Effect of Morning and Evening Exercise on Energy Balance: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Supervised Exercise Interventions
2.3. Body Weight and Composition
2.4. Sleep, Sedentary Behavior, and Physical Activity
2.5. Components of Energy Balance
2.6. Cardiorespiratory Fitness
2.7. Statistical Analyses
3. Results
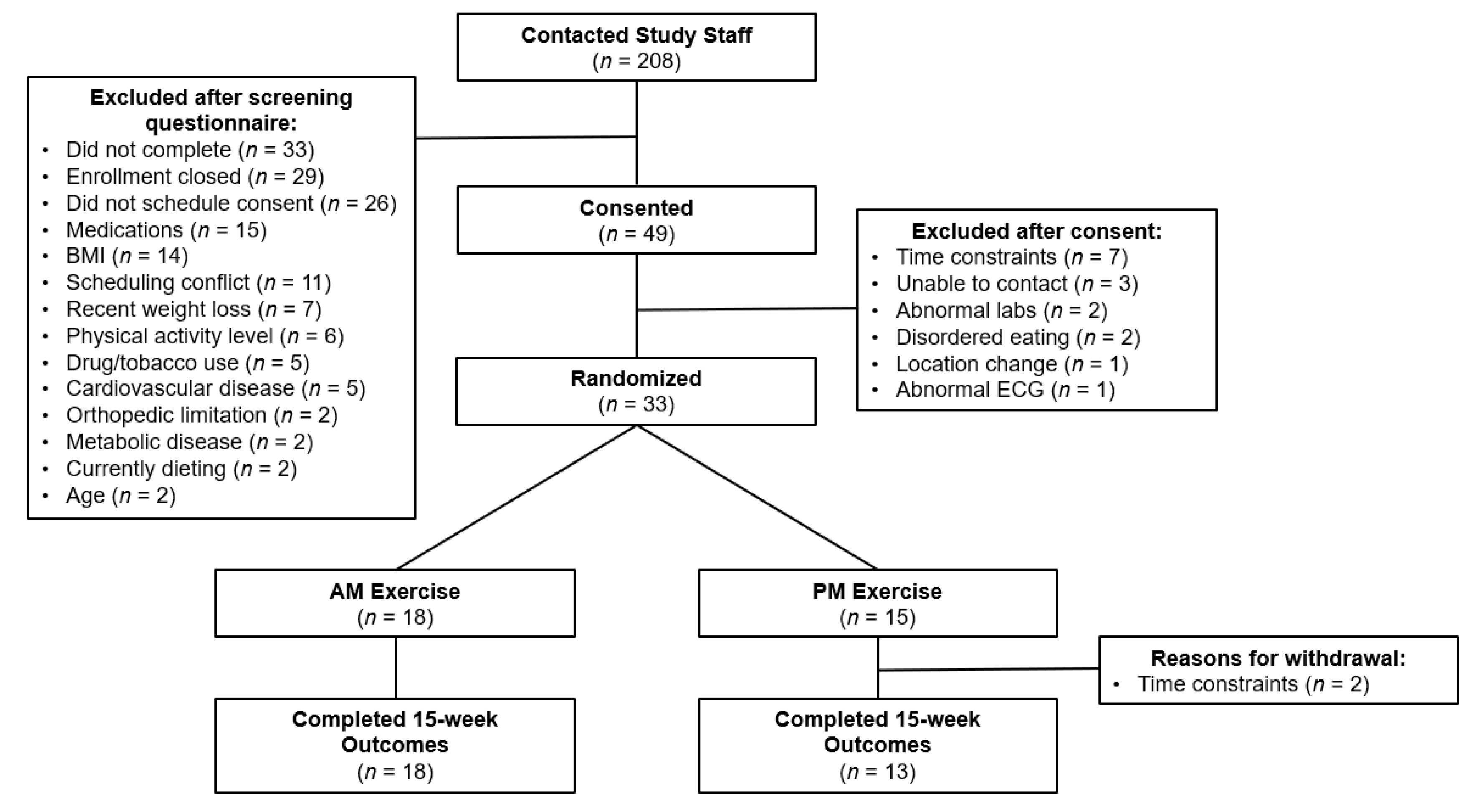
3.1. Enrollment and Retention
3.2. Exercise Intervention Adherence
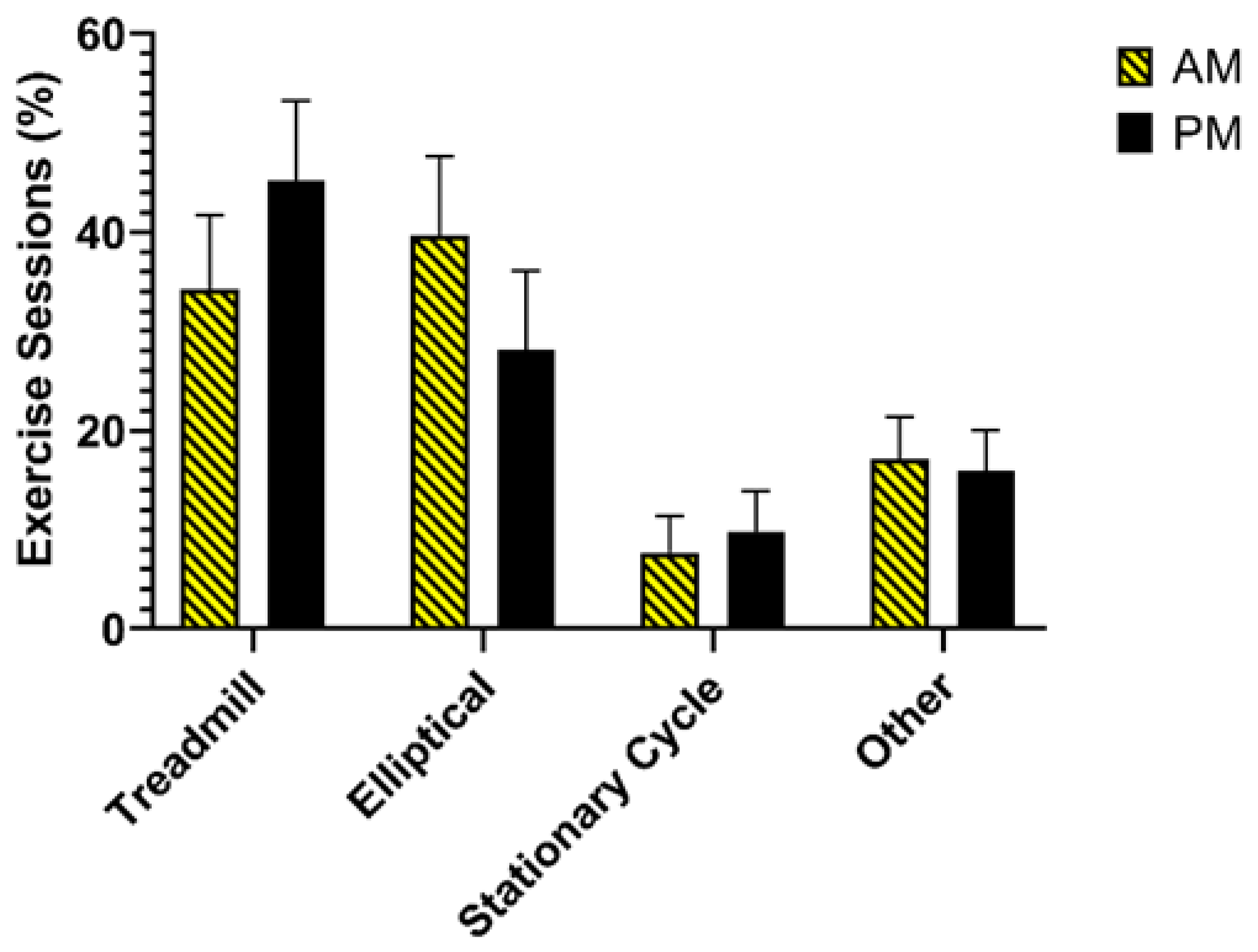
3.3. Exercise Intervention Acceptability
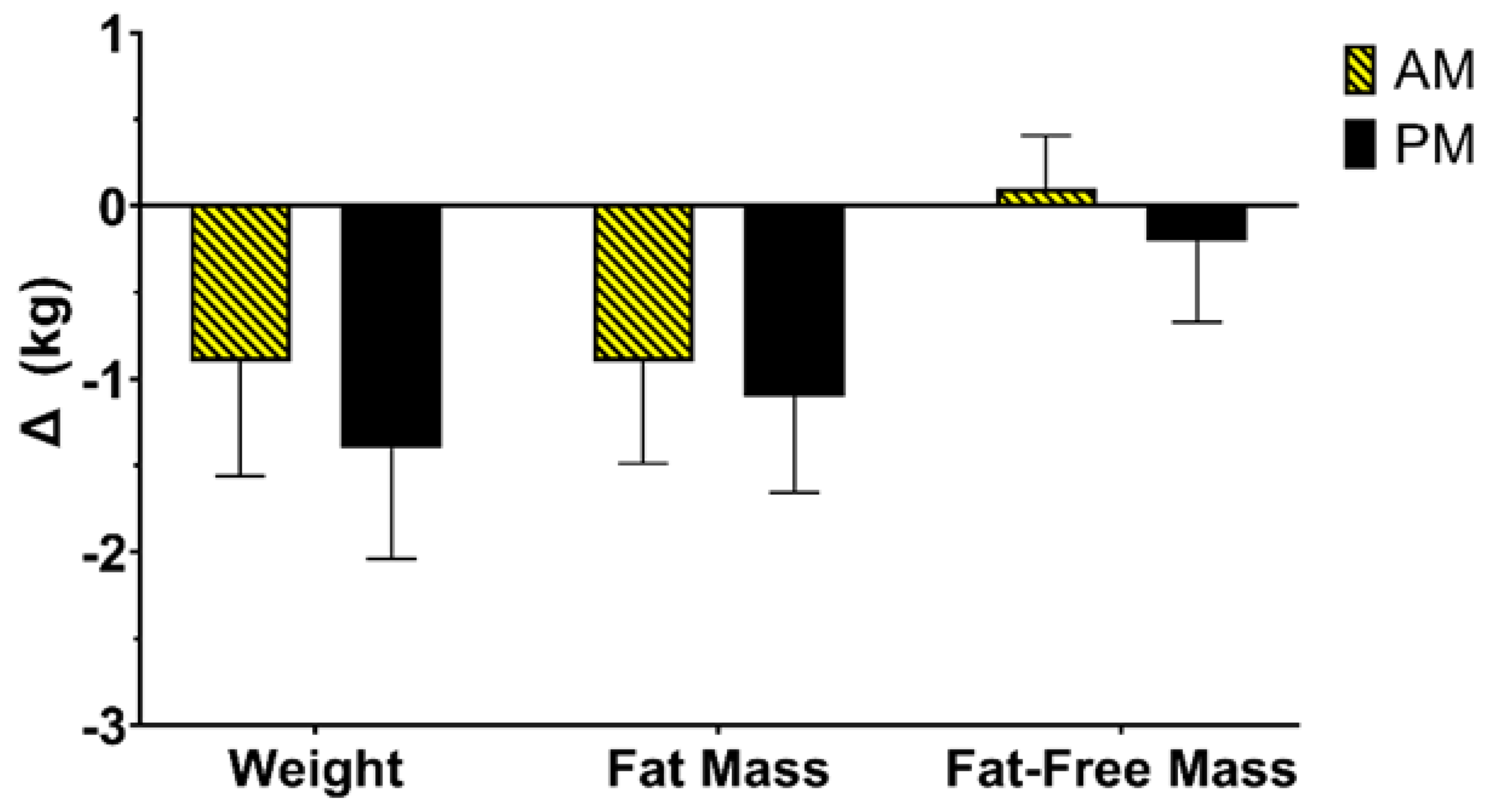
3.4. Change in Weight and Body Composition
3.5. Change in Energy Expenditure and Energy Intake
3.6. Change in Sleep, Sedentary Behavior, and Physical Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, C.K.; Johnson, W.D.; Myers, C.A.; Apolzan, J.W.; Earnest, C.P.; Thomas, D.M.; Rood, J.C.; Johannsen, N.M.; Tudor-Locke, C.; Harris, M.; et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Honas, J.J.; Smith, B.K.; Mayo, M.S.; Gibson, C.A.; Sullivan, D.K.; Lee, J.; Herrmann, S.D.; Lambourne, K.; Washburn, R.A. Aerobic exercise alone results in clinically significant weight loss for men and women: Midwest exercise trial 2. Obesity 2013, 21, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hill, J.O.; Jacobsen, D.J.; Potteiger, J.; Sullivan, D.K.; Johnson, S.L.; Heelan, K.; Hise, M.; Fennessey, P.V.; Sonko, B. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The Midwest Exercise Trial. Arch. Intern. Med. 2003, 163, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Martin, C.K.; Thompson, A.M.; Earnest, C.P.; Mikus, C.R.; Blair, S.N. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS ONE 2009, 4, e4515. [Google Scholar] [CrossRef]
- Slentz, C.A.; Duscha, B.D.; Johnson, J.L.; Ketchum, K.; Aiken, L.B.; Samsa, G.P.; Houmard, J.A.; Bales, C.W.; Kraus, W.E. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—A randomized controlled study. Arch. Intern. Med. 2004, 164, 31–39. [Google Scholar] [CrossRef]
- Melanson, E.L.; Keadle, S.K.; Donnelly, J.E.; Braun, B.; King, N.A. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013, 45, 1600–1609. [Google Scholar] [CrossRef] [Green Version]
- Doucet, É.; McInis, K.; Mahmoodianfard, S. Compensation in response to energy deficits induced by exercise or diet. Obes. Rev. 2018, 19 (Suppl. S1), 36–46. [Google Scholar] [CrossRef]
- Riou, M.E.; Jomphe-Tremblay, S.; Lamothe, G.; Stacey, D.; Szczotka, A.; Doucet, E. Predictors of Energy Compensation during Exercise Interventions: A Systematic Review. Nutrients 2015, 7, 3677–3704. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, S.D.; Willis, E.A.; Honas, J.J.; Lee, J.; Washburn, R.A.; Donnelly, J.E. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity 2015, 23, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Meijer, E.P.; Westerterp, K.R.; Verstappen, F.T. Effect of exercise training on total daily physical activity in elderly humans. Eur. J. Appl. Physiol. 1999, 80, 16–21. [Google Scholar] [CrossRef]
- Morio, B.; Montaurier, C.; Pickering, G.; Ritz, P.; Fellmann, N.; Coudert, J.; Beaufrere, B.; Vermorel, M. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br. J. Nutr. 1998, 80, 511–519. [Google Scholar] [CrossRef]
- Drenowatz, C. Reciprocal Compensation to Changes in Dietary Intake and Energy Expenditure within the Concept of Energy Balance. Adv. Nutr. 2015, 6, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, E.A.; Creasy, S.A.; Honas, J.J.; Melanson, E.L.; Donnelly, J.E. The effects of exercise session timing on weight loss and components of energy balance: Midwest exercise trial 2. Int. J. Obes. 2019, 44, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, Z.; Younespour, S.; Tabesh, M.R.; Haghravan, S. Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: A randomized controlled trial. Clin. Obes. 2017, 7, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Brooker, P.G.; Gomersall, S.R.; King, N.A.; Leveritt, M.D. The feasibility and acceptability of morning versus evening exercise for overweight and obese adults: A randomized controlled trial. Contemp. Clin. Trials Commun. 2019, 14, 100320. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, R.; Brouwers, B.; Schrauwen-Hinderling, V.B.; Hesselink, M.K.C.; Hoeks, J.; Schrauwen, P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep. 2021, 8, e14669. [Google Scholar] [CrossRef]
- Di Blasio, A.; Di Donato, F.; Mastrodicasa, M.; Fabrizio, N.; Di Renzo, D.; Napolitano, G.; Petrella, V.; Gallina, S.; Ripari, P. Effects of the time of day of walking on dietary behaviour, body composition and aerobic fitness in post-menopausal women. J. Sports Med. Phys. Fit. 2010, 50, 196–201. [Google Scholar]
- Blankenship, J.M.; Rosenberg, R.C.; Rynders, C.A.; Melanson, E.L.; Catenacci, V.A.; Creasy, S.A. Examining the Role of Exercise Timing in Weight Management: A Review. Int. J. Sports Med. 2021, 42, 967–978. [Google Scholar] [CrossRef]
- Chomistek, A.K.; Shiroma, E.J.; Lee, I.M. The Relationship Between Time of Day of Physical Activity and Obesity in Older Women. J. Phys. Act. Health 2016, 13, 416–418. [Google Scholar] [CrossRef]
- Schumacher, L.M.; Thomas, J.G.; Raynor, H.A.; Rhodes, R.E.; O’Leary, K.C.; Wing, R.R.; Bond, D.S. Relationship of Consistency in Timing of Exercise Performance and Exercise Levels Among Successful Weight Loss Maintainers. Obesity 2019, 27, 1285–1291. [Google Scholar] [CrossRef]
- Creasy, S.A.; Hibbing, P.R.; Cotton, E.; Lyden, K.; Ostendorf, D.M.; Willis, E.A.; Pan, Z.; Melanson, E.L.; Catenacci, V.A. Temporal patterns of physical activity in successful weight loss maintainers. Int. J. Obes. 2021, 45, 2074–2082. [Google Scholar] [CrossRef]
- Teo, S.Y.M.; Kanaley, J.A.; Guelfi, K.J.; Marston, K.J.; Fairchild, T.J. The Effect of Exercise Timing on Glycemic Control: A Randomized Clinical Trial. Med. Sci. Sports Exerc. 2020, 52, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.Y.M.; Kanaley, J.A.; Guelfi, K.J.; Dimmock, J.A.; Jackson, B.; Fairchild, T.J. Effects of diurnal exercise timing on appetite, energy intake and body composition: A parallel randomized trial. Appetite 2021, 167, 105600. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.M.; Thomas, J.G.; Raynor, H.A.; Rhodes, R.E.; Bond, D.S. Consistent Morning Exercise May Be Beneficial for Individuals with Obesity. Exerc. Sport Sci. Rev. 2020, 48, 201–208. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L.; Swibas, T.; Kohrt, W.M.; Catenacci, V.A.; Creasy, S.A.; Plasqui, G.; Wouters, L.; Speakman, J.R.; Berman, E.S.F. Validation of the doubly labeled water method using off-axis integrated cavity output spectroscopy and isotope ratio mass spectrometry. Am. J. Physiol. Endocrinol. Metab. 2017, 314, E124–E130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.R.; Yamada, Y.; Sagayama, H.; Berman, E.S.F.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep. Med. 2021, 2, 100203. [Google Scholar] [CrossRef]
- Weir, J. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition 1990, 6, 213–221. [Google Scholar] [CrossRef]
- Racette, S.B.; Das, S.K.; Bhapkar, M.; Hadley, E.C.; Roberts, S.B.; Ravussin, E.; Pieper, C.; DeLany, J.P.; Kraus, W.E.; Rochon, J.; et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: The multicenter CALERIE study. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E441–E448. [Google Scholar] [CrossRef] [Green Version]
- Czajkowski, S.M.; Powell, L.H.; Adler, N.; Naar-King, S.; Reynolds, K.D.; Hunter, C.M.; Laraia, B.; Olster, D.H.; Perna, F.M.; Peterson, J.C.; et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015, 34, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Leon, A.C.; Davis, L.L.; Kraemer, H.C. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011, 45, 626–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, Z.; Mostafaee, M.; Mazaheri, R.; Younespour, S. Acute Effect of Morning and Afternoon Aerobic Exercise on Appetite of Overweight Women. Asian J. Sports Med. 2015, 6, e24222. [Google Scholar] [CrossRef] [Green Version]
- Creasy, S.A.; Rogers, R.J.; Davis, K.K.; Gibbs, B.B.; Kershaw, E.E.; Jakicic, J.M. Effects of supervised and unsupervised physical activity programmes for weight loss. Obes. Sci. Pract. 2017, 3, 143–152. [Google Scholar] [CrossRef]
- Riou, M.; Jomphe-Tremblay, S.; Lamothe, G.; Finlayson, G.S.; Blundell, J.E.; Décarie-Spain, L.; Gagnon, J.C.; Doucet, É. Energy Compensation Following a Supervised Exercise Intervention in Women Living with Overweight/Obesity Is Accompanied by an Early and Sustained Decrease in Non-structured Physical Activity. Front. Physiol. 2019, 10, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, T.J.; Blumenthal, J.A.; Dorfman, C.S.; Huffman, K.M.; Edmond, S.N.; Miller, S.N.; Wren, A.A.; Caldwell, D.; Keefe, F.J. Effects of a Weight and Pain Management Program in Patients with Rheumatoid Arthritis With Obesity: A Randomized Controlled Pilot Investigation. J. Clin. Rheumatol. 2021, 28, 7–13. [Google Scholar] [CrossRef]
- Swift, D.L.; Nevels, T.R.; Solar, C.A.; Brophy, P.M.; McGee, J.E.; Brewer, S.B.; Clark, A.; Houmard, J.A.; Lutes, L.D. The Effect of Aerobic Training and Increasing Nonexercise Physical Activity on Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2152–2163. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5, e134270. [Google Scholar] [CrossRef] [Green Version]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P., Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [Green Version]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Week | Sessions/Week | Kcal/Session | Intensity | Kcal/Week |
|---|---|---|---|---|
| 1–2 | 3 supervised, 1 on-own | 187.5 | 70% HRmax | 750 |
| 3–4 | 3 supervised, 1 on-own | 250 | 70% HRmax | 1000 |
| 5–6 | 3 supervised, 1 on-own | 312.5 | 75% HRmax | 1250 |
| 7–8 | 3 supervised, 1 on-own | 375 | 75% HRmax | 1500 |
| 9–10 | 3 supervised, 1 on-own | 437.5 | 80% HRmax | 1750 |
| 11–15 | 3 supervised, 1 on-own | 500 | 80% HRmax | 2000 |
| AM (n = 18) | PM (n = 15) | |
|---|---|---|
| Age (years) | 40.8 ± 8.4 | 36.4 ± 10.8 |
| BMI (kg/m2) | 30.0 ± 3.9 | 30.6 ± 4.7 |
| Female (n, %) | 14, 78% | 9, 60% |
| Race | ||
| White (n, %) | 16, 89% | 12, 80% |
| Black | 1, 6% | 2, 13% |
| Other | 1, 6% | 1, 7% |
| Ethnicity | ||
| Hispanic | 3, 17% | 8, 53% |
| Non-Hispanic | 15, 83% | 7, 47% |
| Week | Attendance (Sessions/Week) | Completed Exercise EE (kcal/Week) | Completed Intensity (% HRmax) | Exercise Prescription (min/Week) | ||||
|---|---|---|---|---|---|---|---|---|
| AM | PM | AM | AM | AM | PM | AM | PM | |
| 1–2 | 3.9 ± 0.3 | 3.6 ± 0.6 | 727 ± 64 | 700 ± 108 | 72 ± 1 | 72 ± 1 | 136 ± 25 | 142 ± 33 |
| 3–4 | 3.9 ± 0.2 | 3.4 ± 0.9 | 994 ± 65 | 938 ± 144 | 72 ± 2 | 72 ± 2 | 181 ± 33 | 189 ± 44 |
| 5–6 | 3.9 ± 0.3 | 3.8 ± 0.5 | 1165 ± 111 | 1154 ± 157 | 77 ± 1 | 76 ± 1 | 170 ± 25 | 170 ± 38 |
| 7–8 | 3.8 ± 0.6 | 3.3 ± 0.7 | 1298 ± 141 | 1190 ± 215 | 77 ± 1 | 76 ± 3 | 212 ± 32 | 212 ± 47 |
| 9–10 | 3.6 ± 0.4 | 3.4 ± 0.7 | 1641 ± 225 | 1689 ± 252 | 81 ± 1 | 80 ± 2 | 218 ± 33 | 200 ± 45 |
| 11–15 | 3.2 ± 0.8 | 3.3 ± 0.7 | 1601 ± 340 | 1696 ± 328 | 80 ± 2 | 79 ± 5 | 249 ± 37 | 229 ± 52 |
| Baseline | Week 15 | Change | |
|---|---|---|---|
| TDEE (kcal/day) | |||
| AM | 2231 ± 341 | 2453 ± 391 | 222 ± 399 |
| PM | 2548 ± 574 | 2638 ± 536 | 90 ± 150 |
| Non-Exercise EE (kcal/day) | |||
| AM | 750 ± 316 | 690 ± 294 | −60 ± 432 |
| PM | 948 ± 277 | 747 ± 228 | −200 ± 152 |
| Resting EE (kcal/day) | |||
| AM | 1457 ± 233 | 1477 ± 206 | 20 ± 117 |
| PM | 1600 ± 385 | 1605 ± 424 | 4 ± 127 |
| Energy Intake (kcal/day) | |||
| AM | 2231 ± 341 | 2330 ± 367 | 99 ± 198 |
| PM | 2548 ± 574 | 2527 ± 615 | −21 ± 156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creasy, S.A.; Wayland, L.; Panter, S.L.; Purcell, S.A.; Rosenberg, R.; Willis, E.A.; Shiferaw, B.; Grau, L.; Breit, M.J.; Bessesen, D.H.; et al. Effect of Morning and Evening Exercise on Energy Balance: A Pilot Study. Nutrients 2022, 14, 816. https://doi.org/10.3390/nu14040816
Creasy SA, Wayland L, Panter SL, Purcell SA, Rosenberg R, Willis EA, Shiferaw B, Grau L, Breit MJ, Bessesen DH, et al. Effect of Morning and Evening Exercise on Energy Balance: A Pilot Study. Nutrients. 2022; 14(4):816. https://doi.org/10.3390/nu14040816
Chicago/Turabian StyleCreasy, Seth A., Liza Wayland, Shelby L. Panter, Sarah A. Purcell, Rebecca Rosenberg, Erik A. Willis, Bethelhem Shiferaw, Laura Grau, Matthew J. Breit, Daniel H. Bessesen, and et al. 2022. "Effect of Morning and Evening Exercise on Energy Balance: A Pilot Study" Nutrients 14, no. 4: 816. https://doi.org/10.3390/nu14040816
APA StyleCreasy, S. A., Wayland, L., Panter, S. L., Purcell, S. A., Rosenberg, R., Willis, E. A., Shiferaw, B., Grau, L., Breit, M. J., Bessesen, D. H., Melanson, E. L., & Catenacci, V. A. (2022). Effect of Morning and Evening Exercise on Energy Balance: A Pilot Study. Nutrients, 14(4), 816. https://doi.org/10.3390/nu14040816






