Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Fat-Supplemented Medium Preparation
2.3. Establishment of a 3D NAFLD Model (Tissue-Engineered Fatty Liver)
2.4. Hematoxylin and Eosin (H&E) Staining and Immunofluorescence
2.5. Cell Viability/Cytotoxicity Assay
2.6. Lactate Dehydrogenase (LDH) Measurement
2.7. Intracellular TG Measurements
2.8. Western Blotting
2.9. Apoptosis Detection
2.10. Mitochondrial Membrane Potential Detection
2.11. Transmission Electron Microscopy (TEM)
2.12. Detection of Chemicals Associated with Oxidative Stress
2.13. Detection of Antioxidant Capacity
2.14. Statistical Analysis
3. Results
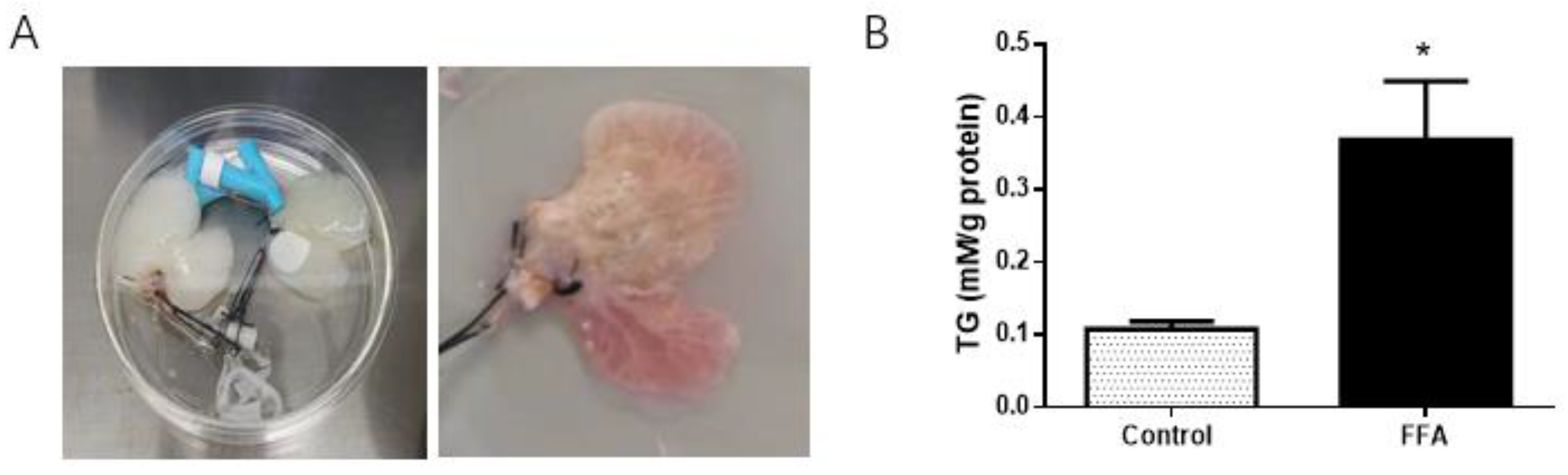
3.1. Successful Establishment of a 3D NAFLD Model
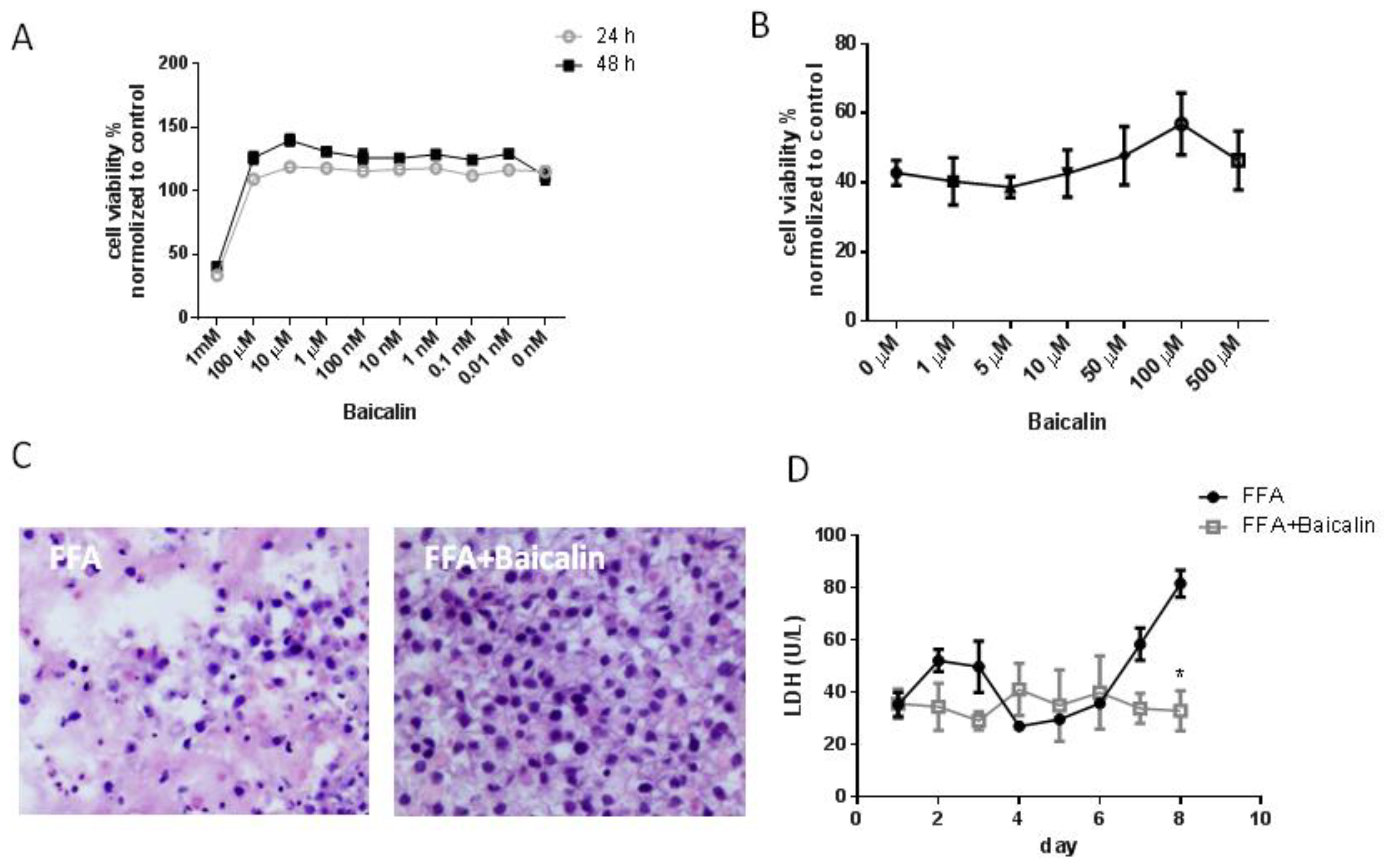
3.2. Baicalin Reduces Cell Damage in the FFA Group
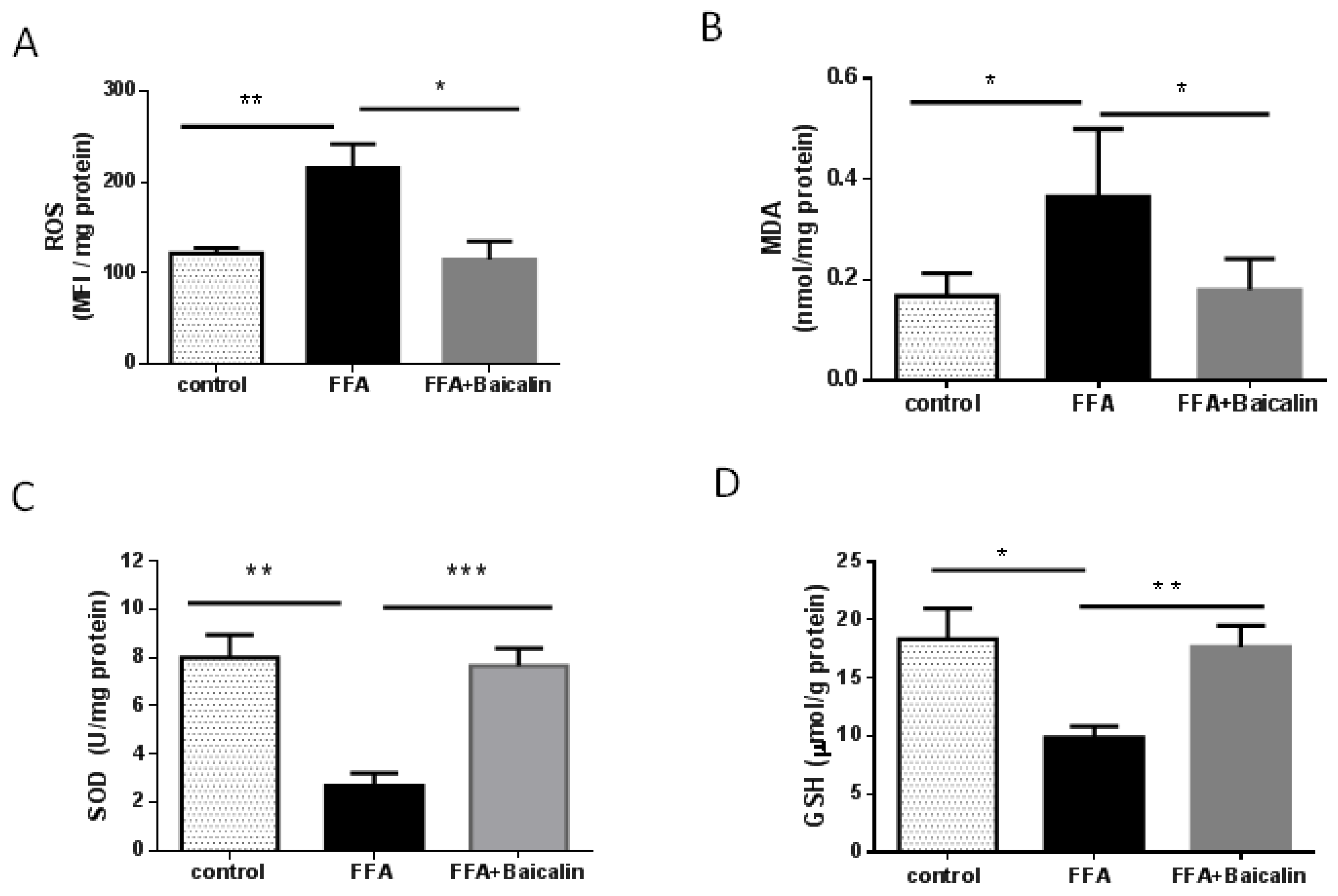
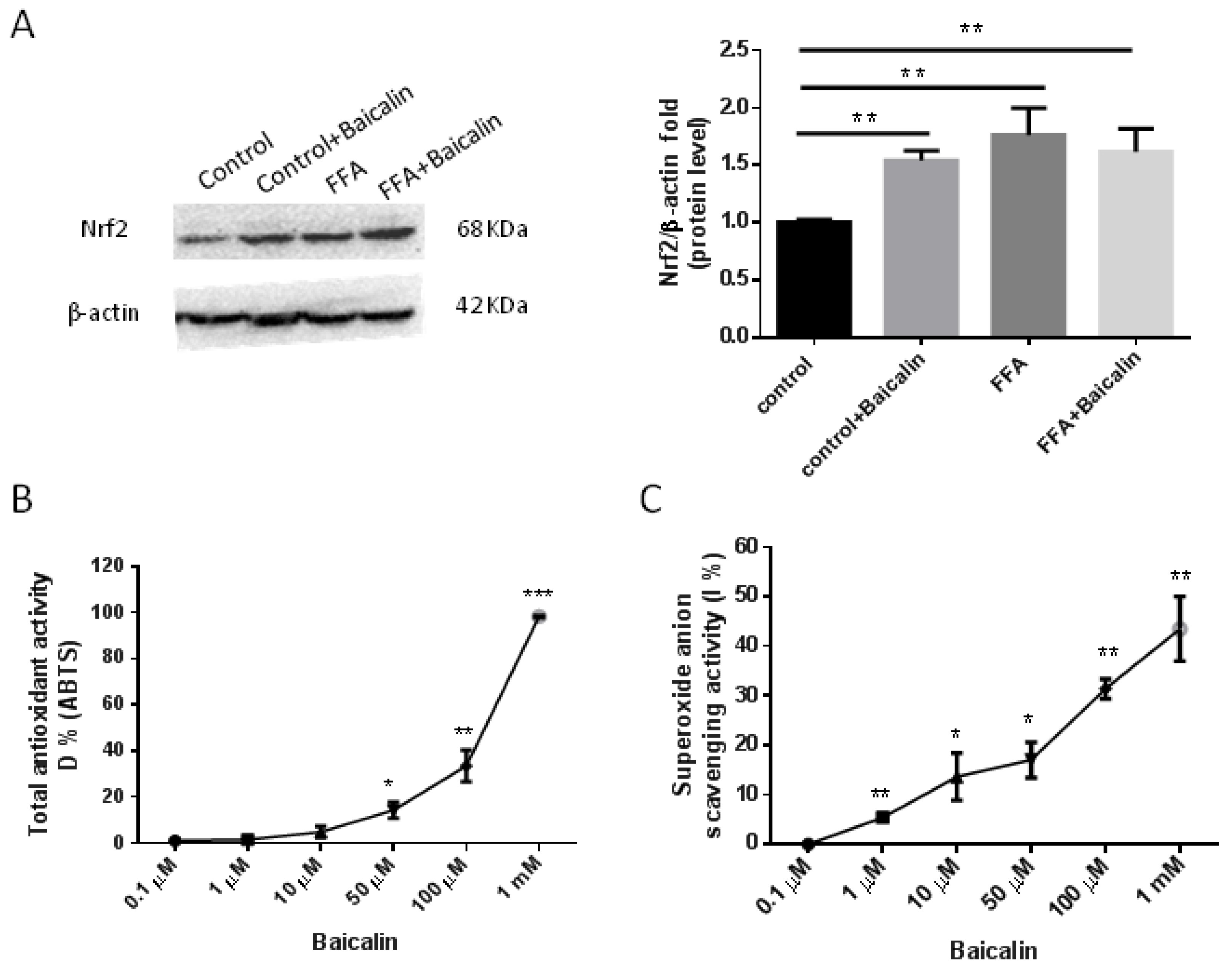
3.3. Baicalin Attenuates Oxidative Stress Process
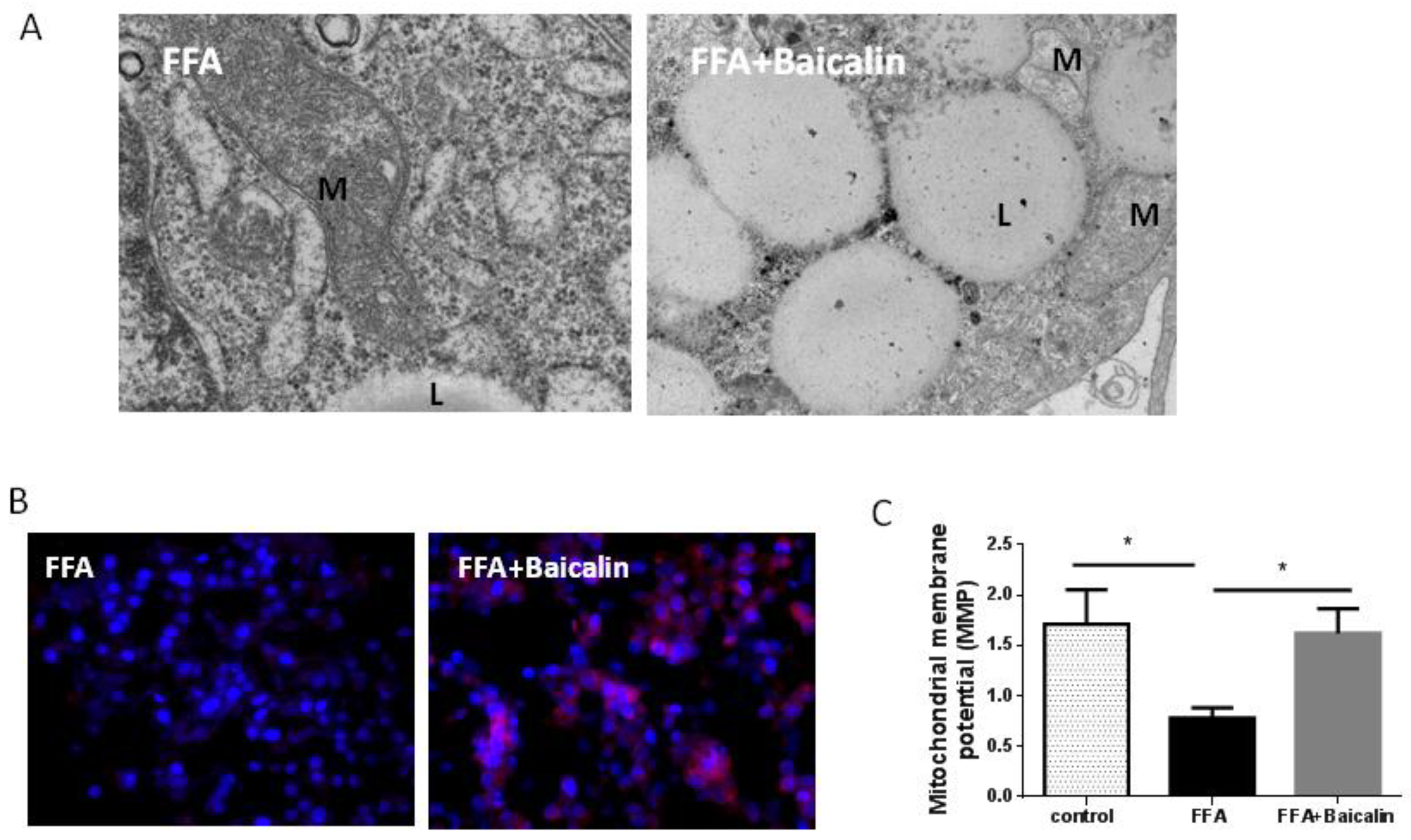
3.4. Protective Effect of Baicalin on the Mitochondria
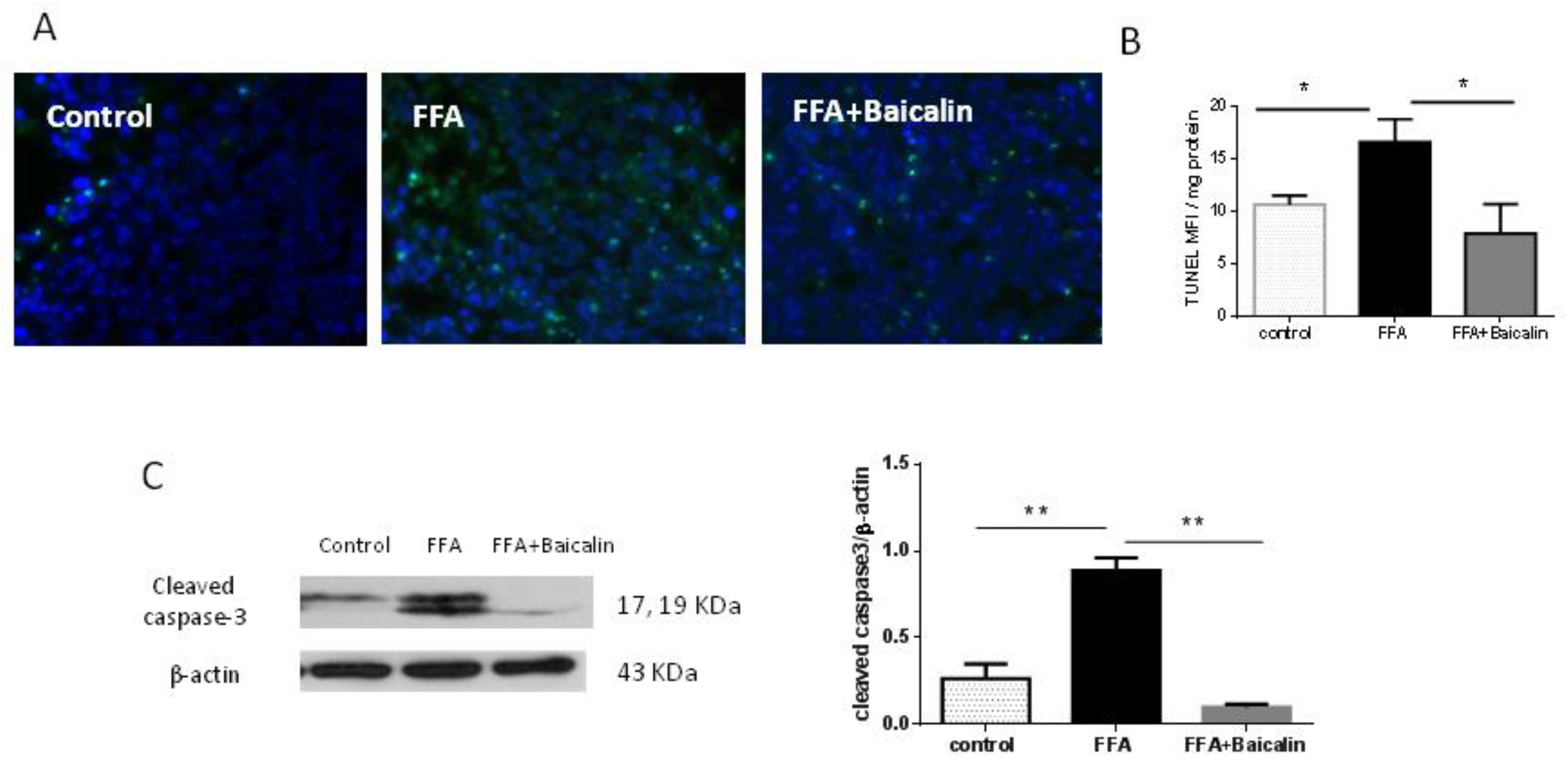
3.5. Baicalin Attenuates Apoptosis Induced by FFA
3.6. Antioxidant Activity of Baicalin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Wu, M.; Li, H.; Dong, S.; Luo, E.; Gu, M.; Shen, X.; Jiang, Y.; Liu, Y.; Liu, H. Baicalin Attenuates High Fat Diet-Induced Obesity and Liver Dysfunction: Dose-Response and Potential Role of CaMKKβ/AMPK/ACC Pathway. Cell Physiol. Biochem. 2015, 35, 2349–2359. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef]
- Huang, B.; Bao, J.; Cao, Y.-R.; Gao, H.-F.; Jin, Y. Cytochrome P450 1A1 (CYP1A1) Catalyzes Lipid Peroxidation of Oleic Acid-Induced HepG2 Cells. Biochemistry 2018, 83, 595–602. [Google Scholar] [CrossRef]
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox Proteomics: Chemical Principles, Methodological Approaches and Biological/Biomedical Promises. Chem. Rev. 2012, 113, 596–698. [Google Scholar] [CrossRef]
- Gao, H.; Cao, Y.; Xia, H.; Zhu, X.; Jin, Y. CYP4A11 is involved in the development of nonalcoholic fatty liver disease via ROS-induced lipid peroxidation and inflammation. Int. J. Mol. Med. 2020, 45, 1121–1129. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Ota, T. Impact of Glucoraphanin-Mediated Activation of Nrf2 on Non-Alcoholic Fatty Liver Disease with a Focus on Mitochondrial Dysfunction. Int. J. Mol. Sci. 2019, 20, 5920. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Cheng, Q.; Wang, L.; Zhang, L. Deep Eutectic Solvent-Based Ultrahigh Pressure Extraction of Baicalin from Scutellaria baicalensis Georgi. Molecules 2018, 23, 3233. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ma, L.; Su, M.; Zhou, Y.; Mao, K.; Li, C.; Peng, G.; Zhou, C.; Shen, B.; Dou, J. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis. 2018, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-Y.; Li, M.; Zhang, C.-L.; Liu, D. Pharmacological properties of baicalin on liver diseases: A narrative review. Pharmacol. Rep. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Hung, C.-H.; Wang, C.-N.; Cheng, H.-H.; Liao, J.-W.; Chen, Y.-T.; Chao, Y.-W.; Jiang, J.; Lee, C.-C. Baicalin Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Planta Med. 2018, 84, 1110–1117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Gao, W.; Guo, Z.; Wang, K.; Liu, S.; Duan, Z.; Chen, Y. Naringin improves lipid metabolism in a tissue-engineered liver model of NAFLD and the underlying mechanisms. Life Sci. 2021, 277, 119487. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Liu, L.; Chen, Y.; Wang, J.; Leng, L.; Yu, Q.; Duan, Z.; Wang, Y. Establishment of an ex Vivo Model of Nonalcoholic Fatty Liver Disease Using a Tissue-Engineered Liver. ACS Biomater. Sci. Eng. 2018, 4, 3016–3026. [Google Scholar] [CrossRef]
- Okina, Y.; Sato-Matsubara, M.; Matsubara, T.; Daikoku, A.; Longato, L.; Rombouts, K.; Thanh Thuy, L.T.; Ichikawa, H.; Minamiyama, Y.; Kadota, M.; et al. TGF-beta1-driven reduction of cytoglobin leads to oxidative DNA damage in stellate cells during non-alcoholic steatohepatitis. J. Hepatol. 2020, 73, 882–895. [Google Scholar] [CrossRef]
- Cai, J.-G.; Luo, L.-M.; Tang, H.; Zhou, L. Cytotoxicity of Malondialdehyde and Cytoprotective Effects of Taurine via Oxidative Stress and PGC-1α Signal Pathway in C2C12 Cells. Mol. Biol. 2018, 52, 532–542. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2020, 41, 770–784. [Google Scholar] [CrossRef]
- Choong, C.-J.; Okuno, T.; Ikenaka, K.; Baba, K.; Hayakawa, H.; Koike, M.; Yokota, M.; Doi, J.; Kakuda, K.; Takeuchi, T.; et al. Alternative mitochondrial quality control mediated by extracellular release. Autophagy 2020, 17, 2962–2974. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2019, 9, 1428. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liang, J.; Gao, L.-R.; Si, Z.-P.; Zhang, X.-T.; Liang, G.; Yan, Y.; Li, K.; Cheng, X.; Bao, Y.; et al. Baicalin administration attenuates hyperglycemia-induced malformation of cardiovascular system. Cell Death Dis. 2018, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Ore, A.; Akinloye, O.A. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina 2019, 55, 26. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.; Lozoya, O.; Wang, Y.; Cardinale, V.; Gaudio, E.; Alpini, G.; Mendel, G.; Wauthier, E.; Barbier, C.; Alvaro, D.; et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011, 53, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Liu, L.; Wang, Y.; Mao, T.; Li, J. Effects of a combination of puerarin, baicalin and berberine on the expression of proliferator-activated receptor-gamma and insulin receptor in a rat model of nonalcoholic fatty liver disease. Exp. Ther. Med. 2016, 11, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.-K.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S.; et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. Mitochondria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol. Cell. Neurosci. 2014, 63, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Esselun, C.; Bruns, B.; Hagl, S.; Grewal, R.; Eckert, G.P. Differential Effects of Silibinin A on Mitochondrial Function in Neuronal PC12 and HepG2 Liver Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1652609. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef]
- Liu, L.-L.; Gong, L.-K.; Wang, H.; Xiao, Y.; Wu, X.-F.; Zhang, Y.-H.; Xue, X.; Qi, X.-M.; Ren, J. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007, 27, 582–591. [Google Scholar] [CrossRef]
- Hwang, J.-M.; Wang, C.-J.; Chou, F.-P.; Tseng, T.-H.; Hsieh, Y.-S.; Hsu, J.-D.; Chu, C.-Y. Protective effect of baicalin on tert-butyl hydroperoxide-induced rat hepatotoxicity. Arch. Toxicol. 2005, 79, 102–109. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Xu, B.; Zhang, Y.; Liu, S.; Duan, Z.; Chen, Y.; Zhang, X. Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species. Nutrients 2022, 14, 541. https://doi.org/10.3390/nu14030541
Gao W, Xu B, Zhang Y, Liu S, Duan Z, Chen Y, Zhang X. Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species. Nutrients. 2022; 14(3):541. https://doi.org/10.3390/nu14030541
Chicago/Turabian StyleGao, Wen, Bin Xu, Yizhi Zhang, Shuang Liu, Zhongping Duan, Yu Chen, and Xiaohui Zhang. 2022. "Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species" Nutrients 14, no. 3: 541. https://doi.org/10.3390/nu14030541
APA StyleGao, W., Xu, B., Zhang, Y., Liu, S., Duan, Z., Chen, Y., & Zhang, X. (2022). Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species. Nutrients, 14(3), 541. https://doi.org/10.3390/nu14030541





