Protective Effect of MFG-E8 on Necroptosis-Induced Intestinal Inflammation and Enteroendocrine Cell Function in Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Estimation of Body Weight, Fasting Blood Glucose (FBG)
2.4. Light Microscopy
2.5. Cell Cultures
2.6. MFG-E8 siRNA and Overexpression Plasmid Transfection
2.7. Cell Viability Assay
2.8. Cell Death Assay
2.9. ELISA Assay
2.10. Quantitative Real-Time PCR
2.11. Western Blot Assay
2.12. Immunocytochemical Staining
2.13. Statistical Analysis
3. Results
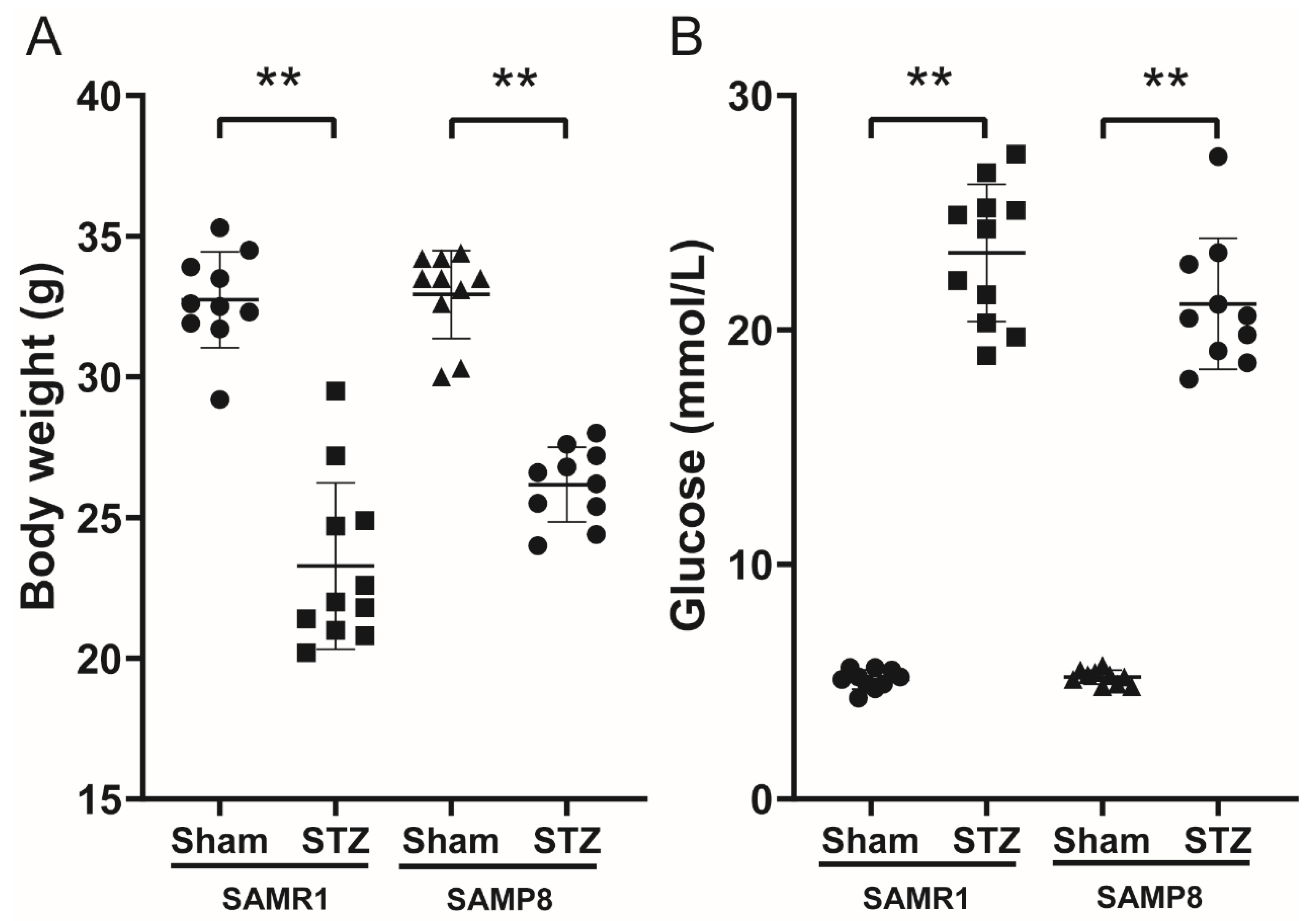
3.1. Body Weight and FBG in Control and Diabetic Mice
3.2. Histological Findings and the Expression of MFG-E8, p-MLKL and HMGB1 in the Intestinal Tissues
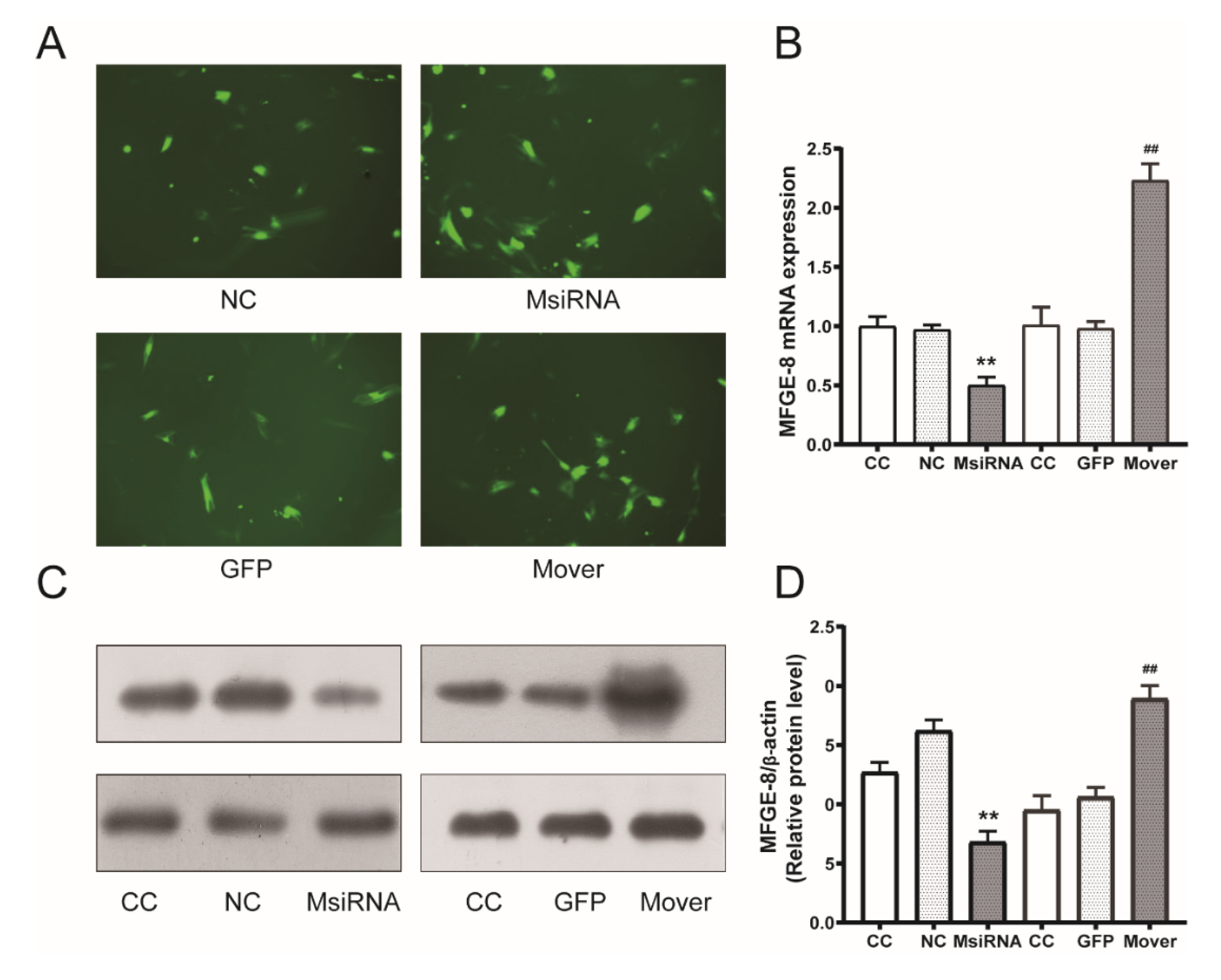
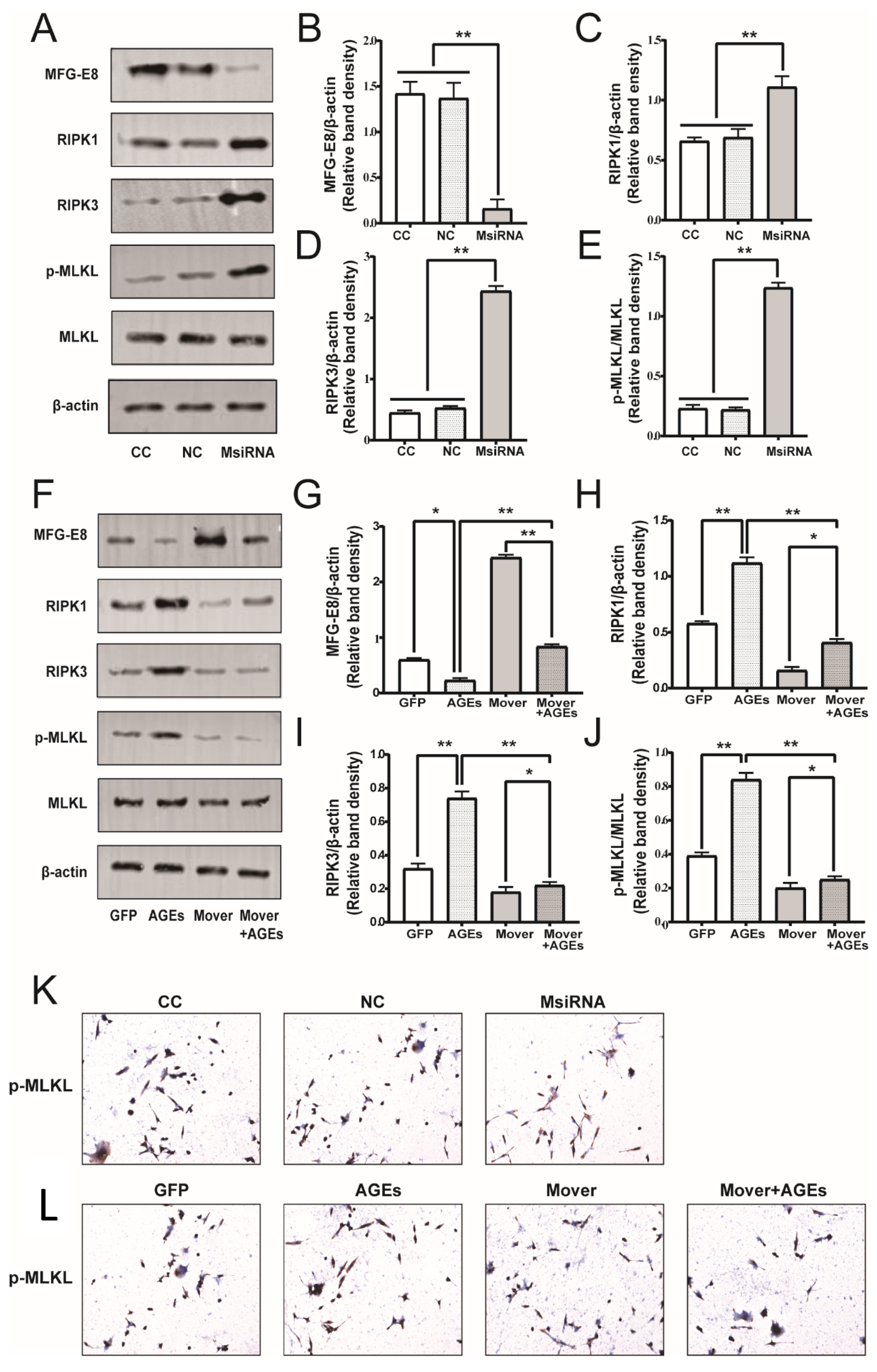
3.3. MFG-E8 siRNA and Overexpression Plasmids Transduction Efficiency
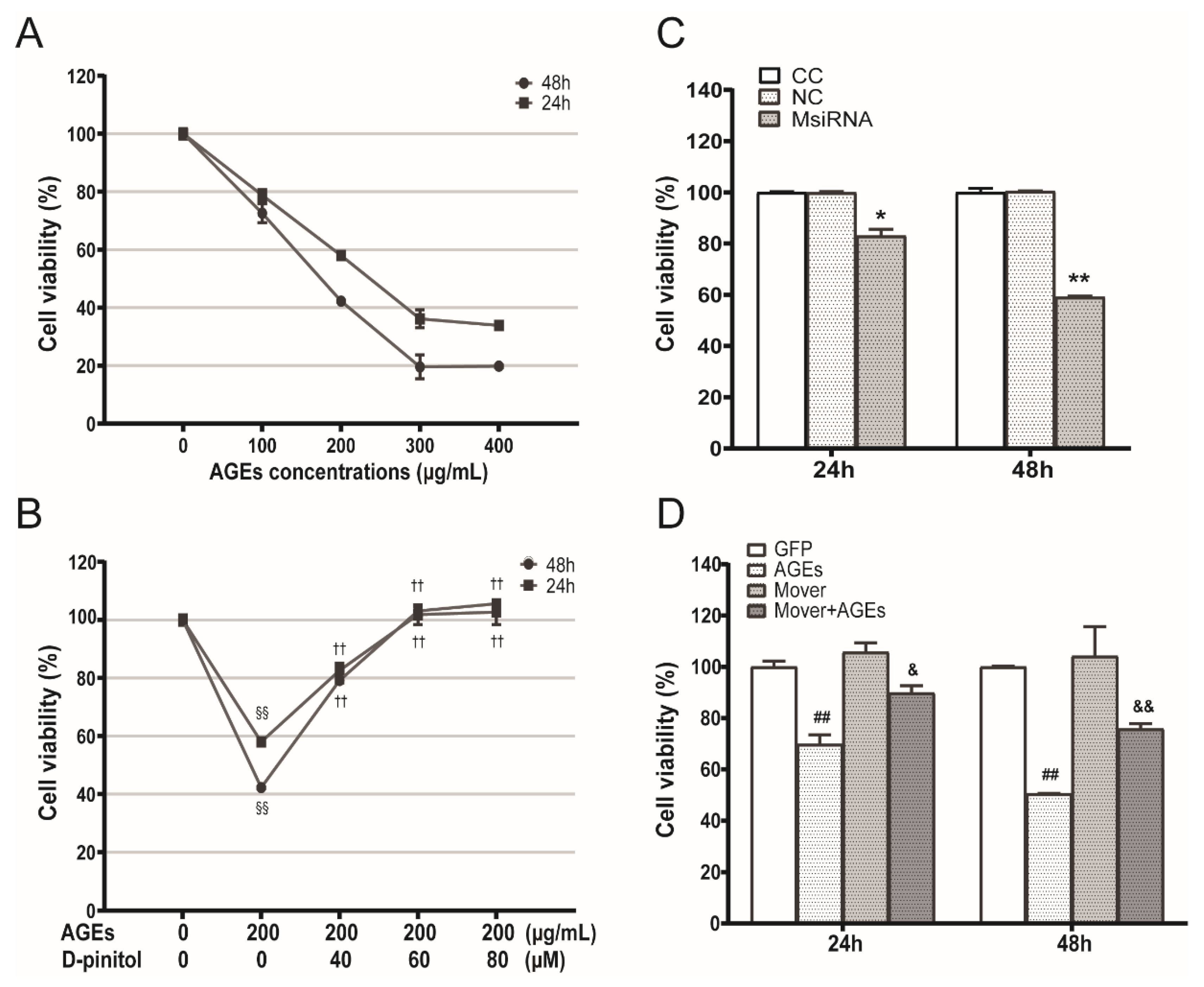
3.4. Effects of AGEs, D-Pinitol, MFG-E8 siRNA and Overexpression Plasmids on the STC-1 Cell Viability
3.5. Role of D-Pinitol on AGEs-Induced Necroptosis and Effects of MFG-E8 siRNA and Overexpression Plasmids
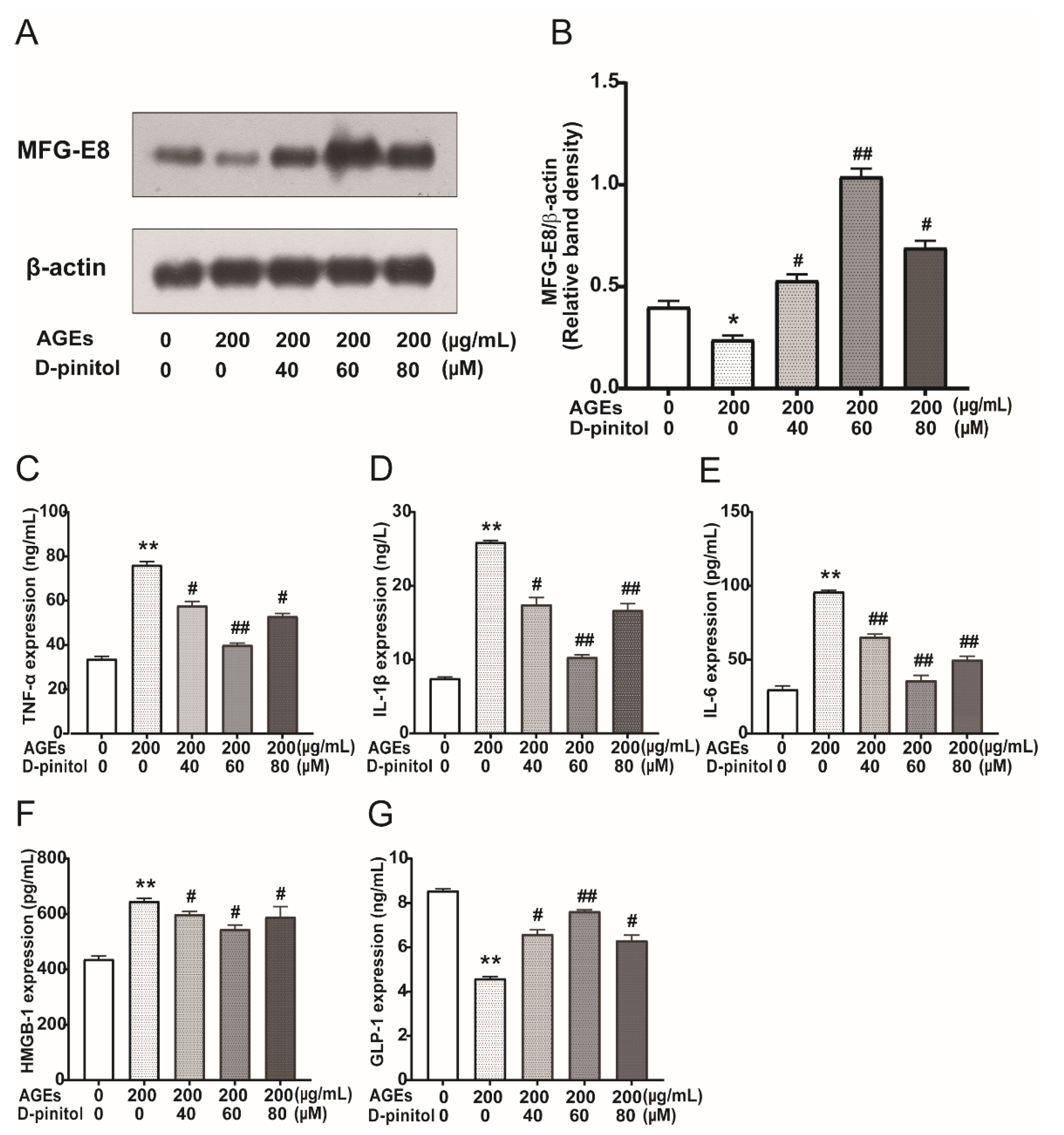
3.6. Effects of D-Pinitol on MFG-E8 and Pro-Inflammatory Cytokines in AGEs Treated STC-1 Cells
3.7. Effect of MFG-E8 on the Expression of DAMPs and Pro-Inflammatory Cytokines in STC-1 Cells
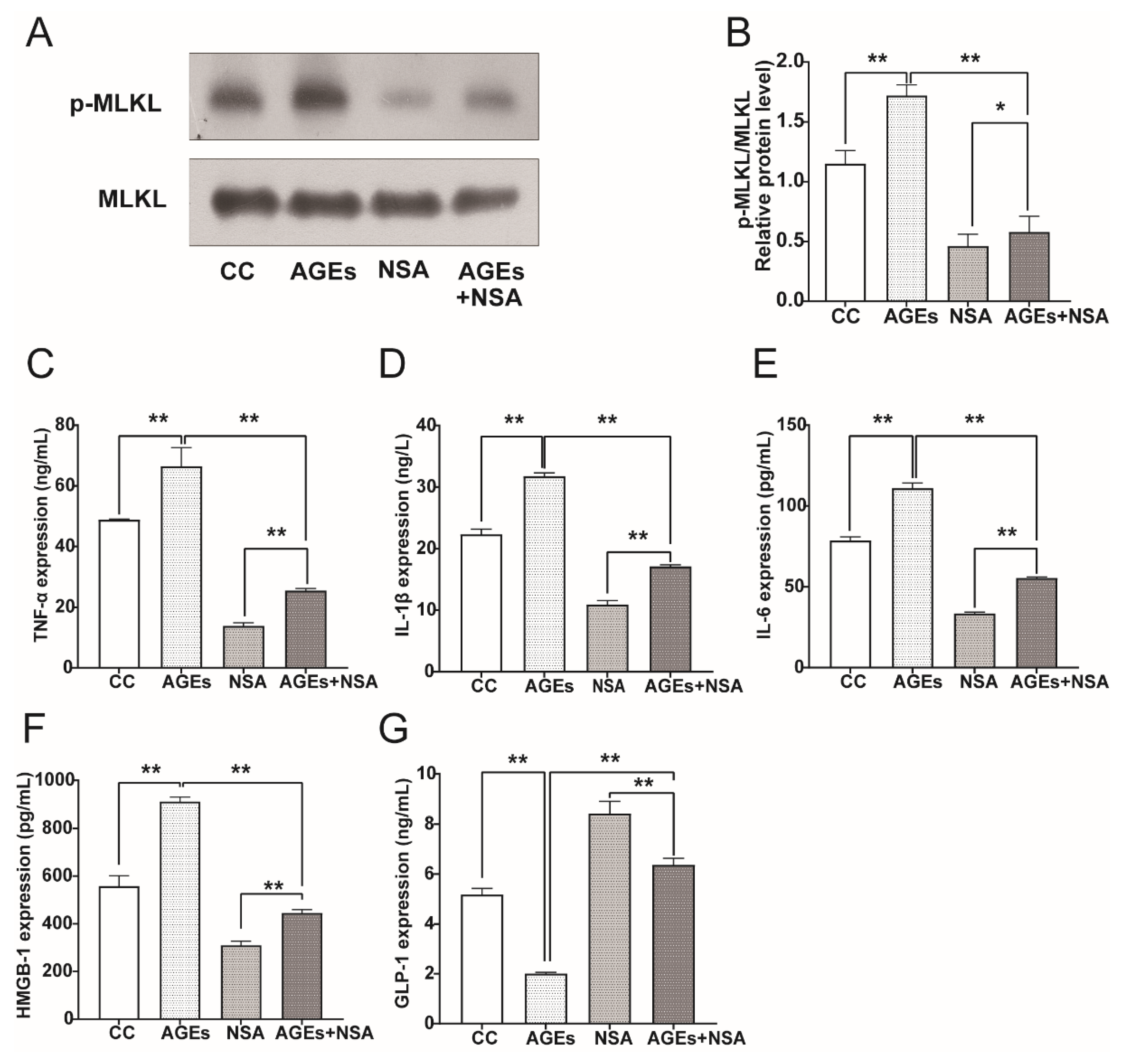
3.8. NSA Reversed AGEs-Induced Phosphorylation of MLKL and Upregulation of Pro-Inflammatory Cytokines
3.9. Effect of MFG-E8 on Necroptosis Pathway Related Protein Expression in STC-1 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.L.; Lin, H.L. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J. Gastroenterol. 2014, 20, 17737–17745. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toft-Nielsen, M.B.; Damholt, M.B.; Madsbad, S.; Hilsted, L.M.; Hughes, T.E.; Michelsen, B.K.; Holst, J.J. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2001, 86, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jin, T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J. Diabetes 2016, 8, 753–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbs, J.D.; Lekutis, C.; Singer, K.L.; Bui, A.; Yuzuki, D.; Srinivasan, U.; Parry, G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. USA 1990, 87, 8417–8421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.S. Functional Role of Milk Fat Globule-Epidermal Growth Factor VIII in Macrophage-Mediated Inflammatory Responses and Inflammatory/Autoimmune Diseases. Mediators. Inflamm. 2016, 2016, 5628486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, H.F.; Zuo, X.L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.D. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 2007, 117, 3673–3683. [Google Scholar] [CrossRef] [Green Version]
- Ajakaiye, M.A.; Jacob, A.; Wu, R.; Yang, W.L.; Nicastro, J.; Coppa, G.F.; Wang, P. Recombinant human MFG-E8 attenuates intestinal injury and mortality in severe whole body irradiation in rats. PLoS ONE 2012, 7, e46540. [Google Scholar] [CrossRef]
- Das, A.; Ghatak, S.; Sinha, M.; Chaffee, S.; Ahmed, N.S.; Parinandi, N.L.; Wohleb, E.S.; Sheridan, J.F.; Sen, C.K.; Roy, S. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. J. Immunol. 2016, 196, 5089–5100. [Google Scholar] [CrossRef] [Green Version]
- Cuchet-Lourenço, D.; Eletto, D.; Wu, C.; Plagnol, V.; Papapietro, O.; Curtis, J.; Ceron-Gutierrez, L.; Bacon, C.M.; Hackett, S.; Alsaleem, B.; et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 2018, 361, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Wallach, D.; Kang, T.B.; Dillon, C.P.; Green, D.R. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016, 352, aaf2154. [Google Scholar] [CrossRef]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jin, C.; Zhang, X.; Jia, W.; Le, J.; Ye, J. Restoration of GLP-1 secretion by Berberine is associated with protection of colon enterocytes from mitochondrial overheating in diet-induced obese mice. Nutr. Diabetes 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Wang, M.; Gucek, M.; Zhang, J.; Wu, J.; Jiang, L.; Monticone, R.E.; Khazan, B.; Telljohann, R.; Mattison, J.; et al. Milk fat globule protein epidermal growth factor-8: A pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ. Res. 2009, 104, 1337–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheyuo, C.; Jacob, A.; Wu, R.; Zhou, M.; Qi, L.; Dong, W.; Ji, Y.; Chaung, W.W.; Wang, H.; Nicastro, J.; et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology 2012, 62, 890–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissette, M.J.; Lepage, S.; Lamonde, A.S.; Sirois, I.; Groleau, J.; Laurin, L.P.; Cailhier, J.F. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS ONE 2012, 7, e36368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.Y.; Tao, T.; Wu, D.; Zhuang, Z.; Lu, Y.; Wu, L.Y.; Liu, G.J.; Zhou, Y.; Zhang, D.D.; Wang, H.; et al. MFG-E8 attenuates inflammation in subarachnoid hemorrhage by driving microglial M2 polarization. Exp. Neurol. 2021, 336, 113532. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death. Discov. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Pandey, B.L.; Tripathi, M.; Pandey, V.B. Anti-inflammatory effect of (+)-pinitol. Fitoterapia 2001, 72, 168–170. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subramanian, S.P. D-pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen. Physiol. Biophys. 2009, 28, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Vereecke, L.; Bertrand, M.J.; Duprez, L.; Berger, S.B.; Divert, T.; Gonçalves, A.; Sze, M.; Gilbert, B.; Kourula, S.; et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014, 513, 95–99. [Google Scholar] [CrossRef]
- Garcia-Carbonell, R.; Yao, S.J.; Das, S.; Guma, M. Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut. Front. Immunol. 2019, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Chan, F.K. Necroptosis-independent signaling by the RIP kinases in inflammation. Cell. Mol. Life Sci. 2016, 73, 2325–2334. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Huang, Z.; Ren, J.; Zhang, Z.; He, P.; Li, Y.; Ma, J.; Chen, W.; Zhang, Y.; Zhou, X.; et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013, 23, 994–1006. [Google Scholar] [CrossRef] [Green Version]
- Popper, B.; Rammer, M.T.; Gasparitsch, M.; Singer, T.; Keller, U.; Döring, Y.; Lange-Sperandio, B. Neonatal obstructive nephropathy induces necroptosis and necroinflammation. Sci. Rep. 2019, 9, 18600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, Y.; Matsumoto, H.; Roh, M.; Giani, A.; Kataoka, K.; Morizane, Y.; Kayama, M.; Thanos, A.; Nakatake, S.; Notomi, S.; et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014, 21, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Welz, P.S.; Wullaert, A.; Vlantis, K.; Kondylis, V.; Fernández-Majada, V.; Ermolaeva, M.; Kirsch, P.; Sterner-Kock, A.; van Loo, G.; Pasparakis, M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011, 477, 330–334. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Negroni, A.; Colantoni, E.; Pierdomenico, M.; Palone, F.; Costanzo, M.; Oliva, S.; Tiberti, A.; Cucchiara, S.; Stronati, L. RIP3 AND pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells. Dig. Liver Dis. 2017, 49, 1201–1210. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, X.; Li, B.; Yu, F.; Zhao, W.; Tan, Y.; Li, X.; Fu, C.; Yu, X.; Gao, H.; Cheng, M. Protective Effect of MFG-E8 on Necroptosis-Induced Intestinal Inflammation and Enteroendocrine Cell Function in Diabetes. Nutrients 2022, 14, 604. https://doi.org/10.3390/nu14030604
Hua X, Li B, Yu F, Zhao W, Tan Y, Li X, Fu C, Yu X, Gao H, Cheng M. Protective Effect of MFG-E8 on Necroptosis-Induced Intestinal Inflammation and Enteroendocrine Cell Function in Diabetes. Nutrients. 2022; 14(3):604. https://doi.org/10.3390/nu14030604
Chicago/Turabian StyleHua, Xiaomin, Baoying Li, Fei Yu, Wenqian Zhao, Yuwei Tan, Xiaoli Li, Chunli Fu, Xin Yu, Haiqing Gao, and Mei Cheng. 2022. "Protective Effect of MFG-E8 on Necroptosis-Induced Intestinal Inflammation and Enteroendocrine Cell Function in Diabetes" Nutrients 14, no. 3: 604. https://doi.org/10.3390/nu14030604
APA StyleHua, X., Li, B., Yu, F., Zhao, W., Tan, Y., Li, X., Fu, C., Yu, X., Gao, H., & Cheng, M. (2022). Protective Effect of MFG-E8 on Necroptosis-Induced Intestinal Inflammation and Enteroendocrine Cell Function in Diabetes. Nutrients, 14(3), 604. https://doi.org/10.3390/nu14030604




