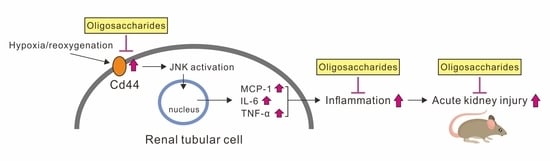
Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Short Interfering (si)RNA Transfection of CD44
2.5. Cell Proliferation Assay
2.6. CD44 Antigenicity Tests
2.7. Experimental Animals and Renal IRI
2.8. Immunohistochemistry Staining
2.9. PAS Staining
2.10. ELISA Assay
2.11. Statistical Tests
3. Results
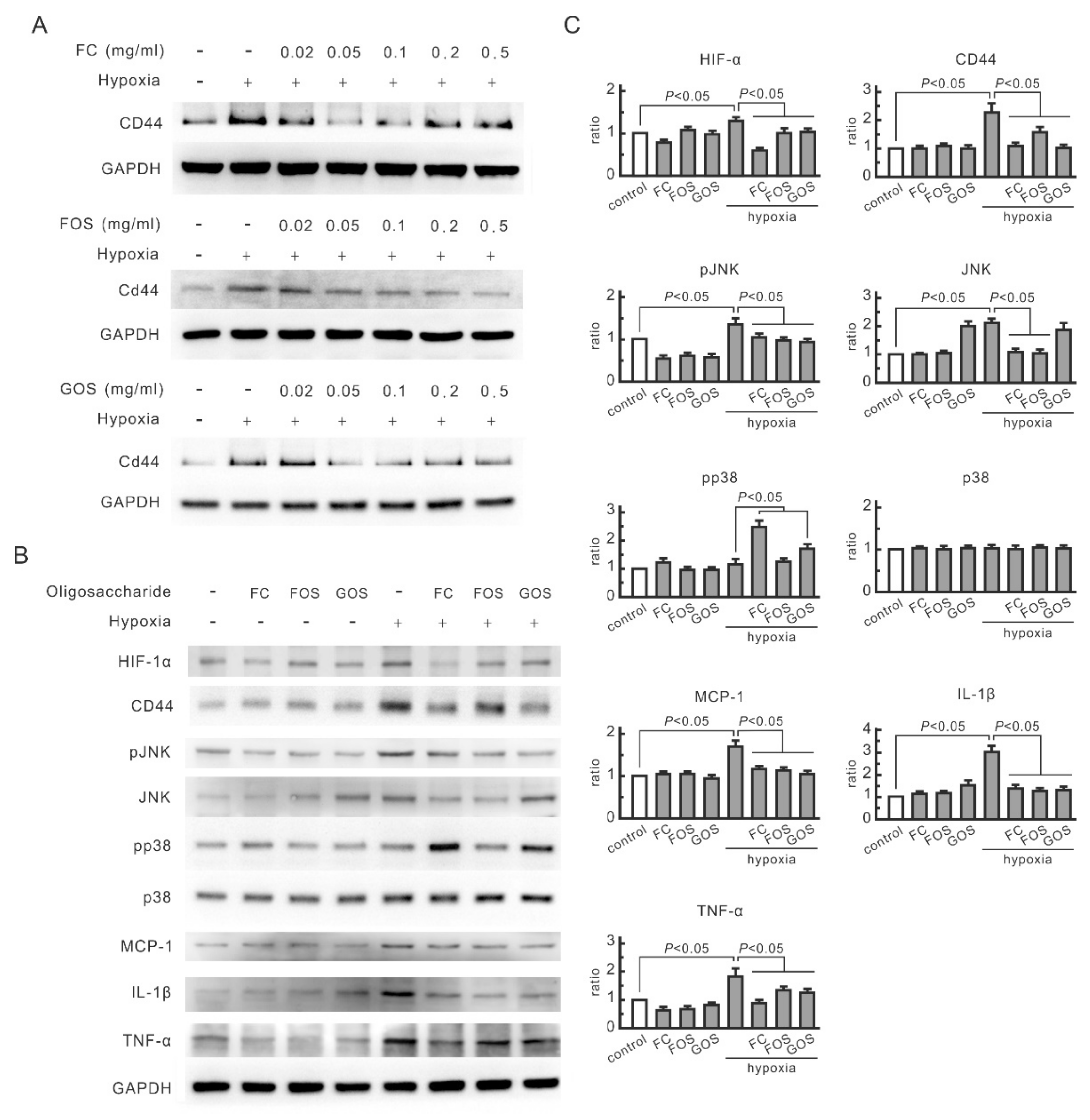
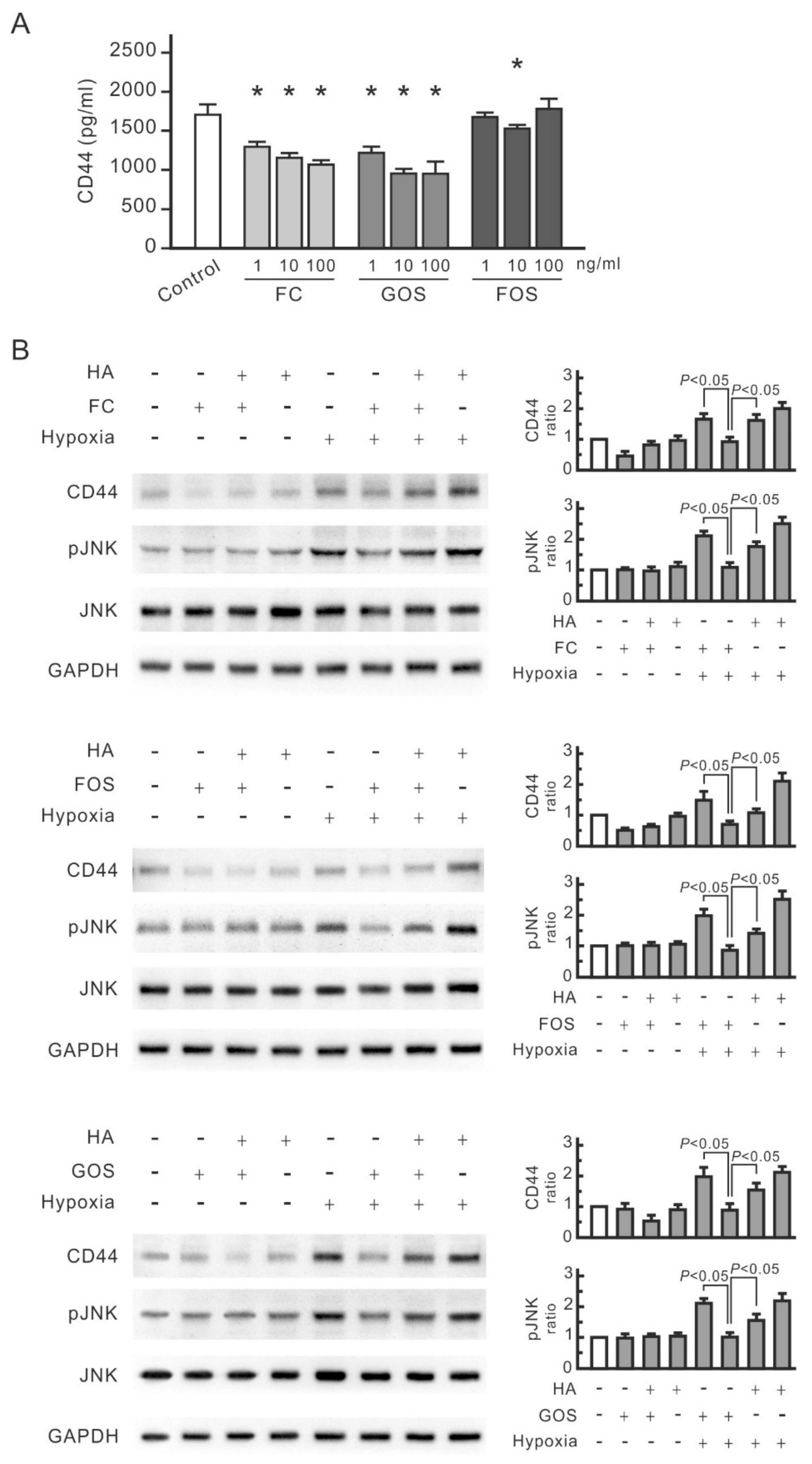
3.1. Oligosaccharides Inhibit the Increase in CD44 and Cytokines in Hypoxic NRK-52E Cells
3.2. CD44 and JNK Play a Critical Role in Cytokine Increase in Hypoxic NRK-52E Cells
3.3. Hypoxia-Induced Cytokine Secretion in Renal Tubular Cells Promotes Macrophage Proliferation
3.4. Oligosaccharides Reduce CD44 Antigenicity
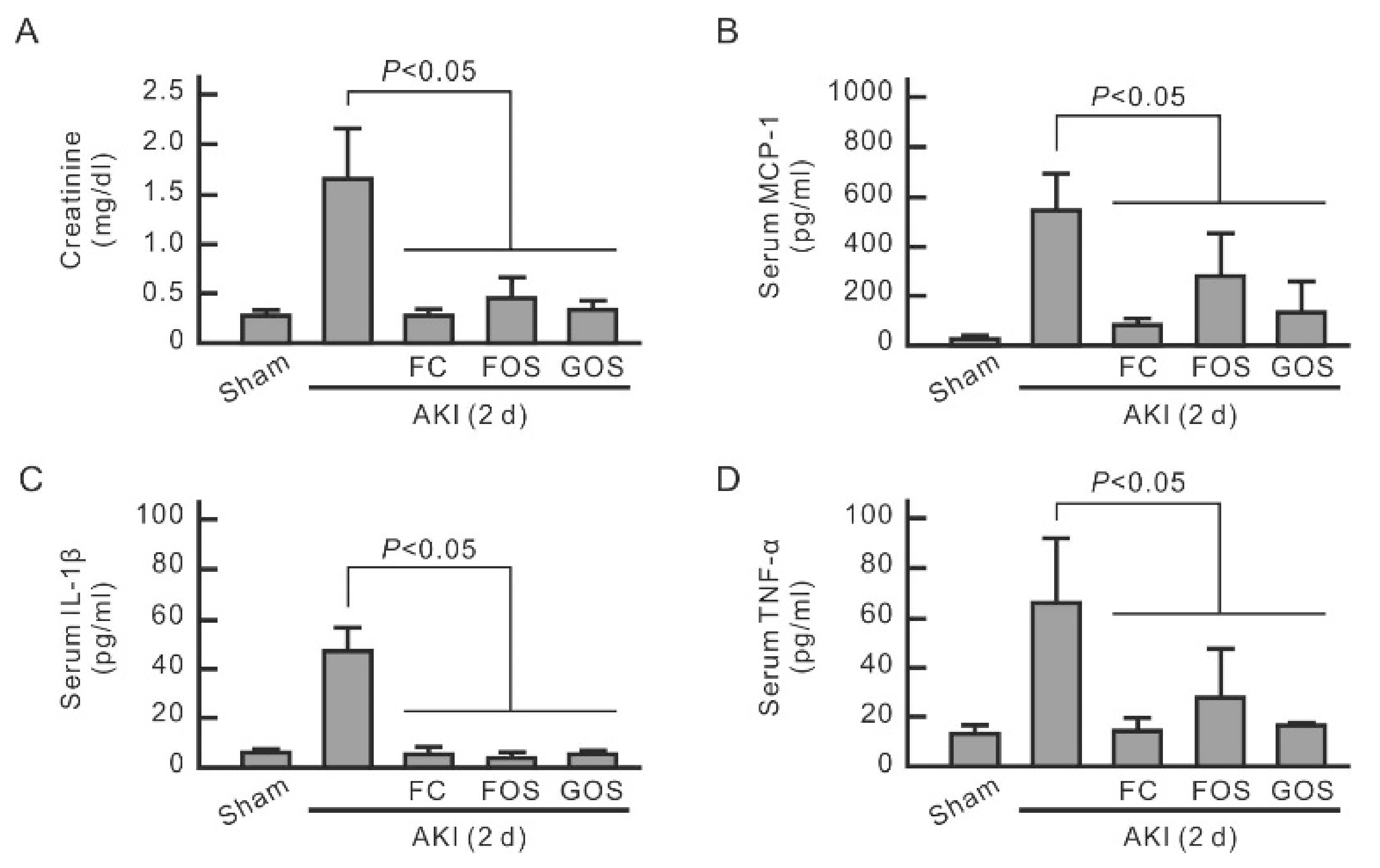
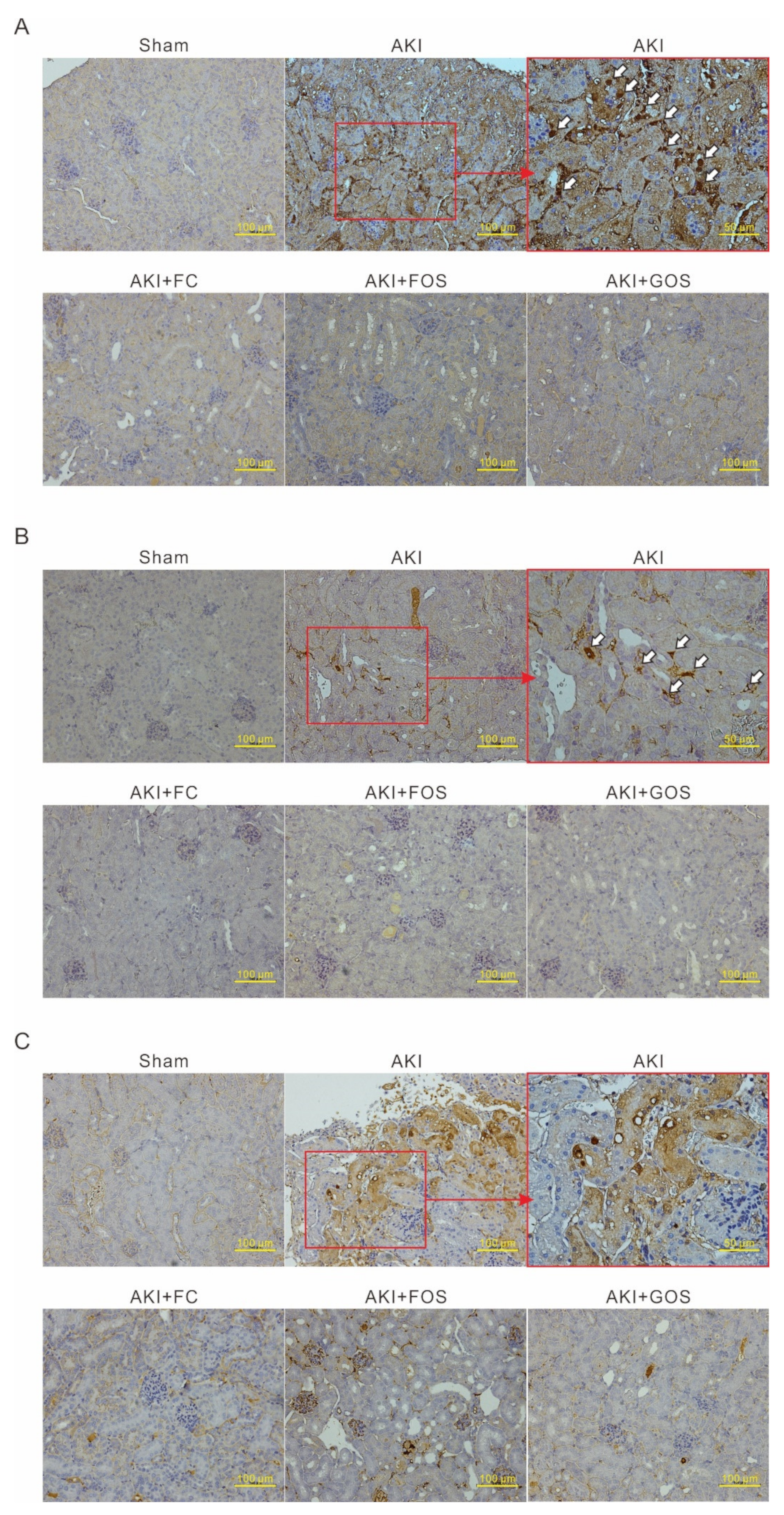
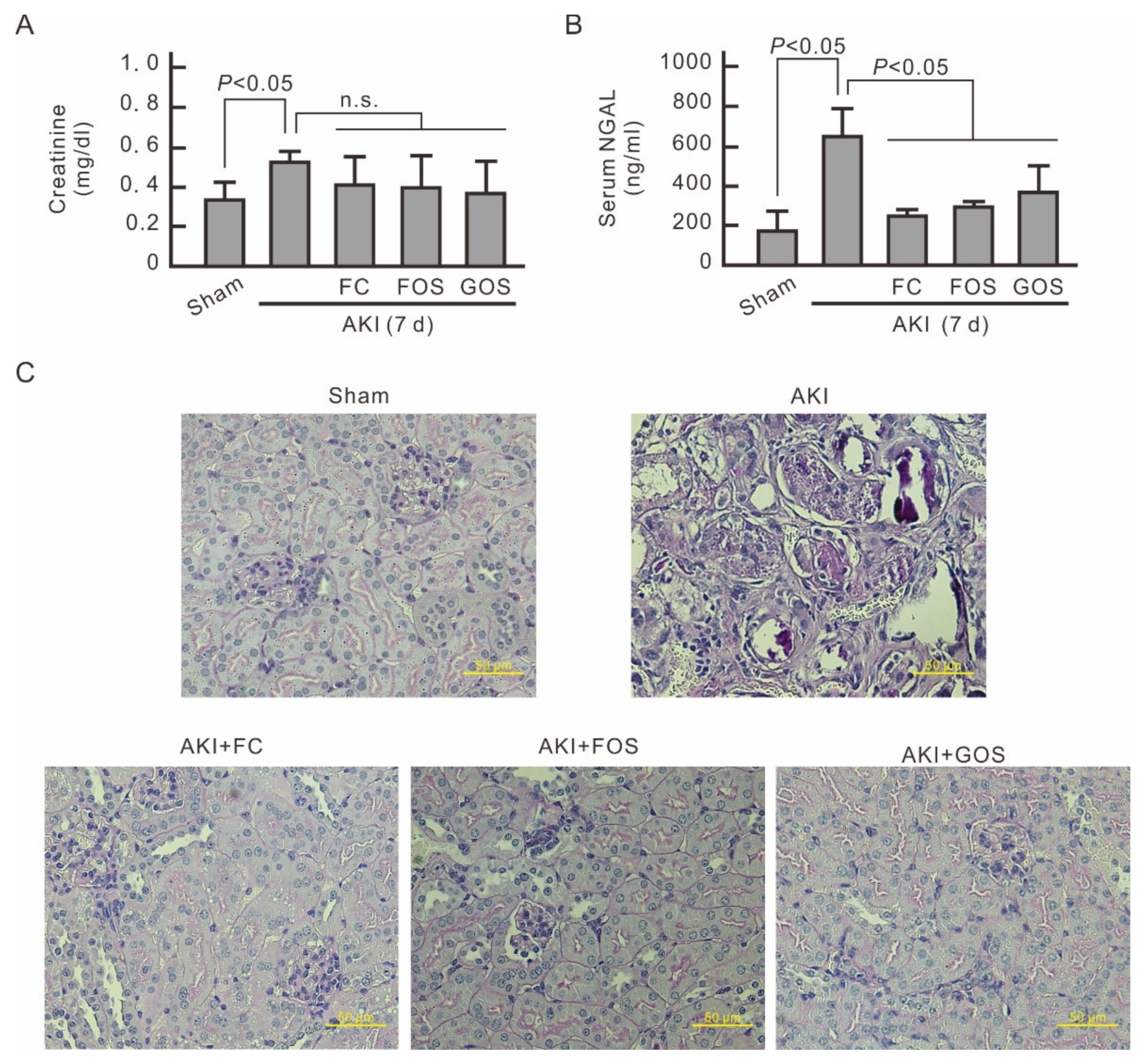
3.5. Oligosaccharides Improve Renal Function and Reduce Inflammation in Mice with Early Stage AKI
3.6. Oligosaccharides Promote Kidney Recovery at Post-AKI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. Causes of Death after a Hospitalization with AKI. J. Am. Soc. Nephrol. 2018, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Druml, W.; Forni, L.G.; Groeneveld, A.B.J.; Honore, P.M.; Hoste, E.; Ostermann, M.; Oudemans-van Straaten, H.M.; Schetz, M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017, 43, 730–749. [Google Scholar] [CrossRef] [PubMed]
- Bijuklic, K.; Jennings, P.; Kountchev, J.; Hasslacher, J.; Aydin, S.; Sturn, D.; Pfaller, W.; Patsch, J.R.; Joannidis, M. Migration of leukocytes across an endothelium-epithelium bilayer as a model of renal interstitial inflammation. Am. J. Physiol. Physiol. 2007, 293, C486–C492. [Google Scholar] [CrossRef]
- Bihorac, A.; Baslanti, T.O.; Cuenca, A.G.; Hobson, C.; Ang, D.; Efron, P.A.; Maier, R.V.; Moore, F.A.; Moldawer, L.L. Acute kidney injury is associated with early cytokine changes after trauma. J. Trauma Acute Care Surg. 2013, 74, 1005–1013. [Google Scholar] [CrossRef]
- Singbartl, K.; Formeck, C.; Kellum, J.A. Kidney-Immune System Crosstalk in AKI. Semin. Nephrol. 2019, 39, 96–106. [Google Scholar] [CrossRef]
- Murugan, R.; Wen, X.; Keener, C.; Pike, F.; Palevsky, P.M.; Unruh, M.; Finkel, K.; Vijayan, A.; Elder, M.; Chen, Y.-F.; et al. Associations between Intensity of RRT, Inflammatory Mediators, and Outcomes. Clin. J. Am. Soc. Nephrol. 2015, 10, 926–933. [Google Scholar] [CrossRef]
- Arana, L.; Ordoñez, M.; Ouro, A.; Rivera, I.; Gangoiti, P.; Trueba, M.; Muñoz, A.G. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: Involvement in ceramide 1-phosphate-stimulated cell migration. Am. J. Physiol. Metab. 2013, 304, E1213–E1226. [Google Scholar] [CrossRef]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Lu, Y.; Chikamoto, N.; Nishida, T. Lipopolysaccharide-Induced Expression of Intercellular Adhesion Molecule-1 and Chemokines in Cultured Human Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2005, 46, 114–120. [Google Scholar] [CrossRef]
- Segerer, S.; Nelson, P.J.; Schlöndorff, D. Chemokines, Chemokine Receptors, and Renal Disease: From Basic Science to Pathophysiologic and Therapeutic Studies. J. Am. Soc. Nephrol. 2000, 11, 152–176. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, L.; Liu, Z.; Wang, Y.; Dong, X.; Qu, L.; Gou, R.; Wang, Y.; Wang, Q.; Liu, Z.; et al. Inhibition of IRE1/JNK pathway in HK-2 cells subjected to hypoxia-reoxygenation attenuates mesangial cells-derived extracellular matrix production. J. Cell. Mol. Med. 2020, 24, 13408–13420. [Google Scholar] [CrossRef] [PubMed]
- Shui, H.; Gao, P.; Si, X.; Ding, G. Mycophenolic acid inhibits albumin-induced MCP-1 expression in renal tubular epithelial cells through the p38 MAPK pathway. Mol. Biol. Rep. 2010, 37, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R. Functional Oligosaccharides: Application and Manufacture. Annu. Rev. Food Sci. Technol. 2010, 1, 305–339. [Google Scholar] [CrossRef] [PubMed]
- Molis, C.; Flourie, B.; Ouarne, F.; Gailing, M.F.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J.P. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef]
- Difilippo, E.; Bettonvil, M.; Willems, R.H.A.M.; Braber, S.; Fink-Gremmels, J.; Jeurink, P.V.; Schoterman, M.H.C.; Gruppen, H.; Schols, H.A. Oligosaccharides in Urine, Blood, and Feces of Piglets Fed Milk Replacer Containing Galacto-oligosaccharides. J. Agric. Food Chem. 2015, 63, 10862–10872. [Google Scholar] [CrossRef]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Chen, C.-H.; Sue, Y.-M.; Cheng, C.-Y.; Chen, Y.-C.; Liu, C.-T.; Hsu, Y.-H.; Hwang, P.-A.; Huang, N.-J.; Chen, T.-H. Oligo-fucoidan prevents renal tubulointerstitial fibrosis by inhibiting the CD44 signal pathway. Sci. Rep. 2017, 7, 40183. [Google Scholar] [CrossRef]
- Chen, L.; Bourguignon, L.Y.W. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol. Cancer 2014, 13, 52. [Google Scholar] [CrossRef]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.-G.; Oh, S.W.; Cho, W.Y.; Im, S.-H.; Jo, S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Bradham, C.; McClay, D.R. p38 MAPK in Development and Cancer. Cell Cycle 2006, 5, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Šuligoj, T.; Vigsnæs, L.K.; Abbeele, P.V.D.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, S.; Pohlentz, G.; Borsch, C.; Lentze, M.J.; Kunz, C. Urinary excretion ofin vivo13C-labelled milk oligosaccharides in breastfed infants. Br. J. Nutr. 2012, 107, 957–963. [Google Scholar] [CrossRef]
- Heldin, P.; Kolliopoulos, C.; Lin, C.-Y.; Heldin, C.-H. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell. Signal. 2020, 65, 109427. [Google Scholar] [CrossRef]
- Lee-Sayer, S.S.M.; Dong, Y.; Arif, A.A.; Olsson, M.; Brown, K.L.; Johnson, P. The Where, When, How, and Why of Hyaluronan Binding by Immune Cells. Front. Immunol. 2015, 6, 150. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef]
- Toole, B.P.; Slomiany, M.G. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008, 18, 244–250. [Google Scholar] [CrossRef]
- Marhaba, R.; Zöller, M. CD44 in Cancer Progression: Adhesion, Migration and Growth Regulation. Histochem. J. 2003, 35, 211–231. [Google Scholar] [CrossRef]
- Legg, J.W.; Lewis, C.A.; Parsons, M.; Ng, T.; Isacke, C. A novel PKC-regulated mechanism controls CD44–ezrin association and directional cell motility. Nat. Cell Biol. 2002, 4, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Fregien, N.; Bourguignon, L.Y. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J. Cell Biol. 1994, 126, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Zhu, H.; Shao, L.; Chen, Y.-W. CD44 Interaction with c-Src Kinase Promotes Cortactin-mediated Cytoskeleton Function and Hyaluronic Acid-dependent Ovarian Tumor Cell Migration. J. Biol. Chem. 2001, 276, 7327–7336. [Google Scholar] [CrossRef] [PubMed]
- Ispanovic, E.; Haas, T. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am. J. Physiol. Physiol. 2006, 291, C579–C588. [Google Scholar] [CrossRef]
- Benoit, B.; Baillet, A.; Poüs, C. Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators. Int. J. Mol. Sci. 2021, 22, 8375. [Google Scholar] [CrossRef]
- Parameswaran, N.; Enyindah-Asonye, G.; Bagheri, N.; Shah, N.B.; Gupta, N. Spatial Coupling of JNK Activation to the B Cell Antigen Receptor by Tyrosine-Phosphorylated Ezrin. J. Immunol. 2013, 190, 2017–2026. [Google Scholar] [CrossRef]
- Harada, T.; Matsuzaki, O.; Hayashi, H.; Sugano, S.; Matsuda, A.; Nishida, E. AKRL1 and AKRL2 activate the JNK pathway. Genes Cells 2003, 8, 493–500. [Google Scholar] [CrossRef]
- Holzer, R.G.; Park, E.-J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solinas, G.; Karin, M. Saturated Fatty Acids Induce c-Src Clustering within Membrane Subdomains, Leading to JNK Activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef]
- Poon, C.L.; Brumby, A.M.; Richardson, H.E. Src Cooperates with Oncogenic Ras in Tumourigenesis via the JNK and PI3K Pathways in Drosophila epithelial Tissue. Int. J. Mol. Sci. 2018, 19, 1585. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018, 9, 5059–5073. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Correction: Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Maldonado-Gómez, M.X.; Hutkins, R.W.; Rose, D.J. Production and in Vitro Fermentation of Soluble, Non-digestible, Feruloylated Oligo- and Polysaccharides from Maize and Wheat Brans. J. Agric. Food Chem. 2014, 62, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Shin, S.Y.; Choi, H.S.; Joo, W.; Cho, S.K.; Li, L.; Kang, J.-H.; Kim, T.-J.; Han, N.S. In vitro digestion and fermentation properties of linear sugar-beet arabinan and its oligosaccharides. Carbohydr. Polym. 2015, 131, 50–56. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-H.; Liu, C.-T.; Cheng, C.-Y.; Sue, Y.-M.; Huang, N.-J.; Chen, C.-H. Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells. Nutrients 2022, 14, 760. https://doi.org/10.3390/nu14040760
Chen T-H, Liu C-T, Cheng C-Y, Sue Y-M, Huang N-J, Chen C-H. Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells. Nutrients. 2022; 14(4):760. https://doi.org/10.3390/nu14040760
Chicago/Turabian StyleChen, Tso-Hsiao, Chung-Te Liu, Chung-Yi Cheng, Yuh-Mou Sue, Nai-Jen Huang, and Cheng-Hsien Chen. 2022. "Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells" Nutrients 14, no. 4: 760. https://doi.org/10.3390/nu14040760
APA StyleChen, T.-H., Liu, C.-T., Cheng, C.-Y., Sue, Y.-M., Huang, N.-J., & Chen, C.-H. (2022). Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells. Nutrients, 14(4), 760. https://doi.org/10.3390/nu14040760