Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Dietary Treatments and Experimental Design
2.2. Samples Collection
2.3. Serum Parameters
2.4. Intestinal Morphology
2.5. mRNA Expression of Inflammatory Cytokines, Host Defense Peptides, Tight Junction Proteins
2.6. Expression of NF-κB and Nrf2 Genes
2.7. Microbial Community
2.8. Production of Lactic Acid and SCFA
2.9. Statistical Analysis
3. Results
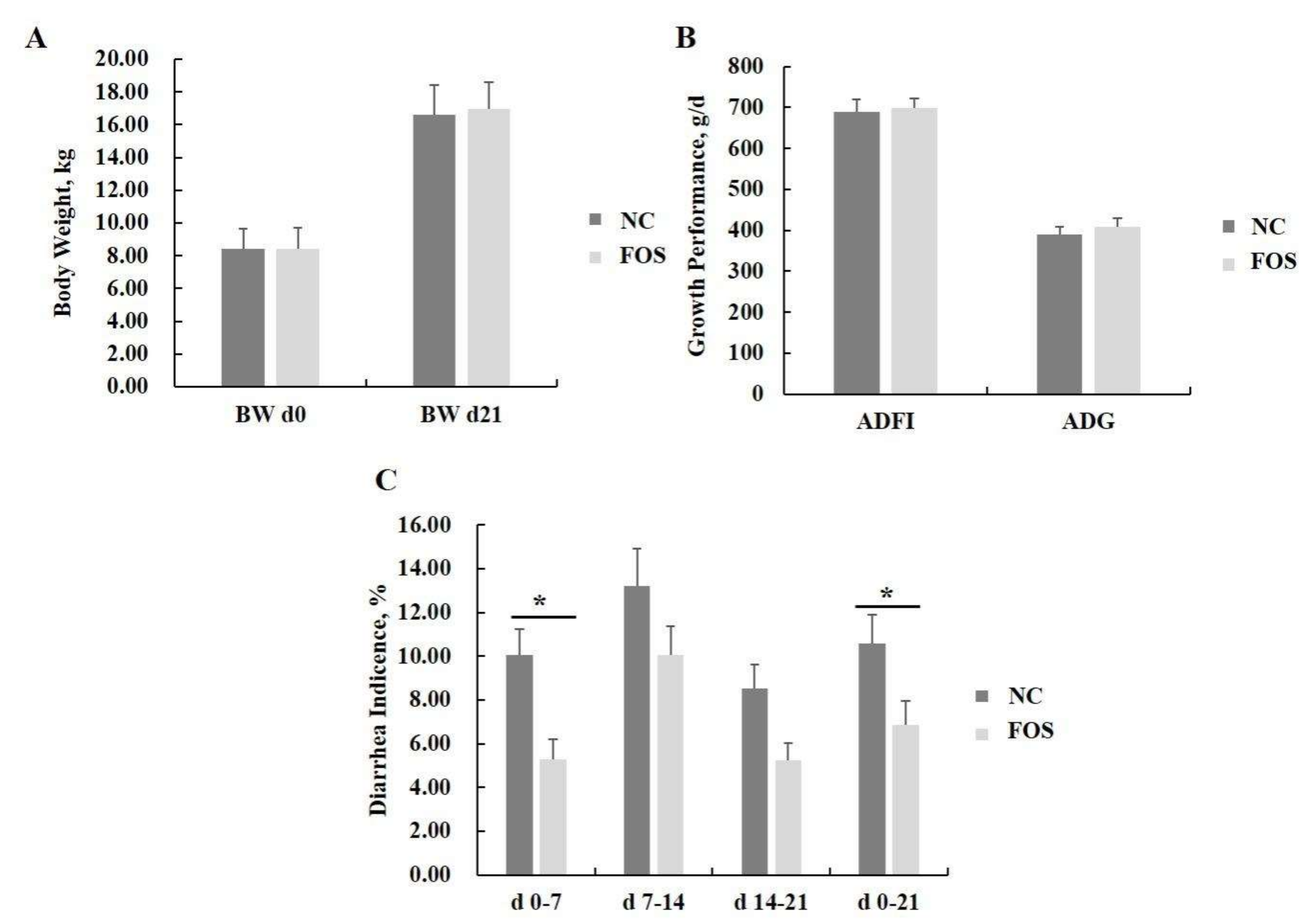
3.1. Effects of FOS Supplementation on Growth Performance and Diarrhea Incidence
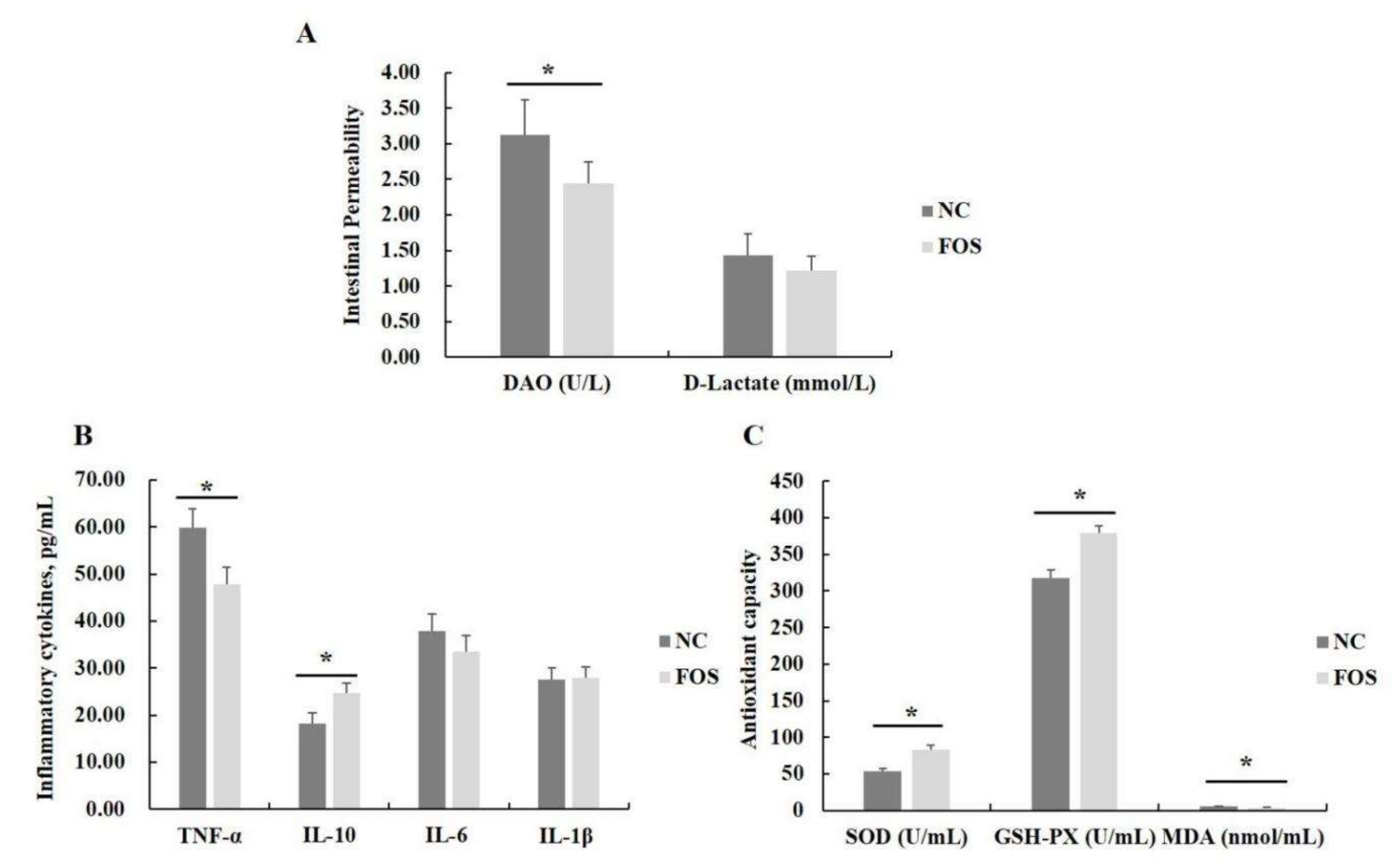
3.2. Effects of FOS Supplementation on Serum Antioxidant Activity, Inflammatory Cytokines, and Intestinal Permeability
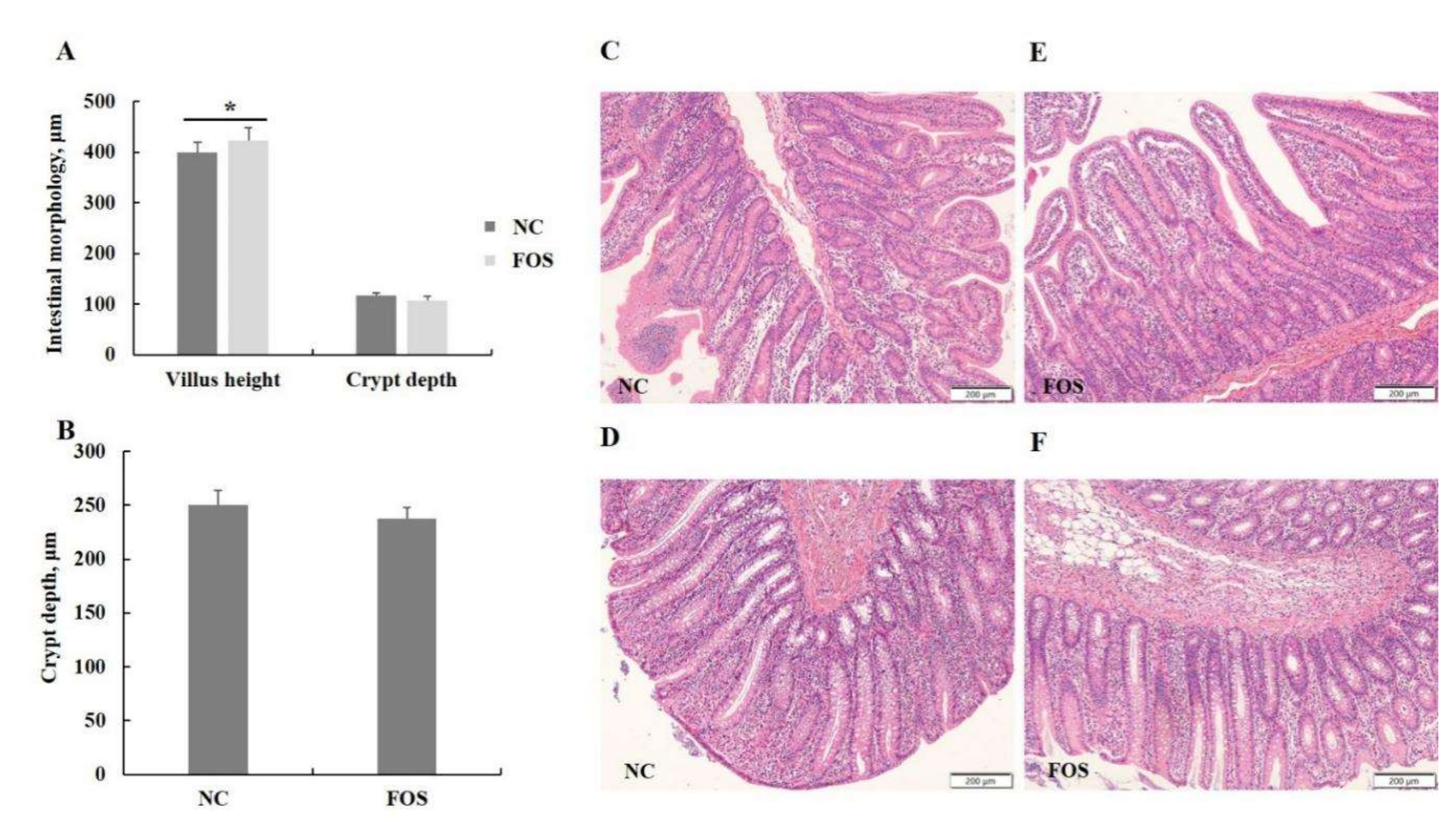
3.3. Effects of FOS Supplementation on Intestinal Morphology
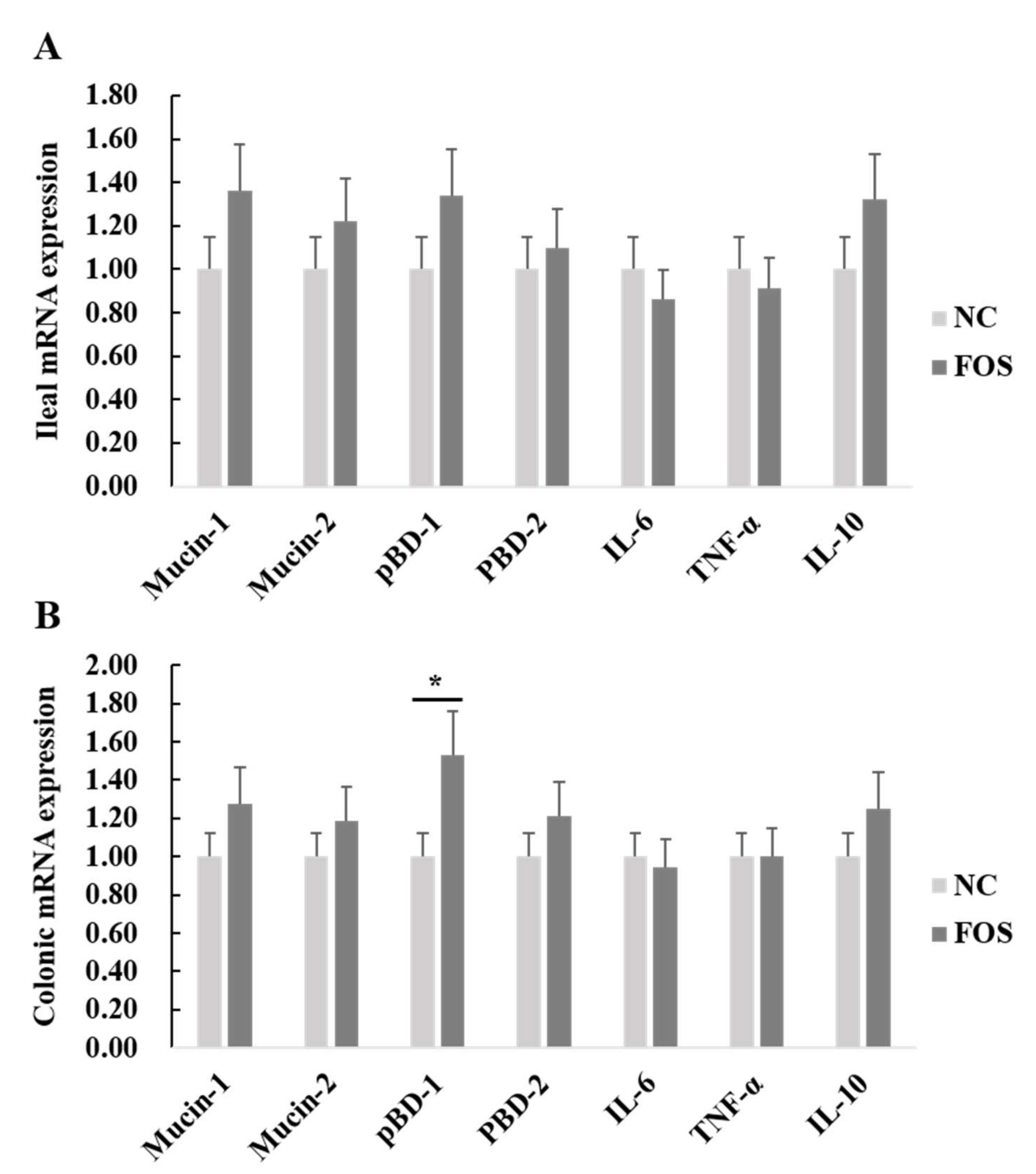
3.4. Effects of FOS Supplementation on mRNA Expression of Intestinal Inflammatory Cytokines and Host Defense Peptides
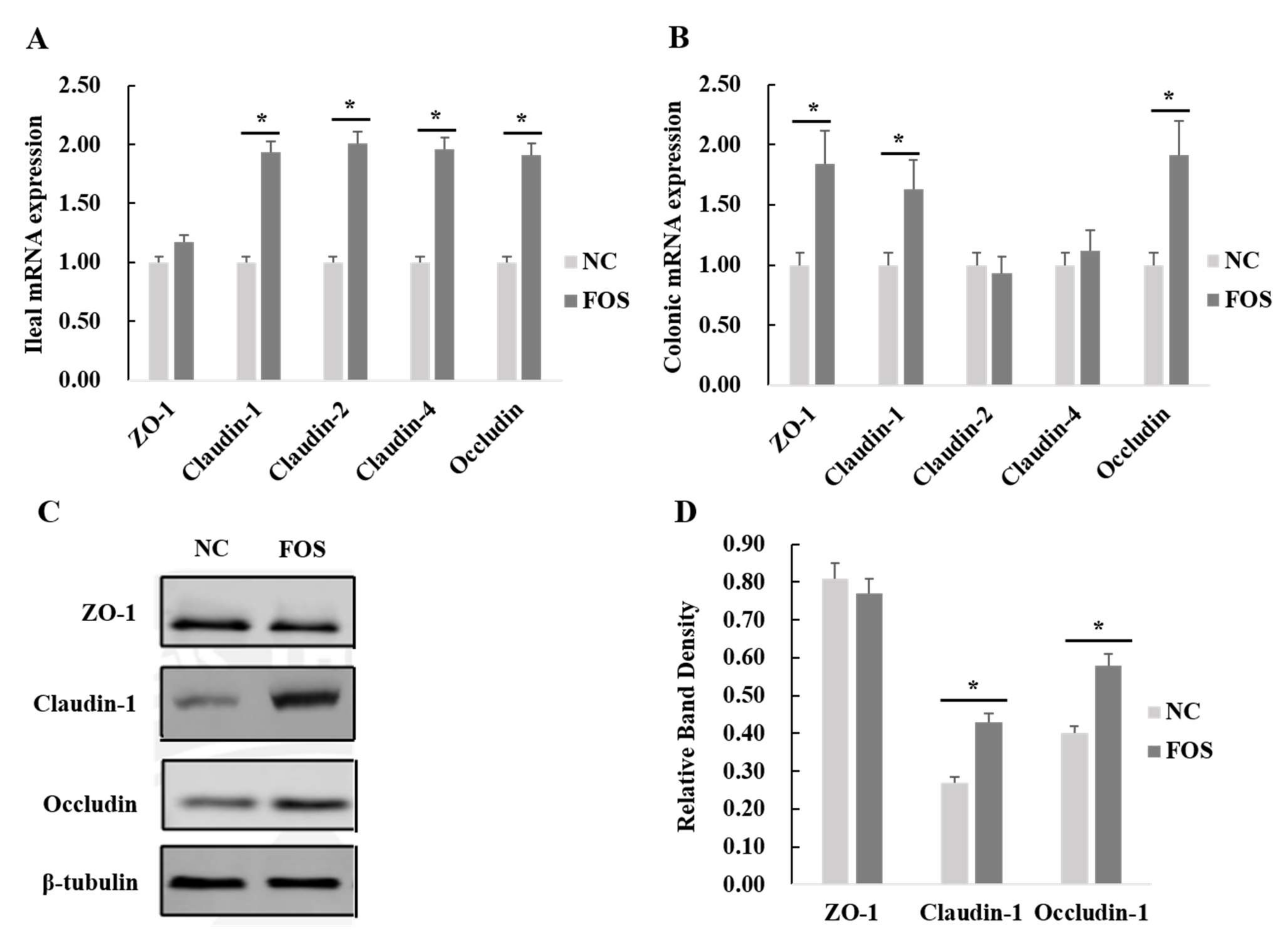
3.5. Effects of FOS Supplementation on Expression of Intestinal Tight Junction Proteins
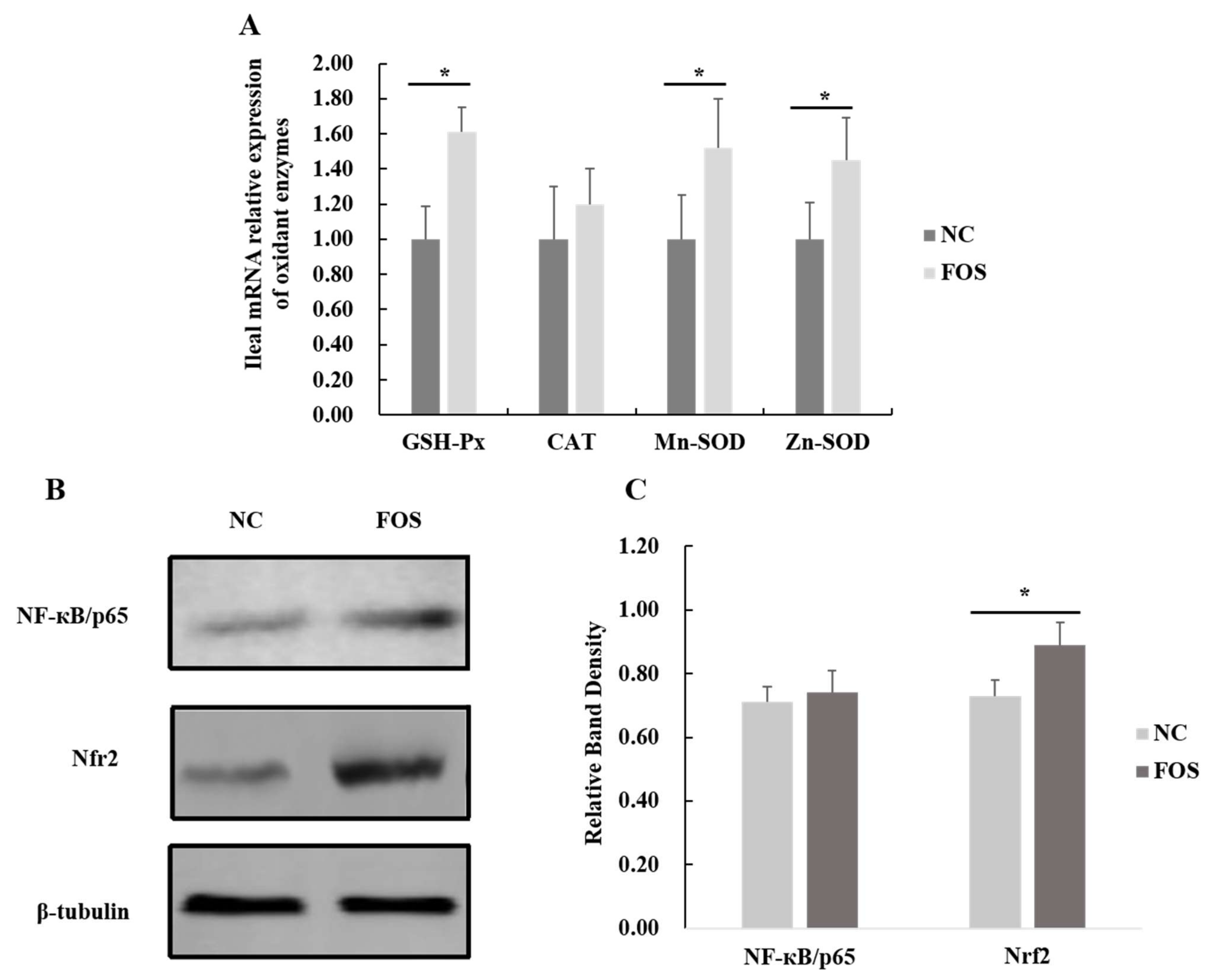
3.6. Effects of FOS Supplementation on Expression of Intestinal Antioxidase Activity
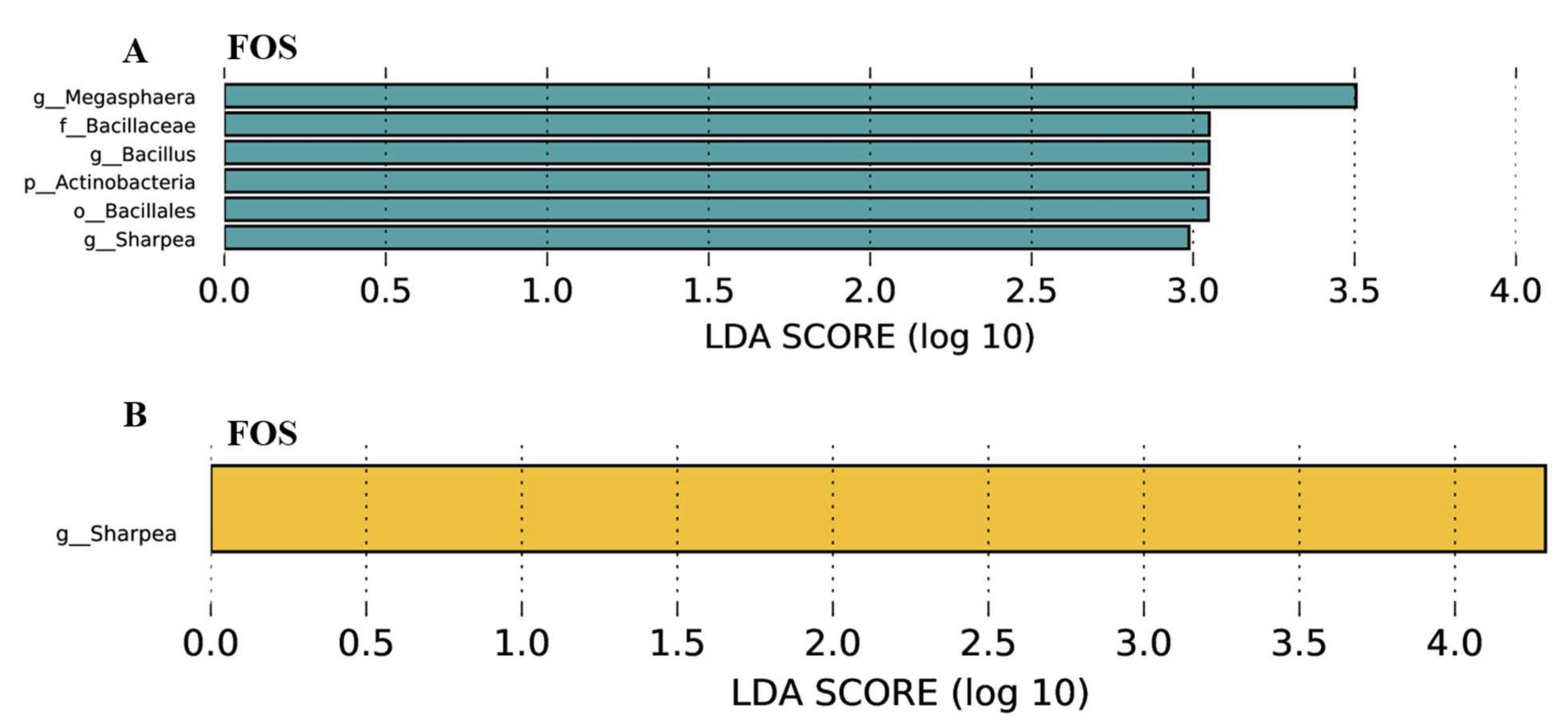
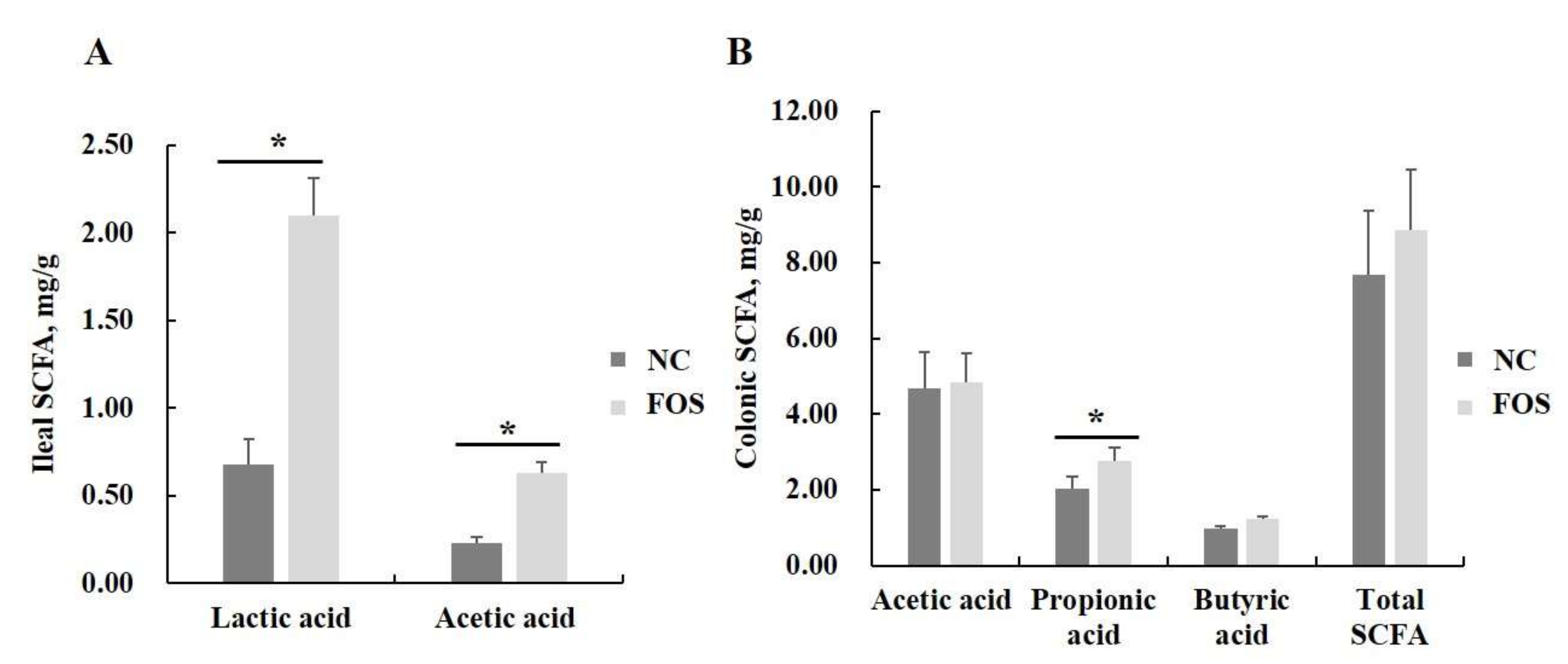
3.7. Effects of FOS Supplementation on Microbial Communities and Their Metabolites
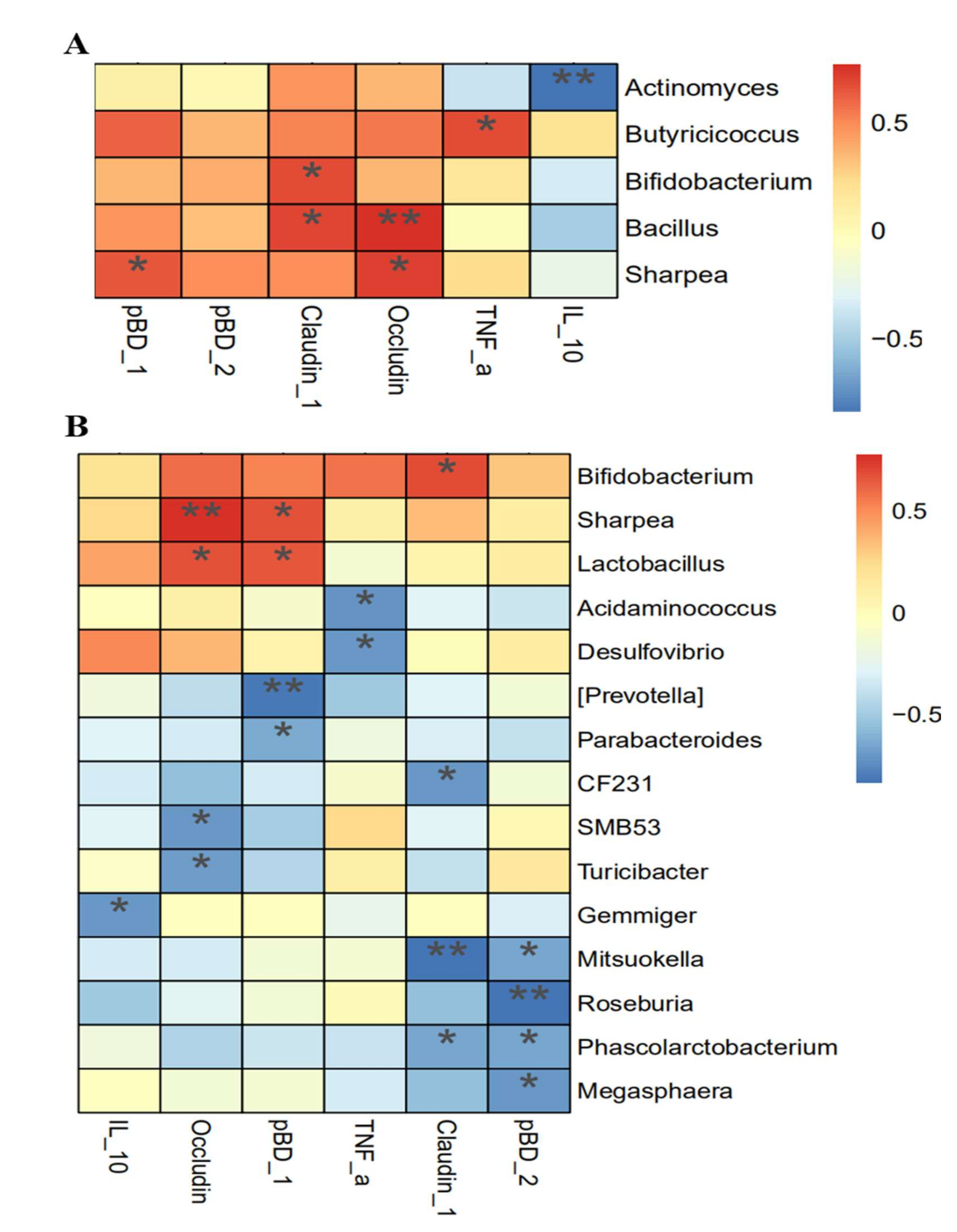
3.8. Association Analysis between Intestinal Integrity and Microbial Community
4. Discussion
4.1. Responses of FOS Supplementation on Pig Performance and Diarrhea Incidence
4.2. Responses of Antioxidase Activity and Intestinal Permeability to FOS Supplementation
4.3. Effects of FOS Supplementation on Intestinal Tight Junction Protein Expression
4.4. Effects of FOS Supplementation on Microbial Composition and Their Metabolites
4.5. Association between Intestinal Function and Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADFI | average daily feed intake |
| ADG | average daily gain |
| FOS | fructooligosaccharide |
| HDAC | histone deacetylase |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| TNF-α | tumor necrosis factor-α |
| SCFA | short-chain fatty acids |
| GPR | G protein-coupled receptors |
| NF-κB | nuclear factor-κB |
| DAO | diamine oxidase |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| OTU | operational taxonomic units |
| LDA | linear discriminant analysis |
| MAPK | mitogen-activated protein kinase |
References
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.S.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, A.R.; Vuaran, M.; Crittenden, R.; Hayakawa, T.; Playne, M.J.; Brown, I.L.; Topping, D.L. Comparative effects of a high-amylose starch and a fructooligosaccharides on fecal Bifidobacteria numbers and short-chain fatty acids in pigs fed Bifidobacterium animalis. Dig. Dis. Sci. 2009, 54, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Le Bourgot, C.; Ferret-Bernard, S.; Le Normand, L.; Savary, G.; Menendez-Aparicio, E.; Blat, S.; Appert-Bossard, E.; Respondek, F.; Le Huërou-Luron, I. Maternal short-chain fructo-oligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS ONE 2014, 9, e107508. [Google Scholar] [CrossRef] [Green Version]
- Le Bourgot, C.; Ferret-Bernard, S.; Blat, S.; Apper, E.; Le Huërou-Luron, I. Short-chain fructooligosaccharide supplementation during gestation and lactation or after weaning differentially impacts pig growth and IgA response to influenza vaccination. J. Funct. Foods 2016, 24, 307–315. [Google Scholar] [CrossRef]
- Schokker, D.; Fledderus, J.; Jansen, R.; Vastenhouw, S.A.; Jansman, A.A.J.M. Supplementation of fructooligosaccharides to suckling piglets affects intestinal microbiota colonization and immune development. J. Anim. Sci. 2018, 96, 2139–2153. [Google Scholar] [CrossRef]
- Nohr, M.K.; Egerod, K.I.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H. Activation of GPR109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammatory and carcinogensis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- United States National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012.
- Yu, Z.; Zhang, S.; Yang, Q.; Peng, Q.; Zhu, J.; Zeng, X.; Qiao, S. Effect of high fiber diets formulated with different fibrous ingredients on performance, nutrient digestibility and faecal microbiota of weaned piglets. Arch. Anim. Nutr. 2016, 70, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Bai, Y.; Li, T.; Li, N.; Wang, J. Original low birth weight deteriorates the hindgut epithelial barrier function in pigs at the growing stage. FASEB J. 2019, 33, 9897–9912. [Google Scholar]
- Tao, S.; Duanmu, Y.; Dong, H.; Tian, J.; Ni, Y.; Zhao, R. A high-concentrate diet induced colonic epithelial barrier disruption is association with the activating of cell apoptosis in lactating goats. BMC Vet. Res. 2014, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhang, X.; Han, D.; Ye, H.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Short administration of combined prebiotics improved microbial colonization, gut barrier, and growth performance of neonatal piglets. ACS Omega 2020, 5, 20506–20516. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, J.; Ma, X. Sodium decanoate improves intestinal epithelial barrier and antioxidation via activating G protein-coupled receptor-43. Nutrients 2021, 13, 2756. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Levesque, C.; Chen, Y.; Zhao, J.; Zhang, J.; et al. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, J.; Wang, W.; Guo, P.; Lu, W.; Wang, C.; Liu, L.; Johnston, L.J.; Zhao, Y.; Wu, X.; et al. Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Front. Microbiol. 2018, 9, 2090. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Tao, S.; Zhang, G.; Wang, J.; Liu, L.; Zhang, S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods 2019, 57, 266–274. [Google Scholar] [CrossRef]
- Xu, C.; Chen, X.; Ji, C.; Ma, Q.; Hao, K. Study of the application of fructooligosaccharides in piglets. Asian-Australas. J. Anim. Sci. 2005, 18, 1011–1016. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.L.; Li, D.F.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, J.D.; Menoyo, D.; Guzmán, P.; Saldaña, B.; Cámara, L.; Mateos, G.G. Effects of fiber inclusion on growth performance and nutrient digestibility of piglets reared under optimal or poor hygienic conditions. J. Anim. Sci. 2015, 93, 3919–3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukudome, K.U.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Baba, R.; Kumagai, N.; Oba, K.; Fujita, M.; et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2014, 47, 100. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Naylor, J.M.; Zello, G.A. D-lactate in human and ruminant metabolism. J. Nutr. 2005, 135, 1619–1625. [Google Scholar] [CrossRef]
- Ji, J.; Gu, Z.; Li, H.; Su, L.; Liu, Z. Cryptdin-2 predicts intestinal 643 injury during heatstroke in mice. Int. J. Mol. Med. 2018, 41, 137–146. [Google Scholar]
- Karabulut, K.U.; Narei, H.; Gul, M.; Dundar, Z.D.; Cander, B.; Girisgin, A.S.; Erdem, S. Diamine oxidase in diagnosis of acute mesenteric schemia. Am. J. Emerg. Med. 2013, 31, 309. [Google Scholar] [CrossRef]
- Yang, C.M.; Ferket, P.R.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglets gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechno. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Shen, W.Z.; Wen, X.X.; Cao, S.C.; Ou, S.Y.; Wu, Y.H.; Liu, G.S. Pilot study on the antioxidation activity in vitro and mechanism of feruloy oligosaccharides extracted from maize. Acta Nutr. Sin. 2012, 34, 483–487. [Google Scholar]
- Veenashri, B.R.; Muralikrishna, G. In vitro anti-oxidant activity of xylo-oligosaccharides derived from cereal and millet brans: A comparative study. Food Chem. 2011, 126, 1475–1481. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Zhang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 2018, 8, 13482–13492. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier function in health and disease. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, X.; Han, J.; Song, W.; Dong, N.; Xu, X.; Wei, Z.; Sham, A. Sodium butyrate improves porcine host defense peptide expression and relieves the inflammatory response upon Toll-like receptor 2 activation and histone deacetylase inhibition in porcine kidney cell. Oncotarget 2017, 8, 26532–26551. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhai, Q.; Li, D.; Mao, B.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Restoration of cefixime-induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol. Res. 2017, 200, 14–24. [Google Scholar] [CrossRef]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Asanuma, N.; Hino, T. Fructose bisphosphate aldolase activity and glycolytic intermediate concentrations in relation to lactate production in Streptococcus bovis. Anaerobe 2002, 8, 1–8. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Backhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.B.; Verstegen, M.W.A.; Kim, I.H.; Kwon, O.S.; Verdonk, J.M.A.J. Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Arch. Anim. Nutr. 2005, 59, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Jørgensen, H. Carbohydrates and carbohydrate utilization in swine. In Sustainable Swine Nutrition; Chiba, L.I., Ed.; John Wiley and Sons: Ames, IA, USA, 2013; pp. 109–135. [Google Scholar]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–733. [Google Scholar] [CrossRef]
- Cani, P.D. Interactions between gut microbes and host cells control gut barrier and metabolism. Int. J. Obes. 2016, 6, S28–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.C.H.; Wang, J.T.; Wei, S.C.; Ni, Y.H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2016, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay, K.M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.M. The influence of the gut microbiota on host physiology: In pursuit of mechanisms. Yale J. Biol. Med. 2016, 89, 285–297. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, G.; Zhang, S.; Zhao, J. Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression. Nutrients 2022, 14, 512. https://doi.org/10.3390/nu14030512
Zhang Z, Zhang G, Zhang S, Zhao J. Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression. Nutrients. 2022; 14(3):512. https://doi.org/10.3390/nu14030512
Chicago/Turabian StyleZhang, Zeyu, Ge Zhang, Shuai Zhang, and Jinbiao Zhao. 2022. "Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression" Nutrients 14, no. 3: 512. https://doi.org/10.3390/nu14030512
APA StyleZhang, Z., Zhang, G., Zhang, S., & Zhao, J. (2022). Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression. Nutrients, 14(3), 512. https://doi.org/10.3390/nu14030512







