Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of RG
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.2.1. Chemical and Reagent
2.2.2. Standard Solution and Sample Preparation
2.2.3. HPLC Analysis Method
2.3. Animals and CUMS Procedure
2.4. Animal Experimental Design
- (1)
- Control: The group was administered orally the same volume of saline as that administered in the other groups without the CUMS procedure.
- (2)
- CUMS: The group was administered orally the same volume of saline as that administered in the CUMS procedure.
- (3)
- CUMS/RG40: The group was administered orally RG at 40 mg/kg daily with the CUMS procedure.
- (4)
- CUMS/RG90: The group was administered orally RG at 90 mg/kg daily with the CUMS procedure.
- (5)
- CUMS/RG140: The group was administered orally RG at 140 mg/kg daily with the CUMS procedure.
- (6)
- CUMS/GABA: The group was administered orally GABA at 30 mg/kg daily with the CUMS procedure.
- (7)
- CUMS/Theanine: The group was administered orally theanine at 50 mg/kg daily with the CUMS procedure.
2.5. Forced Swimming Test (FST)
2.6. Tail-Suspension Test (TST)
2.7. Enzyme-Linked Immunosorbent Assay
2.8. 3,3′-Diaminobenzidine (DAB) Staining
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. GABA Content in RG
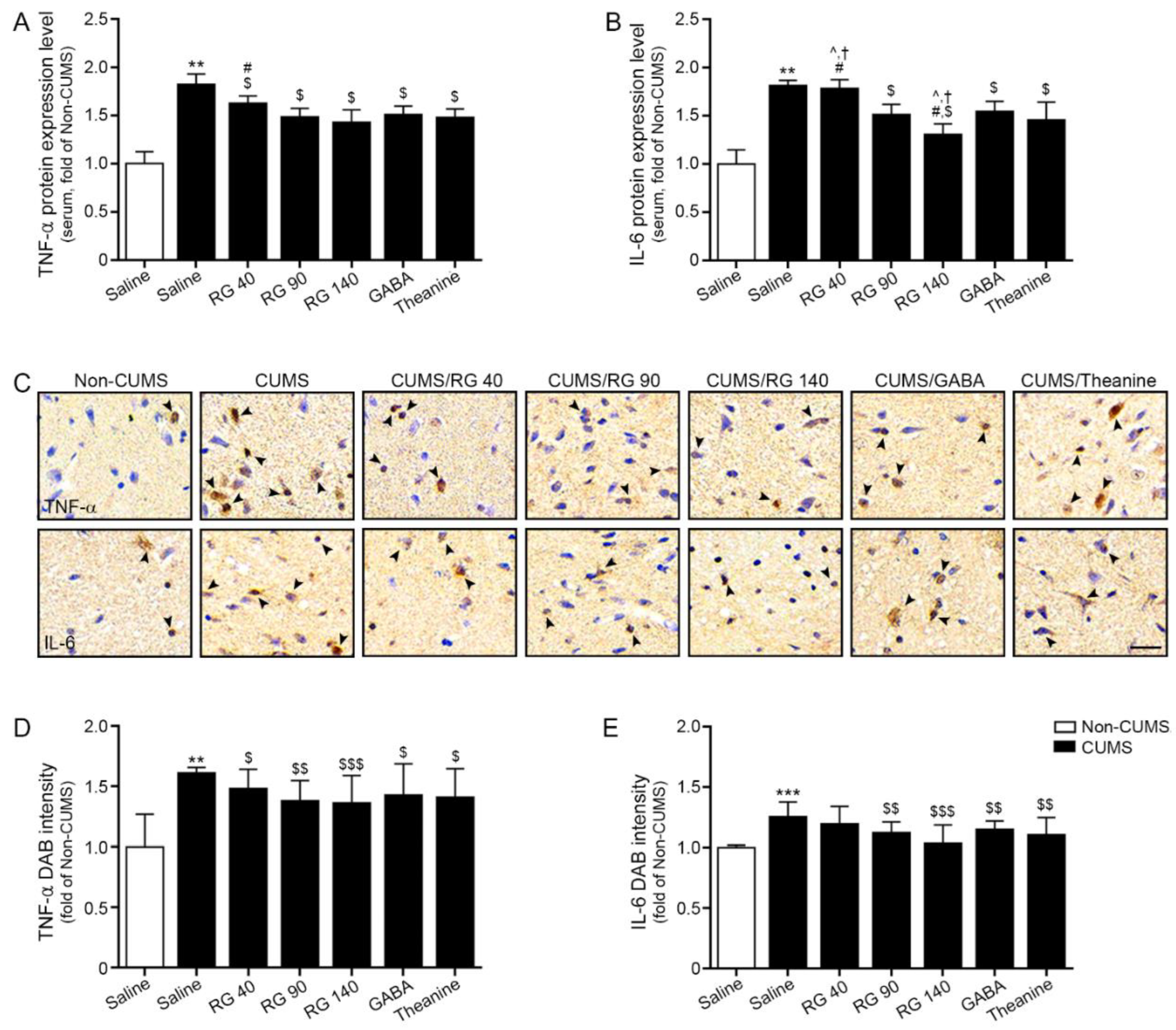
3.2. RG Decreased Expression Levels of TNF-α and IL-6 in Serum and Hypothalamic of CUMS Mice
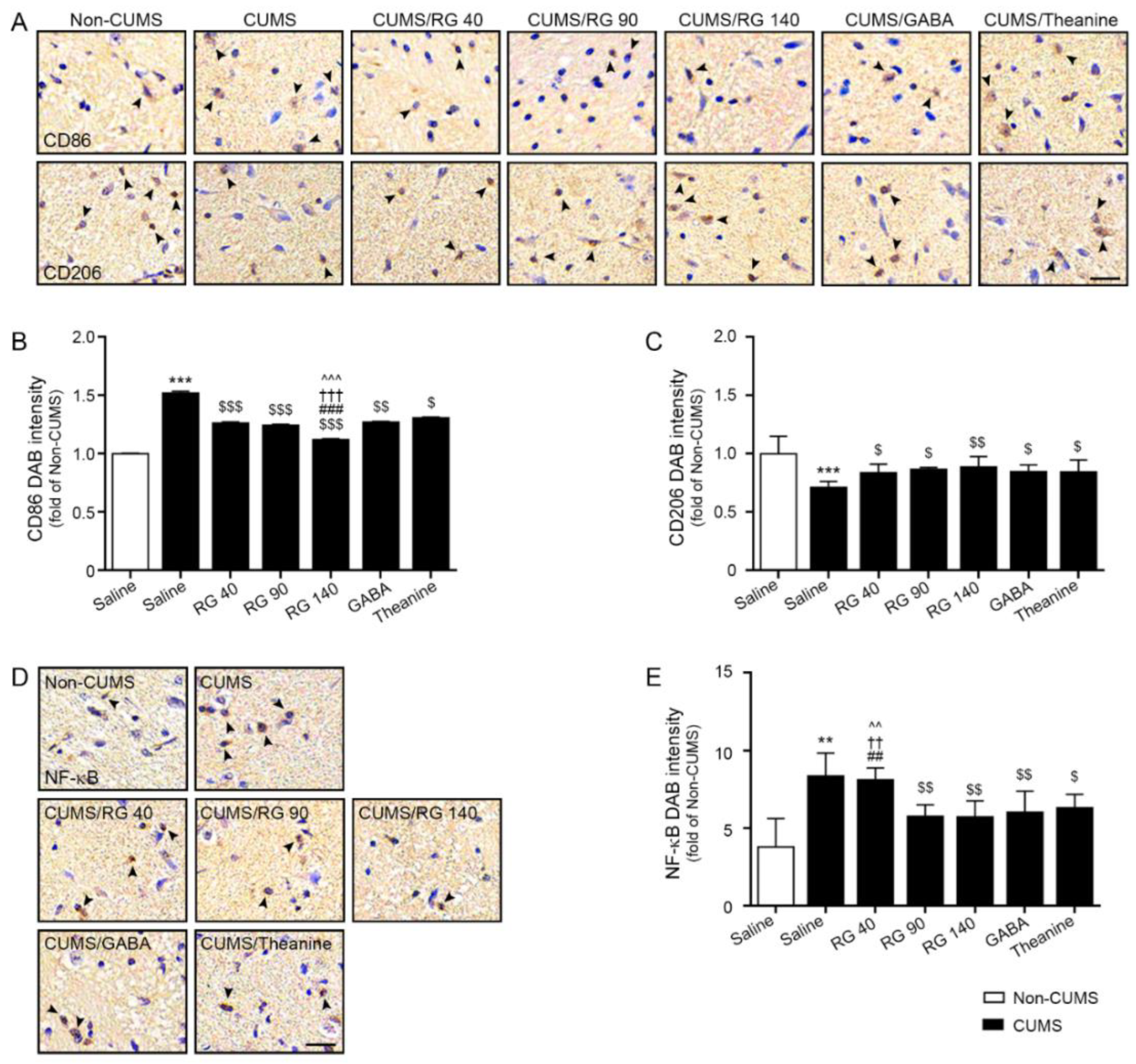
3.3. RG Decreased M1 Polarization and NF-κB Expression
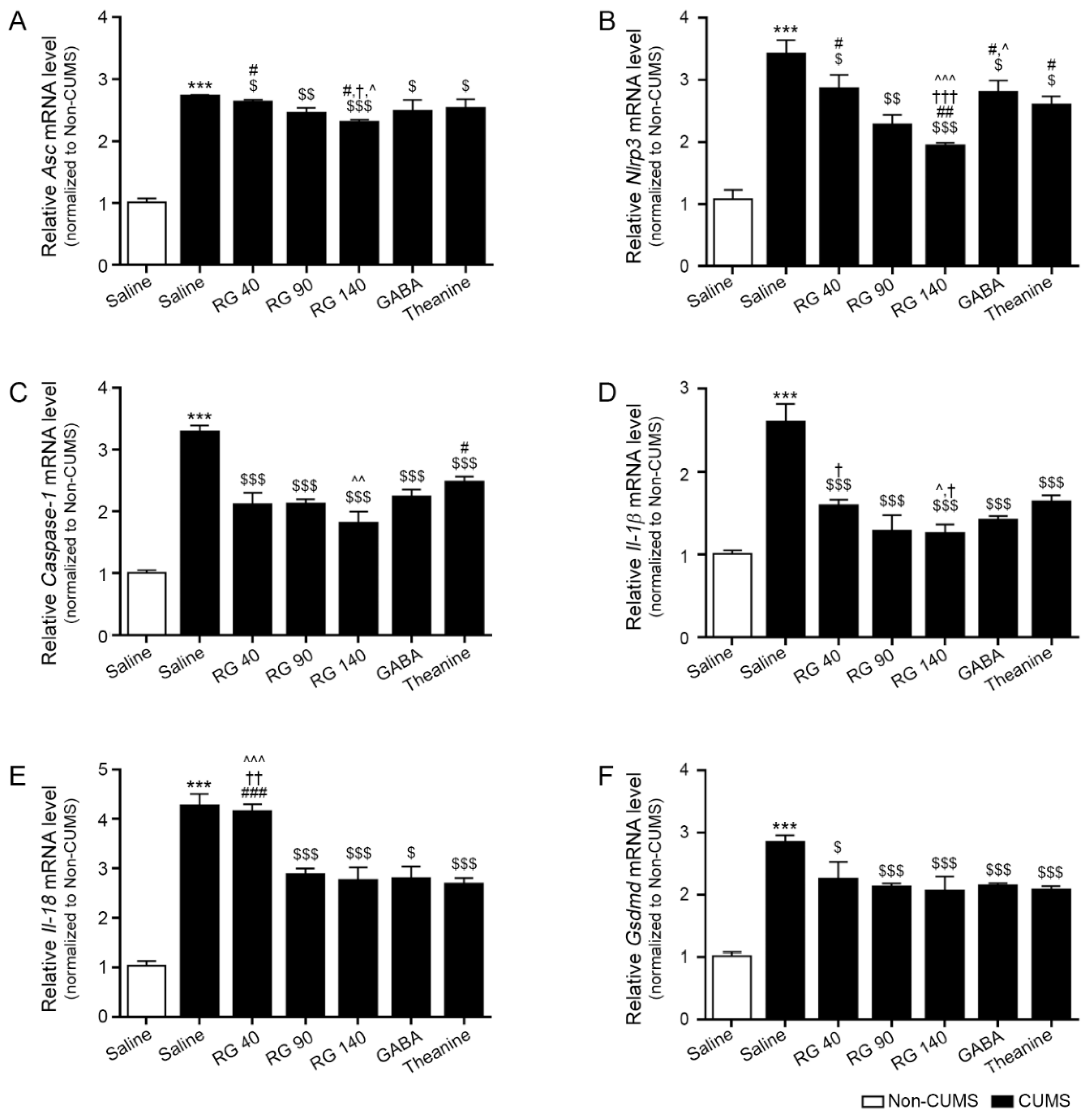
3.4. RG Decreased the Expression Levels of the NLRP3 Inflammasome Complex, Caspase-1, IL-1β, and IL-18
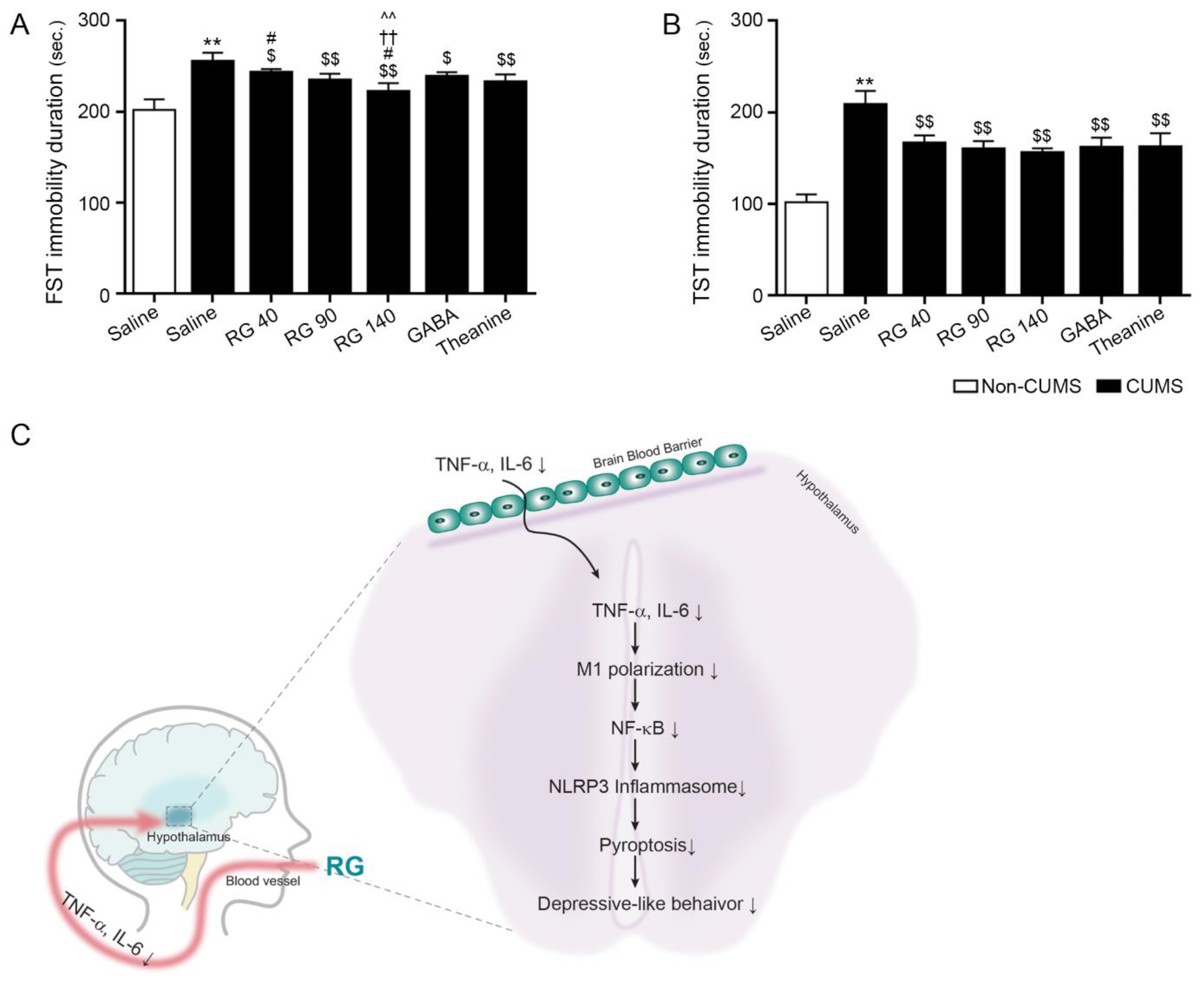
3.5. RB Attenuated Depressive-like Behavior in CUMS Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harwood, N.E.; Batista, F.D. The antigen expressway: Follicular conduits carry antigen to B cells. Immunity 2009, 30, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.L.; Samuels, B.C.; Fitz, S.D.; Federici, L.M.; Hammes, N.; Early, M.C.; Truitt, W.; Lowry, C.A.; Shekhar, A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol. Behav. 2012, 107, 733–742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Scharpé, S.; Meltzer, H.Y.; Bosmans, E.; Suy, E.; Calabrese, J.; Cosyns, P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res 1993, 49, 11–27. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, Y.; Li, Z.; Li, X. TLR4-NF-κB Signal Involved in Depressive-Like Behaviors and Cytokine Expression of Frontal Cortex and Hippocampus in Stressed C57BL/6 and ob/ob Mice. Neural Plast. 2018, 2018, 7254016. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Xu, M.; Hu, L.; Yan, T.; He, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandrin rescues depressive-like behaviors induced by chronic unpredictable mild stress via GDNF/ERK1/2/ROS and PI3K/AKT/NOX signaling pathways in mice. Psychiatry Res. 2017, 257, 230–237. [Google Scholar] [CrossRef]
- Leonard, B.E. The concept of depression as a dysfunction of the immune system. Curr. Immunol. Rev. 2010, 6, 205–212. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Wu, K.; Yan, W.; Tian, T.; Wang, Y.; Yang, H. Paraquat modulates microglia M1/M2 polarization via activation of TLR4-mediated NF-κB signaling pathway. Chem. Biol. Interact. 2019, 310, 108743. [Google Scholar] [CrossRef]
- Varnum, M.M.; Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch. Immunol. Ther. Exp. 2012, 60, 251–266. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.P.; Frausto, R.; Rose-John, S.; Campbell, I.L. Analysis of IL-6/gp130 family receptor expression reveals that in contrast to astroglia, microglia lack the oncostatin M receptor and functional responses to oncostatin M. Glia 2015, 63, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- De Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014, 34, 369–375. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Wu, D.; Han, R.; Deng, S.; Liu, T.; Zhang, T.; Xie, H.; Xu, Y. Protective Effects of Flagellin A N/C Against Radiation-Induced NLR Pyrin Domain Containing 3 Inflammasome-Dependent Pyroptosis in Intestinal Cells. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 107–117. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, J.; Xing, F. ‘Hints’ in the killer protein gasdermin D: Unveiling the secrets of gasdermins driving cell death. Cell Death Differ. 2017, 24, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Rathkey, J.K.; Yang, J.; Dubyak, G.R.; Abbott, D.W.; Xiao, T.S. Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition. Structure 2018, 26, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.; Jelinek, H.F.; Viengkhou, V.; Johnston, G.A.; Matthews, S. Effect of GABA-Fortified Oolong Tea on Reducing Stress in a University Student Cohort. Front. Nutr. 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.I.; Haramoto, M.; Fukuda, T.; Mizuno, H.; Tanaka, A.; Nishimura, M.; Nishihira, J. Optimization of a γ -aminobutyric Acid (GABA) Enrichment Process for Hokkaido White Rice and the Effects of GABA-enriched White Rice on Stress Relief in Humans. J. Jpn. Socn Food Sci. 2015, 62, 95–103. [Google Scholar] [CrossRef]
- Ahn, J.H.; Im, C.; Park, J.H.; Choung, S.Y.; Lee, S.; Choi, J.; Won, M.-H.; Kang, I.-J. Hypnotic effect of GABA from rice germ and/or tryptophan in a mouse model of pentothal-induced sleep. Food Sci. Biotechnol 2014, 23, 1683–1688. [Google Scholar] [CrossRef]
- Rondanelli, M.; Peroni, G.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Naso, M. Effectiveness of Rice Germ Supplementation on Body Composition, Metabolic Parameters, Satiating Capacity, and Amino Acid Profiles in Obese Postmenopausal Women: A Randomized, Controlled Clinical Pilot Trial. Nutrients 2021, 13, 439. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Choi, Y.H.; Park, E.K.; Jeon, Y.J.; Lee, B.J.; Kang, C.H.; Kim, G.Y. Fermented Oyster Extract Promotes Osteoblast Differentiation by Activating the Wnt/β-Catenin Signaling Pathway, Leading to Bone Formation. Biomolecules 2019, 9, 711. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activity and γaminobutyric acid (GABA) content in organic substances.sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Roberts, E. Gamma-aminobutyric acid and nervous system function--a perspective. Biochem. Pharmacol. 1974, 23, 2637–2649. [Google Scholar] [CrossRef]
- Kuriyama, K.; Sze, P.Y. Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, H.Y.; Lee, H.J.; Shim, I.; Hahm, D.H. Wound healing activity of gamma-aminobutyric Acid (GABA) in rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar] [PubMed]
- Prud’homme, G.J.; Glinka, Y.; Wang, Q. GABA exerts anti-inflammatory and immunosuppressive effects (P5175). J. Immunol. 2013, 190 (Suppl. S1), 68.15. [Google Scholar] [CrossRef]
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brennan, M.; Li, S.; Zhao, H.; Lange, K.W.; Brennan, C. How does the tea L-theanine buffer stress and anxiety. Food Sci. Hum. 2022, 11, 467–475. [Google Scholar] [CrossRef]
- Cha, J.Y.; Je, J.Y.; Kim, Y.M. GABA-enriched Fermented Laminaria japonica Protects against Alcoholic Hepatotoxicity in Sprague-Dawley Rats. Fish Aquat. Sci. 2011, 14, 79–88. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 29, e3638. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Wiersma, J.E.; van Oppen, P.; van Schaik, D.J.; van der Does, A.J.; Beekman, A.T.; Penninx, B.W. Psychological characteristics of chronic depression: A longitudinal cohort study. J. Clin. Psychiatry 2011, 72, 288–294. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Vialou, V.; Feng, J.; Robison, A.J.; Nestler, E.J. Epigenetic mechanisms of depression and antidepressant action. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Isingrini, E.; Camus, V.; Le Guisquet, A.M.; Pingaud, M.; Devers, S.; Belzung, C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: A model of fluoxetine resistance in mice. PLoS ONE 2010, 5, 10404. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Radhakrishnan, M.; Jindal, A.; Devadoss, T.; Dhar, A.K. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stress-induced changes in behavioural and brain oxidative stress parameters in mice. Indian J. Pharmacol. 2014, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Pesarico, A.P.; Sartori, G.; Brüning, C.A.; Mantovani, A.C.; Duarte, T.; Zeni, G.; Nogueira, C.W. A novel isoquinoline compound abolishes chronic unpredictable mild stress-induced depressive-like behavior in mice. Behav. Brain Res. 2016, 307, 73–83. [Google Scholar] [CrossRef]
- Shepard, R.; Coutellier, L. Changes in the Prefrontal Glutamatergic and Parvalbumin Systems of Mice Exposed to Unpredictable Chronic Stress. Mol. Neurobiol. 2018, 55, 2591–2602. [Google Scholar] [CrossRef]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of Chronic Cannabidiol Treatment in the Rat Chronic Unpredictable Mild Stress Model of Depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Cryan, J.F. Towards translational rodent models of depression. Cell Tissue Res. 2013, 354, 141–153. [Google Scholar] [CrossRef]
- Hill, M.N.; Hellemans, K.G.; Verma, P.; Gorzalka, B.B.; Weinberg, J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci. Biobehav. Rev. 2012, 36, 2085–2117. [Google Scholar] [CrossRef]
- Cosma, N.C.; Üsekes, B.; Otto, L.R.; Gerike, S.; Heuser, I.; Regen, F.; Hellmann-Regen, J. M1/M2 polarization in major depressive disorder: Disentangling state from trait effects in an individualized cell-culture-based approach. Brain Behav. Immun. 2021, 94, 185–195. [Google Scholar] [CrossRef]
- Wachholz, S.; Eßlinger, M.; Plümper, J.; Manitz, M.P.; Juckel, G.; Friebe, A. Microglia activation is associated with IFN-α induced depressive-like behavior. Brain Behav. Immun. 2016, 55, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Johnson, J.D.; Barnard, D.F.; Kulp, A.C.; Mehta, D.M. Neuroendocrine Regulation of Brain Cytokines After Psychological Stress. J. Endocr. Soc. 2019, 3, 1302–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 21, 114–121. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Song, M.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 2021, 6, 146852. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef]
- Su, W.J.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.J.; Peng, W.; Liu, Y.Z.; Wang, Y.X.; You, Z.L.; Feng, S.J.; et al. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef]
- Maslanik, T.; Mahaffey, L.; Tannura, K.; Beninson, L.; Greenwood, B.N.; Fleshner, M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2013, 28, 54–62. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.; van Reedt Dortland, A.K.; Schoevers, R.A.; Giltay, E.J.; de Jonge, P.; Penninx, B.W. Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 2014, 39, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune targets for therapeutic development in depression: Towards precision medicine. Nat. Rev. Drug Discov. 2022, 21, 224–244. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsukh, S.; Oh, S.; Rheu, K.; Lee, B.-J.; Park, C.-H.; Son, K.H.; Byun, K. Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation. Nutrients 2022, 14, 5382. https://doi.org/10.3390/nu14245382
Batsukh S, Oh S, Rheu K, Lee B-J, Park C-H, Son KH, Byun K. Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation. Nutrients. 2022; 14(24):5382. https://doi.org/10.3390/nu14245382
Chicago/Turabian StyleBatsukh, Sosorburam, Seyeon Oh, Kyoungmin Rheu, Bae-Jin Lee, Chul-Hyun Park, Kuk Hui Son, and Kyunghee Byun. 2022. "Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation" Nutrients 14, no. 24: 5382. https://doi.org/10.3390/nu14245382
APA StyleBatsukh, S., Oh, S., Rheu, K., Lee, B.-J., Park, C.-H., Son, K. H., & Byun, K. (2022). Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation. Nutrients, 14(24), 5382. https://doi.org/10.3390/nu14245382




