314 |
| Total Insulin Clearance [pmol/L] | 1.37 ± 0.093 | 1.56 ± 0.09 | 0.19 ± 0.001 | 0.01 | 1.11 ± 0.09 | 1.07 ± 0.09 | −0.04 ± 0.002 | 0.21 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipe, A.T.; Neuhouser, M.L.; Breymeyer, K.L.; Utzschneider, K.M. Effect of Dietary Glycemic Index on β-Cell Function in Prediabetes: A Randomized Controlled Feeding Study. Nutrients 2022, 14, 887. https://doi.org/10.3390/nu14040887
Sipe AT, Neuhouser ML, Breymeyer KL, Utzschneider KM. Effect of Dietary Glycemic Index on β-Cell Function in Prediabetes: A Randomized Controlled Feeding Study. Nutrients. 2022; 14(4):887. https://doi.org/10.3390/nu14040887
Chicago/Turabian StyleSipe, Alexander T., Marian L. Neuhouser, Kara L. Breymeyer, and Kristina M. Utzschneider. 2022. "Effect of Dietary Glycemic Index on β-Cell Function in Prediabetes: A Randomized Controlled Feeding Study" Nutrients 14, no. 4: 887. https://doi.org/10.3390/nu14040887
APA StyleSipe, A. T., Neuhouser, M. L., Breymeyer, K. L., & Utzschneider, K. M. (2022). Effect of Dietary Glycemic Index on β-Cell Function in Prediabetes: A Randomized Controlled Feeding Study. Nutrients, 14(4), 887. https://doi.org/10.3390/nu14040887






