Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China
Abstract
:1. Introduction
2. Materials and Methods
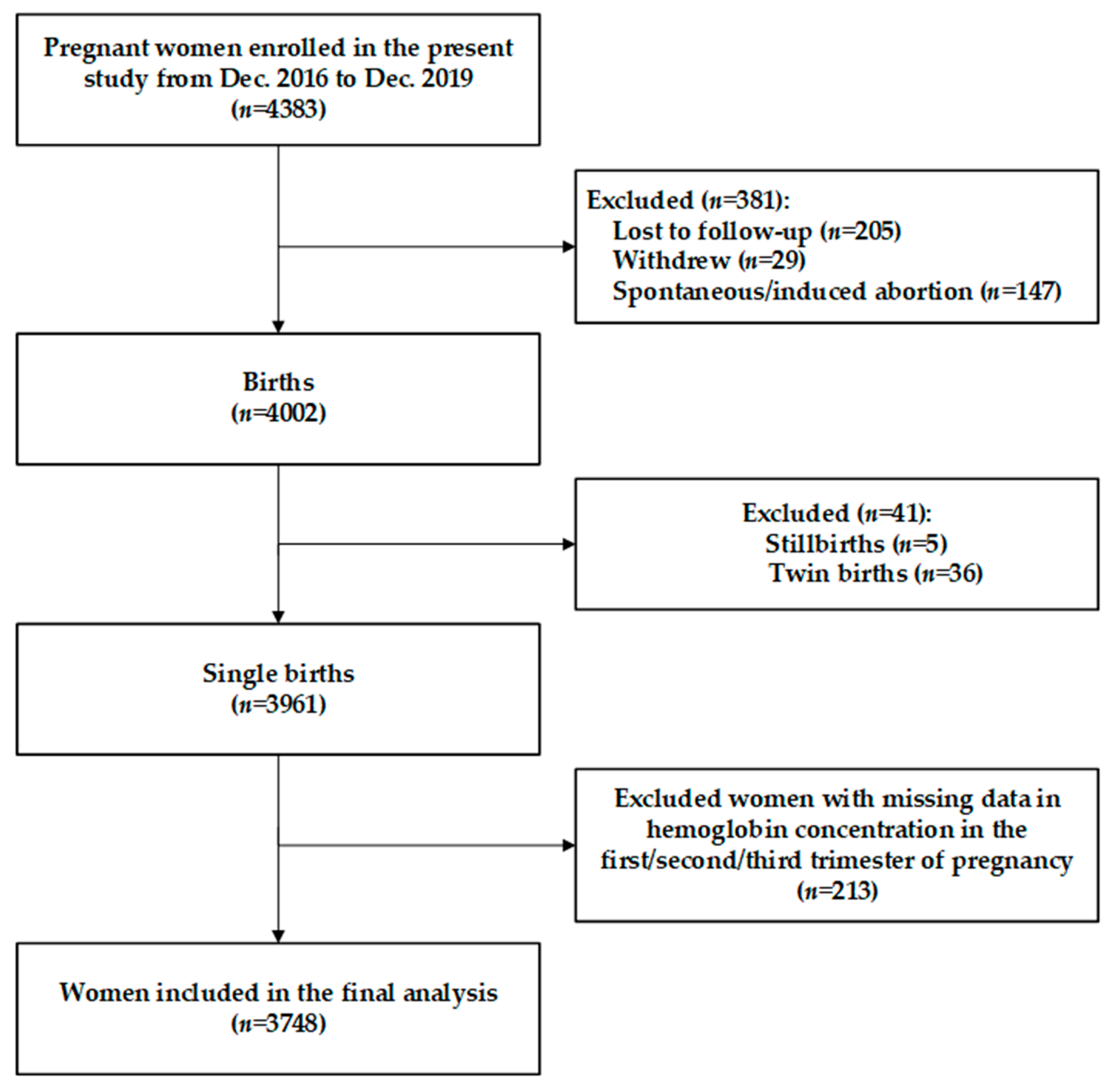
2.1. Study Design and Participants
2.2. Sample Size
2.3. Data Collection
2.4. Hemoglobin Measurement
2.5. Outcome Assessment
2.6. Covariate Assessment
2.7. Statistical Analysis
3. Results
3.1. Maternal Baseline Characteristics and Neonatal Birth Outcomes
3.2. Maternal Hemoglobin Status in Different Trimesters of Pregnancy
3.3. Associations between Maternal Hemoglobin Concentrations during Pregnancy and Birth Weight-Related Outcomes
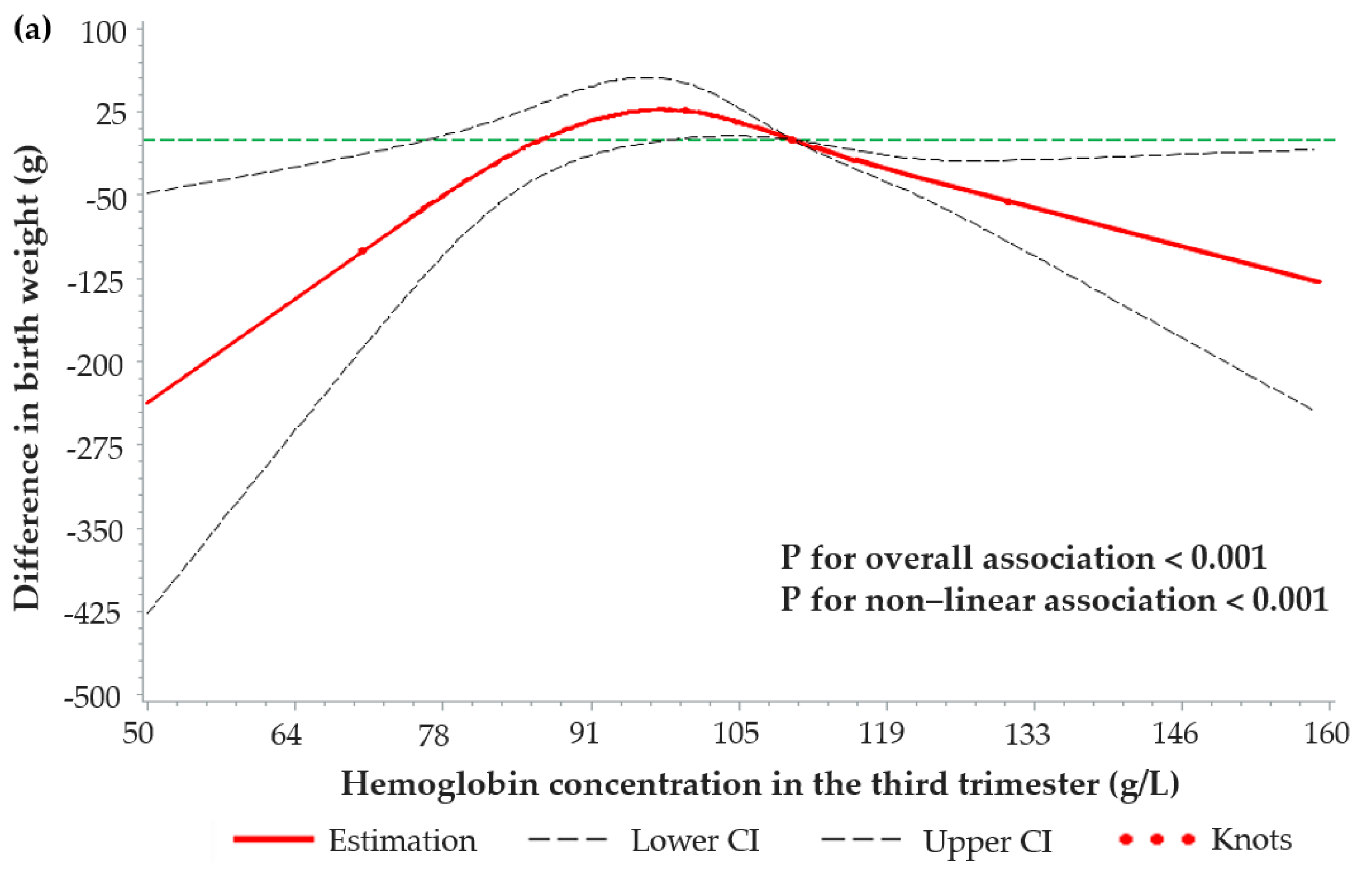
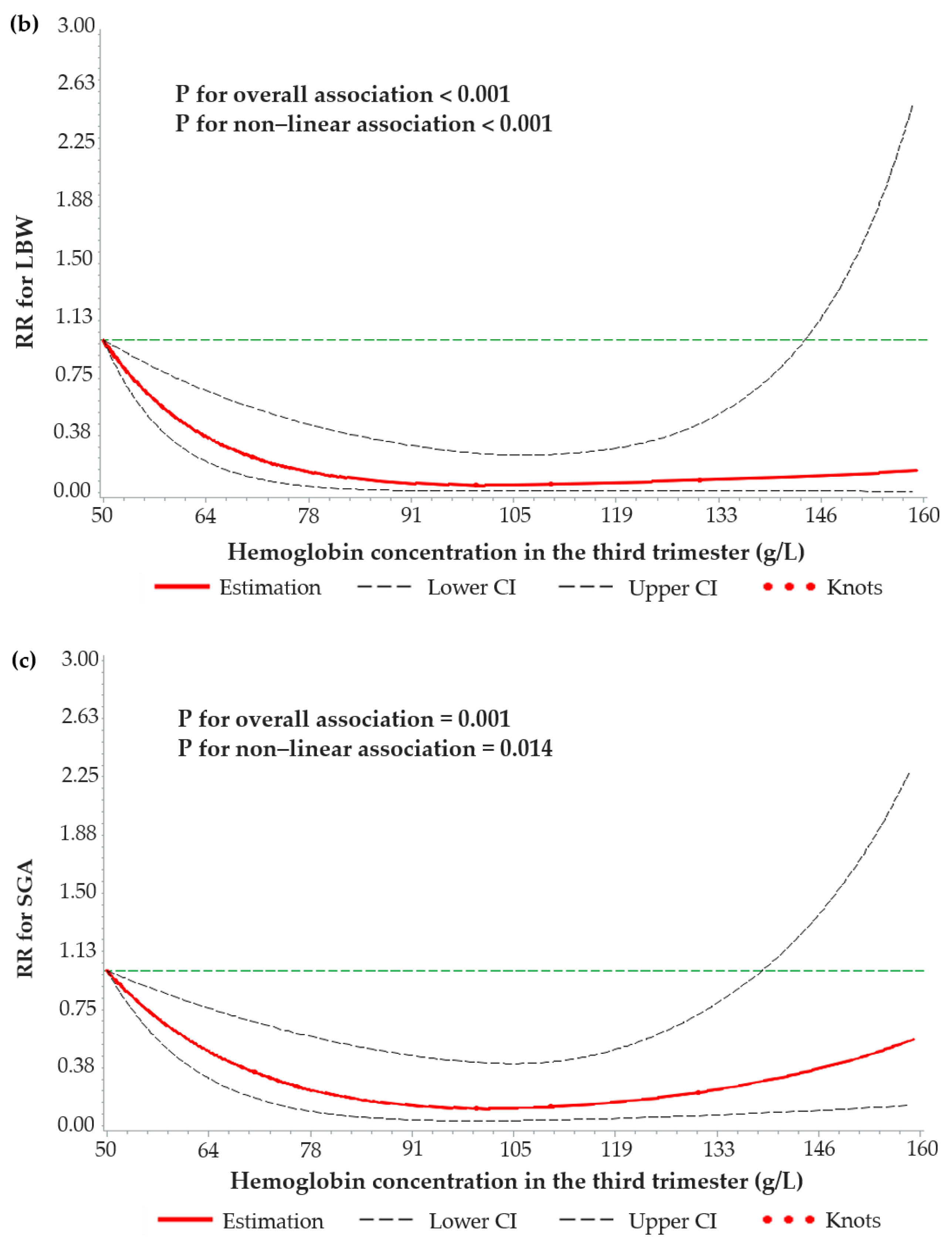
3.4. Dose–Response Relationships between Maternal Hemoglobin Concentrations and Birth Weight-Related Outcomes
3.5. Associations between Maternal Hemoglonbin in the Third Trimester and Birth Weight-Related Outcomes According to Hemoglobin Concentration and Maternal Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dobbins, T.A.; Sullivan, E.A.; Roberts, C.L.; Simpson, J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 2012, 197, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, R.L.; Culhane, J.F. Low birth weight in the United States. Am. J. Clin. Nutr. 2007, 85, 584s–590s. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef] [Green Version]
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21 standard: Analysis of CHERG datasets. BMJ 2017, 358, j3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shorer, D.T.; Wainstock, T.; Sheiner, E.; Landau, D.; Pariente, G. Long-term endocrine outcome of small for gestational age infants born to mothers with and without gestational diabetes mellitus. Gynecol. Endocrinol. 2019, 35, 1003–1009. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.; Oian, P. First- and second-trimester hemoglobin levels. Relation to birth weight and gestational age. Acta Obstet. Gynecol. Scand. 1993, 72, 246–251. [Google Scholar] [CrossRef]
- Steer, P.; Alam, M.A.; Wadsworth, J.; Welch, A. Relation between maternal haemoglobin concentration and birth weight in different ethnic groups. BMJ 1995, 310, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Oaks, B.M. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am. J. Clin. Nutr. 2017, 106, 1694S–1702S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Goonewardene, M.; Shehata, M.; Hamad, A. Anaemia in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Haider, B.A.; Olofin, I.; Wang, M.; Spiegelman, D.; Ezzati, M.; Fawzi, W.W. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2013, 346, f3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Abe, S.K.; Rahman, M.S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, H.; Hakkarainen, K.; Heinonen, S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin. Nutr. 2003, 22, 271–275. [Google Scholar] [CrossRef]
- Ren, A.; Wang, J.; Ye, R.W.; Li, S.; Liu, J.M.; Li, Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int. J. Gynaecol. Obstet. 2007, 98, 124–128. [Google Scholar] [CrossRef]
- Scanlon, K.S.; Yip, R.; Schieve, L.A.; Cogswell, M.E. High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstet. Gynecol. 2000, 96, 741–748. [Google Scholar] [CrossRef]
- Zhou, L.M.; Yang, W.W.; Hua, J.Z.; Deng, C.Q.; Tao, X.; Stoltzfus, R.J. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am. J. Epidemiol. 1998, 148, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, G.F.; Steenland, K.; Tapia, V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1477–R1485. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Buekens, P.; Alexander, S.; Demianczuk, N.; Wollast, E. Anemia during pregnancy and birth outcome: A meta-analysis. Am. J. Perinatol. 2000, 17, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; O’Brien, K.O.; Nathanson, M.S.; Mancini, J.; Witter, F.R. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J. Nutr. 2003, 133, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Ahmad, T.; Macri, C.; Aly, H. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J. Perinat. Med. 2012, 40, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Oaks, B.M.; Tandon, S.; Martorell, R.; Dewey, K.G.; Wendt, A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 47–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Ananth, C.V.; Li, Z.; Smulian, J.C. Maternal anaemia and preterm birth: A prospective cohort study. Int. J. Epidemiol. 2009, 38, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.A.; Tikmani, S.S.; Saleem, S.; Patel, A.B.; Hibberd, P.L.; Goudar, S.S.; Dhaded, S.; Derman, R.J.; Moore, J.L.; McClure, E.M.; et al. Hemoglobin concentrations and adverse birth outcomes in South Asian pregnant women: Findings from a prospective Maternal and Neonatal Health Registry. Reprod. Health 2020, 17, 154. [Google Scholar] [CrossRef]
- Li, S.; Mi, B.; Qu, P.; Liu, D.; Lei, F.; Wang, D.; Zeng, L.; Kang, Y.; Shen, Y.; Pei, L.; et al. Association of antenatal vitamin B complex supplementation with neonatal vitamin B status: Evidence from a cluster randomized controlled trial. Eur. J. Nutr. 2021, 60, 1031–1039. [Google Scholar] [CrossRef]
- Perinatal medicine branch of Chinese Medical Association. Chinese clinical guidelines for the diagnosis and treatment of maternal anemia during pregnancy. Zhonghua Wei Chan Yi Xue Za Zhi 2014, 7, 451–454. [Google Scholar]
- Wang, L.; Mei, Z.; Li, H.; Zhang, Y.; Liu, J.; Serdula, M.K. Modifying effects of maternal Hb concentration on infant birth weight in women receiving prenatal iron-containing supplements: A randomised controlled trial. Br. J. Nutr. 2016, 115, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Randall, D.A.; Patterson, J.A.; Gallimore, F.; Morris, J.M.; Simpson, J.M.; McGee, T.M.; Ford, J.B. Haemoglobin trajectories during pregnancy and associated outcomes using pooled maternity and hospitalization data from two tertiary hospitals. Vox Sang. 2019, 114, 842–852. [Google Scholar] [CrossRef]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. WHO. 2011. Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 7 December 2021).
- Pena-Rosas, J.P.; De-Regil, L.M.; Dowswell, T.; Viteri, F.E. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2012, 12, CD004736. [Google Scholar] [PubMed]
- Zhu, L.; Zhang, R.; Zhang, S.; Shi, W.; Yan, W.; Wang, X.; Lyu, Q.; Liu, L.; Zhou, Q.; Qiu, Q.; et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi 2015, 53, 97–103. [Google Scholar] [PubMed]
- Li, S.; Liu, D.; Zhang, R.; Lei, F.; Liu, X.; Cheng, Y.; Li, C.; Xiao, M.; Guo, L.; Li, M.; et al. The association of maternal dietary folate intake and folic acid supplementation with small-for-gestational-age births: A cross-sectional study in Northwest China. Br. J. Nutr. 2019, 122, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Xue, H.; Zhao, W.; Yang, X.; Zhu, X.; Zhao, L.; Yang, Y. Understanding the China Blue Paper on Obesity Prevention and Control and policy implications and recommendations for obesity prevention and control in China. Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 875–884. [Google Scholar]
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef]
- Roux, A.V.D. Neighborhoods and health: Where are we and were do we go from here? Rev. Epidemiol. Sante Publique 2007, 55, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- WHO. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; WHO: Geneva, Switzerland, 2008; pp. 1–51. [Google Scholar]
- Lin, L.; Wei, Y.; Zhu, W.; Wang, C.; Su, R.; Feng, H.; Yang, H. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: A multicentre retrospective study. BMC Pregnancy Childbirth 2018, 18, 111. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dang, S.; Cheng, Y.; Qiu, H.; Mi, B.; Jiang, Y.; Qu, P.; Zeng, L.; Wang, Q.; Li, Q.; et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017, 20, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Jwa, S.C.; Fujiwara, T.; Yamanobe, Y.; Kozuka, K.; Sago, H. Changes in maternal hemoglobin during pregnancy and birth outcomes. BMC Pregnancy Childbirth 2015, 15, 80. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Guo, X.F.; Liu, S.; Long, J.H.; Zhang, G.Q.; Huang, M.C.; Qiu, X.Q. Impact and changes of maternal hemoglobin on birth weight in pregnant women of Zhuang Nationality, in Guangxi. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 154–157. [Google Scholar] [PubMed]
- Steer, P.J. Maternal hemoglobin concentration and birth weight. Am. J. Clin. Nutr. 2000, 71, 1285S–1287S. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Rahman, M.M.; Rahman, M.S.; Swe, K.T.; Islam, M.R.; Rahman, M.O.; Akter, S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Grantz, K.L.; Kim, S.; Grobman, W.A.; Newman, R.; Owen, J.; Skupski, D.; Grewal, J.; Chien, E.K.; Wing, D.A.; Wapner, R.J.; et al. Fetal growth velocity: The NICHD fetal growth studies. Am. J. Obstet. Gynecol. 2018, 219, 285.e1–285.e36. [Google Scholar] [CrossRef] [Green Version]
- Singla, P.N.; Tyagi, M.; Kumar, A.; Dash, D.; Shankar, R. Fetal growth in maternal anaemia. J. Trop. Pediatr. 1997, 43, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Zondervan, H.A.; Voorhorst, F.J.; Robertson, E.A.; Kurver, P.H.; Massen, C. Is maternal whole blood viscosity a factor in fetal growth? Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 20, 145–151. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, E.; Guo, J.; Pan, L.; Li, B.; Wang, P.; Liu, J.; Wang, Y.; Liu, G.; Baccarelli, A.A.; et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS ONE 2013, 8, e82310. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Socio-demographic characteristics | |
| Age (years) | 26.2 ± 4.1 |
| <25 | 1369 (36.5) |
| 25–34 | 2235 (59.6) |
| ≥35 | 144 (3.8) |
| Education | |
| Junior high school or below | 2040 (54.4) |
| Senior high school | 1179 (31.5) |
| College or above | 529 (14.1) |
| Farmers | 3292 (87.8) |
| Per capita annual household income (RMB) | |
| Low (<5000) | 872 (23.3) |
| Medium (5000–9999) | 1519 (40.5) |
| High (≥10,000) | 1357 (36.2) |
| Health-related characteristics | |
| Primipara | 1914 (51.1) |
| Gestational age at enrolment (weeks) | 14.4 ± 6.1 |
| ≤12 | 1580 (42.2) |
| >12 | 2168 (57.8) |
| Height (cm) | 159.8 ± 4.8 |
| Weight at enrolment (kg) | 55.5 ± 8.11 |
| BMI at enrolment (kg/m2) | |
| Underweight (<18.5) | 408 (10.9) |
| Normal weight (18.5–23.9) | 2662 (71.0) |
| Overweight (24.0–27.9) | 553 (14.8) |
| Obesity (≥28.0) | 125 (3.3) |
| More than five antenatal visits | 1229 (32.8) |
| Micronutrient supplementation | |
| Folic acid | 1363 (36.3) |
| Folic acid + iron | 1130 (30.1) |
| Folic acid + vitamin B complex | 1255 (33.4) |
| Birth outcomes | |
| Birth weight (g) | 3233.4 ± 418.3 |
| Gestational age at delivery (weeks) | 39.7 ± 1.3 |
| Gender, male | 1936 (51.7) |
| LBW | 99 (2.6) |
| SGA | 501 (13.4) |
| Hemoglobin (g/L) | Mean ± SD or n (%) |
|---|---|
| First trimester | |
| Average hemoglobin concentration | 123.2 ± 14.4 |
| Anemia (<110) | 624 (16.6) |
| Severe anemia (<70) | 0 (0.0) |
| Moderate anemia (70–99) | 203 (5.4) |
| Mild anemia (100–109) | 421 (11.2) |
| Normal (110–129) | 1883 (50.2) |
| Hemoglobin ≥ 130 | 1241 (33.1) |
| Second trimester | |
| Average hemoglobin concentration | 115.6 ± 13.4 |
| Anemia (<110) | 1157 (30.9) |
| Severe anemia (<70) | 0 (0.0) |
| Moderate anemia (70–99) | 427 (11.4) |
| Mild anemia (100–109) | 730 (19.5) |
| Normal (110–129) | 2039 (54.4) |
| Hemoglobin ≥ 130 | 552 (14.7) |
| Third trimester | |
| Average hemoglobin concentration | 110.8 ± 13.9 |
| Anemia (<110) | 1720 (45.9) |
| Severe anemia (<70) | 12 (0.3) |
| Moderate anemia (70–99) | 719 (19.2) |
| Mild anemia (100–109) | 989 (26.4) |
| Normal (110–129) | 1720 (45.9) |
| Hemoglobin ≥ 130 | 308 (8.2) |
| Outcomes | Hemoglobin (g/L) | Mean (SD) or n (%) | Unadjusted Model | Adjusted Model 2 |
|---|---|---|---|---|
| Mean (SD) | Changes (95% CI) | Changes (95% CI) | ||
| Birth weight | First trimester | |||
| <70 | - | - | - | |
| 70–99 | 3240.6 (415.7) | 21.0 (−51.0, 92.9) | 26.6 (−34.0, 87.2) | |
| 100–109 | 3238.1 (418.0) | 18.4 (−29.5, 66.2) | 25.1 (−20.5, 70.7) | |
| 110–129 | 3221.1 (410.1) | Ref. | Ref. | |
| ≥130 | 3249.1 (430.8) | 29.0 (1.7, 56.4) | 26.5 (0.2, 52.8) | |
| Second trimester | ||||
| <70 | - | - | - | |
| 70–99 | 3258.0 (426.1) | 27.5 (−3.3, 58.3) | 30.8 (−3.2, 64.8) | |
| 100–109 | 3221.8 (412.1) | −9.3 (−47.9, 29.4) | −5.0 (−44.6, 34.7) | |
| 110–129 | 3230.1 (424.8) | Ref. | Ref. | |
| ≥130 | 3241.5 (395.4) | 10.9 (−26.9, 48.6) | 11.5 (−22.8, 45.7) | |
| Third trimester | ||||
| <70 | 2988.3 (549.6) | −230.5 (−455.2, −5.7) | −216.3 (−426.7, −5.9) | |
| 70–99 | 3253.3 (413.5) | 35.9 (−0.2, 71.9) | 45.8 (9.9, 81.7) | |
| 100–109 | 3259.2 (440.5) | 42.1 (5.4, 78.8) | 46.5 (7.6, 85.3) | |
| 110–129 | 3220.0 (409.0) | Ref. | Ref. | |
| ≥130 | 3193.4 (419.7) | −29.2 (−72.5, 14.2) | −30.7 (−76.1, 14.7) | |
| n (%) | RR (95% CI) | RR (95% CI) | ||
| LBW | First trimester | |||
| <70 | - | - | - | |
| 70–99 | 4 (2.0) | 0.67 (0.22, 2.06) | 0.72 (0.24, 2.16) | |
| 100–109 | 9 (2.1) | 0.77 (0.39, 1.54) | 0.80 (0.39, 1.63) | |
| 110–129 | 54 (2.9) | Ref. | Ref. | |
| ≥130 | 32 (2.6) | 0.91 (0.61, 1.38) | 0.85 (0.54, 1.33) | |
| Second trimester | ||||
| <70 | - | - | - | |
| 70–99 | 12 (2.8) | 0.95 (0.44, 2.02) | 1.10 (0.52, 2.29) | |
| 100–109 | 17 (2.3) | 0.79 (0.47, 1.34) | 0.90 (0.52, 1.55) | |
| 110–129 | 59 (2.9) | Ref. | Ref. | |
| ≥130 | 11 (2.0) | 0.70 (0.35, 1.41) | 0.53 (0.20, 1.37) | |
| Third trimester | ||||
| <70 | 2 (16.7) | 6.36 (2.07, 19.59) | 7.47 (2.53, 22.08) | |
| 70–99 | 21 (2.9) | 1.10 (0.76, 1.61) | 1.20 (0.76, 1.91) | |
| 100–109 | 23 (2.3) | 0.88 (0.57, 1.37) | 0.94 (0.58, 1.51) | |
| 110–129 | 42 (2.4) | Ref. | Ref. | |
| ≥130 | 11 (3.6) | 1.51 (0.64, 3.54) | 1.41 (0.49, 4.03) | |
| SGA | First trimester | |||
| <70 | - | - | - | |
| 70–99 | 27 (13.3) | 0.97 (0.68, 1.39) | 0.98 (0.67, 1.44) | |
| 100–109 | 51 (12.2) | 0.89 (0.65, 1.23) | 0.91 (0.65, 1.29) | |
| 110–129 | 256 (13.6) | Ref. | Ref. | |
| ≥130 | 167 (13.5) | 0.99 (0.82, 1.20) | 0.99 (0.81, 1.21) | |
| Second trimester | ||||
| <70 | - | - | - | |
| 70–99 | 56 (13.1) | 0.94 (0.77, 1.16) | 1.03 (0.83, 1.28) | |
| 100–109 | 90 (12.4) | 0.89 (0.75, 1.06) | 0.91 (0.76, 1.09) | |
| 110–129 | 285 (14.0) | Ref. | Ref. | |
| ≥130 | 70 (12.7) | 0.91 (0.74, 1.11) | 0.95 (0.75, 1.20) | |
| Third trimester | ||||
| <70 | 2 (16.7) | 1.12 (0.31, 4.11) | 1.32 (0.35, 4.98) | |
| 70–99 | 91 (12.7) | 0.86 (0.70, 1.05) | 0.87 (0.70, 1.09) | |
| 100–109 | 104 (10.5) | 0.72 (0.61, 0.84) | 0.73 (0.61, 0.87) | |
| 110–129 | 252 (14.7) | Ref. | Ref. | |
| ≥130 | 52 (16.9) | 1.16 (0.88, 1.53) | 1.16 (0.87, 1.55) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, S.; Zhang, B.; Kang, Y.; Cheng, Y.; Zeng, L.; Chen, F.; Mi, B.; Qu, P.; Zhao, D.; et al. Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China. Nutrients 2022, 14, 858. https://doi.org/10.3390/nu14040858
Liu D, Li S, Zhang B, Kang Y, Cheng Y, Zeng L, Chen F, Mi B, Qu P, Zhao D, et al. Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China. Nutrients. 2022; 14(4):858. https://doi.org/10.3390/nu14040858
Chicago/Turabian StyleLiu, Danmeng, Shanshan Li, Binyan Zhang, Yijun Kang, Yue Cheng, Lingxia Zeng, Fangyao Chen, Baibing Mi, Pengfei Qu, Doudou Zhao, and et al. 2022. "Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China" Nutrients 14, no. 4: 858. https://doi.org/10.3390/nu14040858
APA StyleLiu, D., Li, S., Zhang, B., Kang, Y., Cheng, Y., Zeng, L., Chen, F., Mi, B., Qu, P., Zhao, D., Zhu, Z., Yan, H., Wang, D., & Dang, S. (2022). Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China. Nutrients, 14(4), 858. https://doi.org/10.3390/nu14040858







