Plasma Cholesterol- and Body Fat-Lowering Effects of Chicken Protein Hydrolysate and Oil in High-Fat Fed Male Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Amino Acids in the Diet
2.3. Fatty Acid Composition of the Chicken Oil
2.4. Quantification of Plasma Lipids and Carnitine Metabolites
2.5. Mitochondrial Fatty Acid Oxidation and Hepatic Enzyme Activities
2.6. Plasma Inflammatory Markers and Antioxidant Capacity
2.7. Statistical Analysis
3. Results
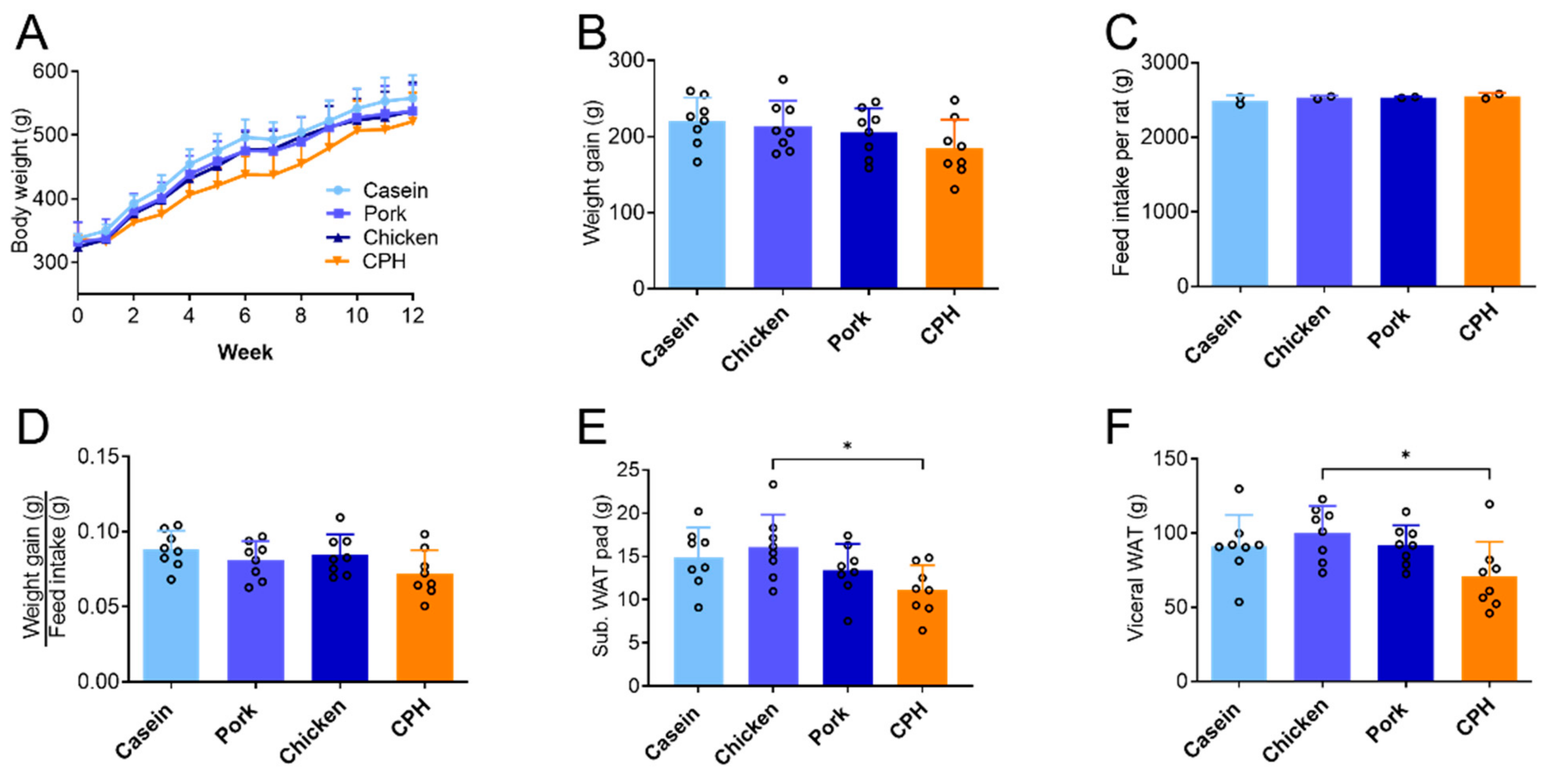
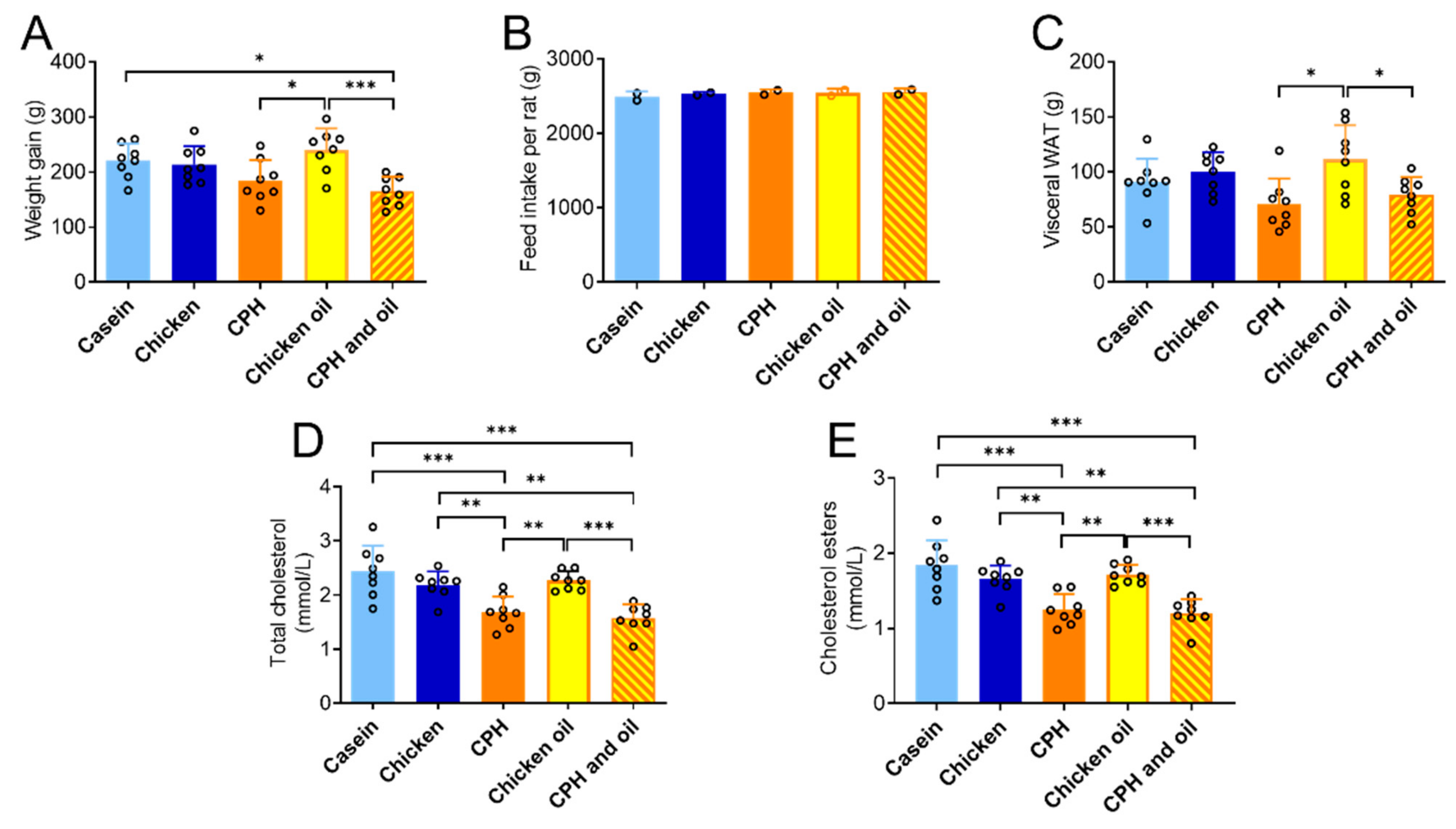
3.1. The Effect of Different Protein Sources on Body Weight, Adipose Tissue and Liver Mass
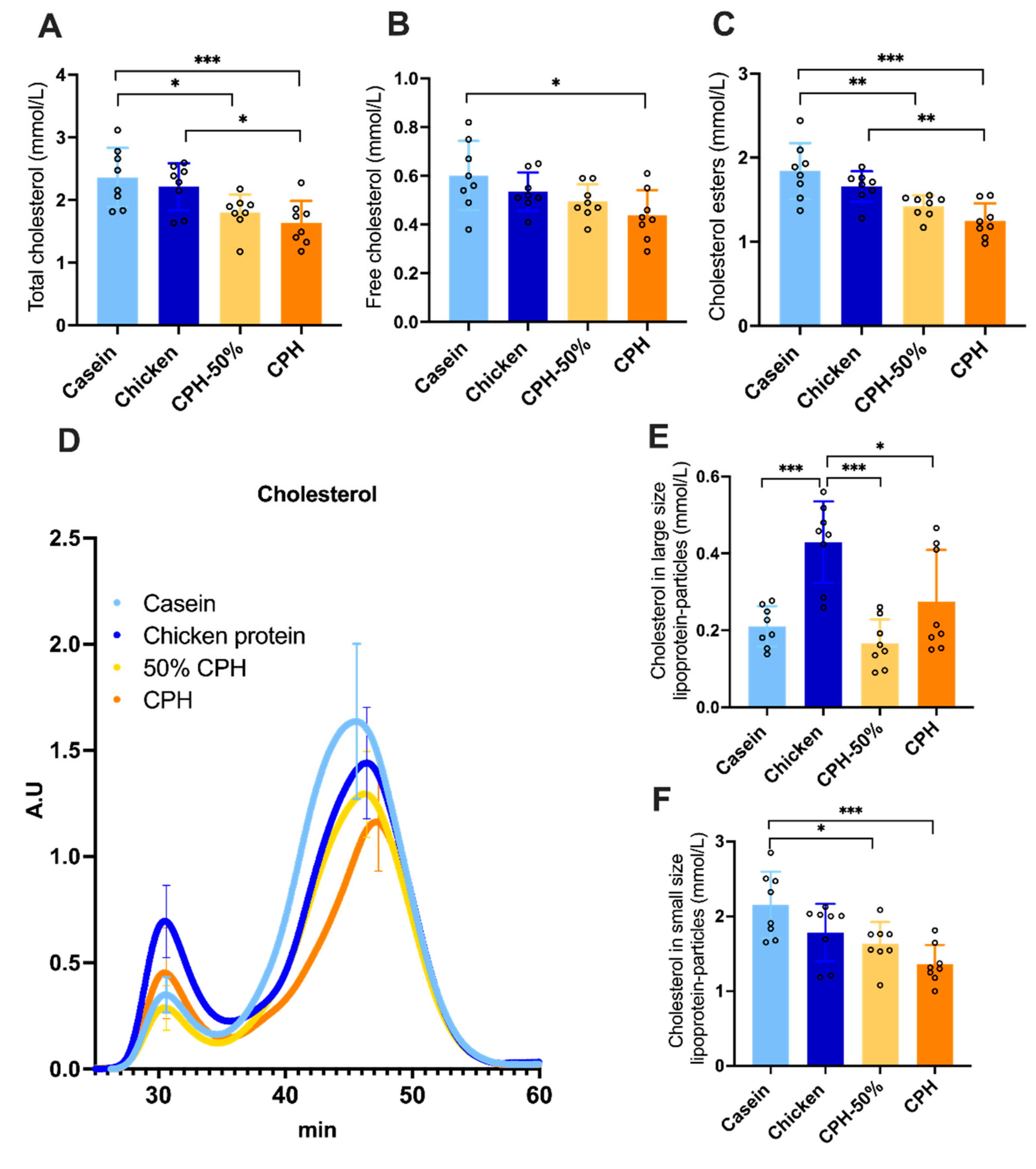
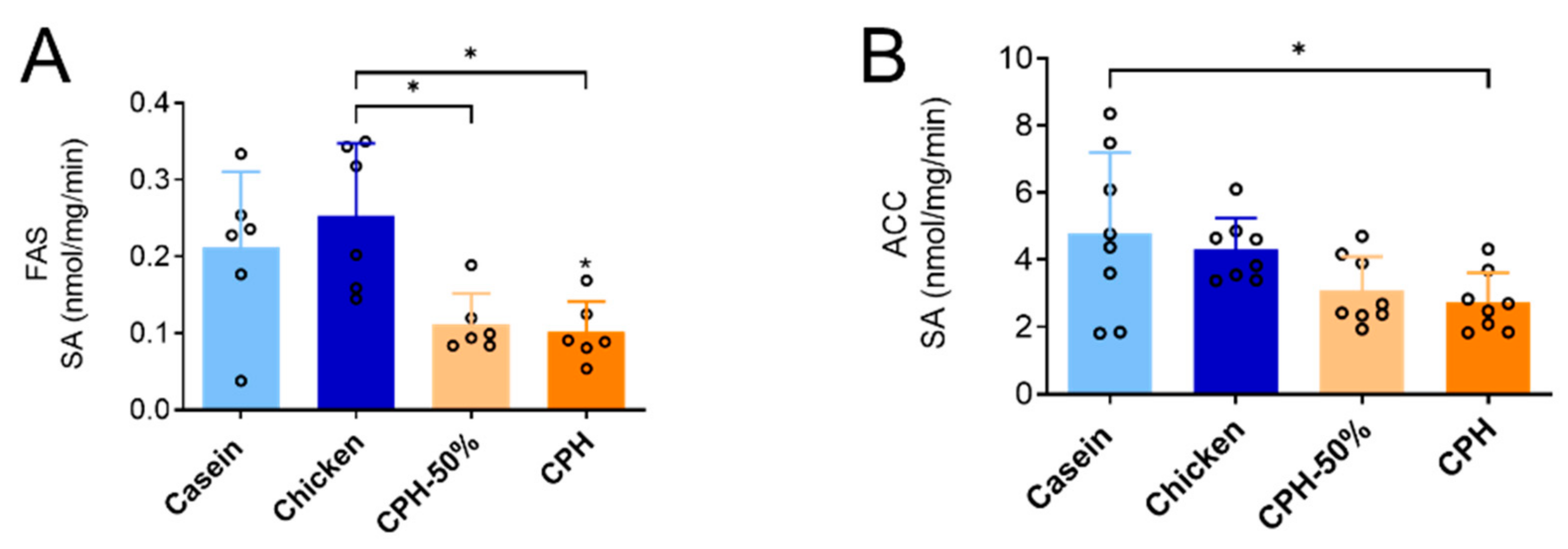
3.2. Lowering of Cholesterol, Cholesterol Esters and Hepatic Lipogenesis by Different Doses of Chicken Protein Hydrolysate
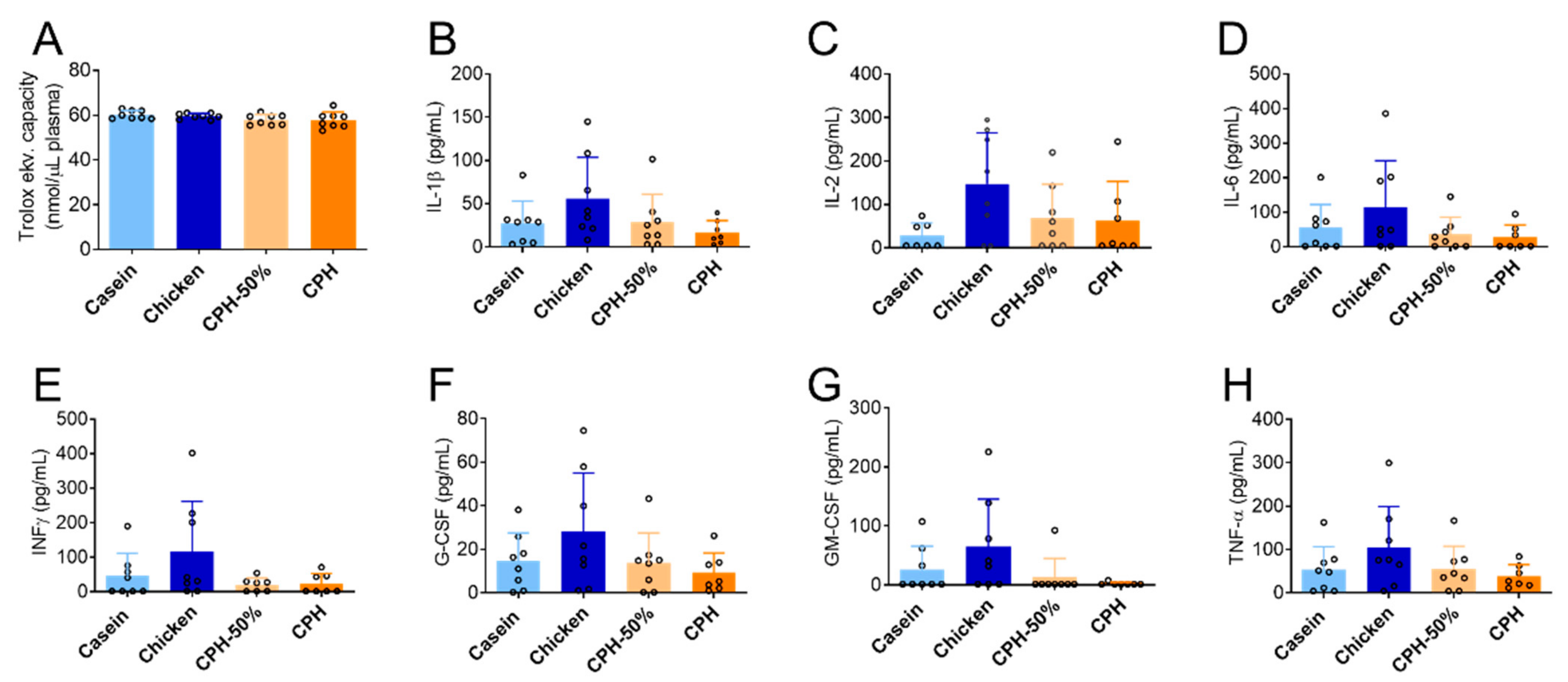
3.3. The Effect of Chicken Protein Hydrolysate on Systemic Inflammation and Total Antioxidant Status
3.4. Effects on Weight and Plasma Cholesterol in Rats Fed a Combination of CPH and Chicken Oil
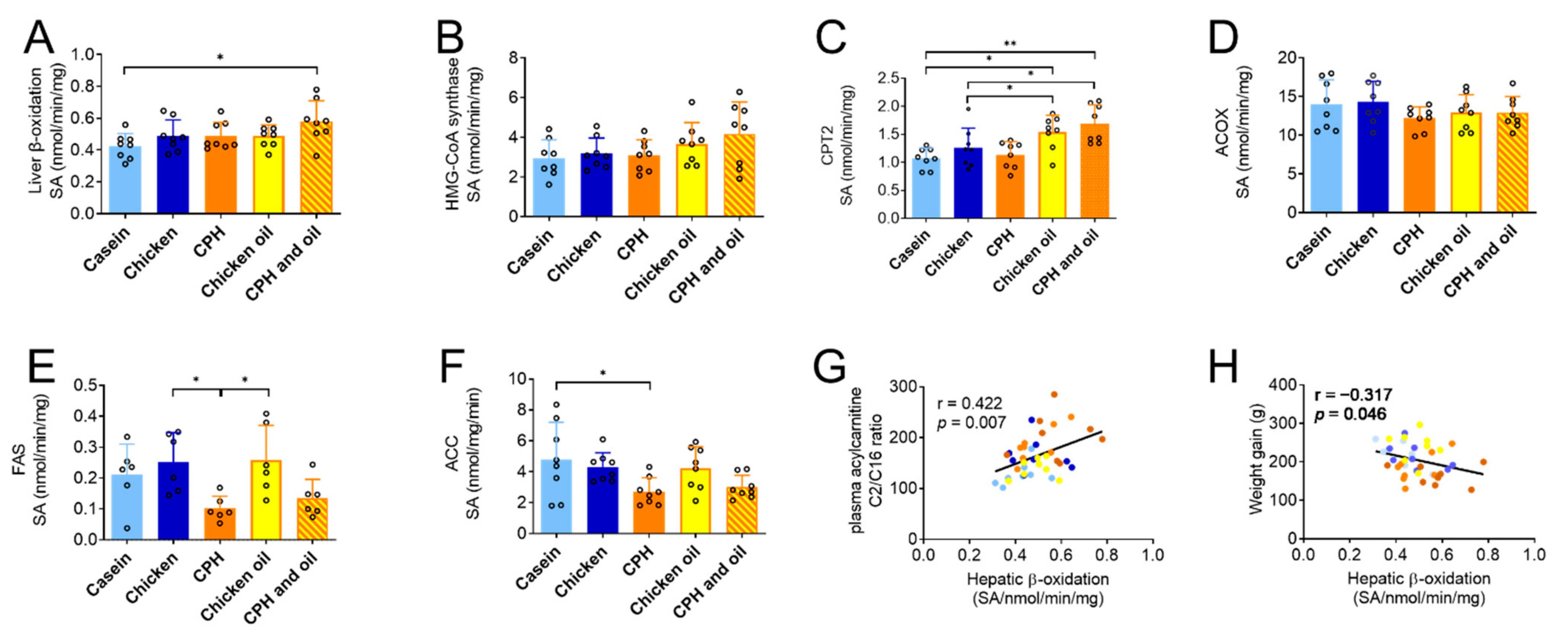
3.5. Effects on Plasma Carnitine and Acylcarnitines, TMAO and Precursors, and Hepatic Lipid Metabolism in Rats Fed a Combination of CPH and Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beavers, K.M.; Nesbit, B.A.; Kiel, J.R.; Sheedy, J.L.; Arterburn, L.M.; Collins, A.E.; Ford, S.A.; Henderson, R.M.; Coleman, C.D.; Beavers, D.P. Effect of an Energy-Restricted, Nutritionally Complete, Higher Protein Meal Plan on Body Composition and Mobility in Older Adults With Obesity: A Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Luhovyy, B.L.; El Khoury, D.; Anderson, G.H. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef] [PubMed]
- Liisberg, U.; Myrmel, L.S.; Fjaere, E.; Ronnevik, A.K.; Bjelland, S.; Fauske, K.R.; Holm, J.B.; Basse, A.L.; Hansen, J.B.; Liaset, B.; et al. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte 2016, 5, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Grytten, E.; Laupsa-Borge, J.; Bohov, P.; Bjorndal, B.; Strand, E.; Skorve, J.; Nordrehaug, J.E.; Berge, R.K.; Rostrup, E.; Mellgren, G.; et al. Changes in lipoprotein particle subclasses, standard lipids, and apolipoproteins after supplementation with n-3 or n-6 PUFAs in abdominal obesity: A randomized double-blind crossover study. Clin. Nutr. 2021, 40, 2556–2575. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef]
- Jia, X.; Kohli, P.; Virani, S.S. Omega-3 Fatty Acid and Cardiovascular Outcomes: Insights From Recent Clinical Trials. Curr. Atheroscler. Rep. 2019, 21, 1. [Google Scholar] [CrossRef]
- Takata, Y.; Zhang, X.; Li, H.; Gao, Y.T.; Yang, G.; Gao, J.; Cai, H.; Xiang, Y.B.; Zheng, W.; Shu, X.O. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am. J. Epidemiol. 2013, 178, 46–57. [Google Scholar] [CrossRef]
- Butler, L.J.; Janulewicz, P.A.; Carwile, J.L.; White, R.F.; Winter, M.R.; Aschengrau, A. Childhood and adolescent fish consumption and adult neuropsychological performance: An analysis from the Cape Cod Health Study. Neurotoxicol. Teratol. 2017, 61, 47–57. [Google Scholar] [CrossRef]
- Khankari, N.K.; Bradshaw, P.T.; Steck, S.E.; He, K.; Olshan, A.F.; Shen, J.; Ahn, J.; Chen, Y.; Ahsan, H.; Terry, M.B.; et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer 2015, 121, 2244–2252. [Google Scholar] [CrossRef]
- Qin, B.; Plassman, B.L.; Edwards, L.J.; Popkin, B.M.; Adair, L.S.; Mendez, M.A. Fish intake is associated with slower cognitive decline in Chinese older adults. J. Nutr. 2014, 144, 1579–1585. [Google Scholar] [CrossRef]
- Moller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: A randomized controlled trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef]
- Shukla, A.; Bettzieche, A.; Hirche, F.; Brandsch, C.; Stangl, G.I.; Eder, K. Dietary fish protein alters blood lipid concentrations and hepatic genes involved in cholesterol homeostasis in the rat model. Br. J. Nutr. 2006, 96, 674–682. [Google Scholar] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Ann. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjorndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in ApoE-deficient mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef]
- Bjorndal, B.; Berge, C.; Ramsvik, M.S.; Svardal, A.; Bohov, P.; Skorve, J.; Berge, R.K. A fish protein hydrolysate alters fatty acid composition in liver and adipose tissue and increases plasma carnitine levels in a mouse model of chronic inflammation. Lipids Health Dis. 2013, 12, 143. [Google Scholar] [CrossRef]
- Wergedahl, H.; Gudbrandsen, O.A.; Rost, T.H.; Berge, R.K. Combination of fish oil and fish protein hydrolysate reduces the plasma cholesterol level with a concurrent increase in hepatic cholesterol level in high-fat-fed Wistar rats. Nutrition 2009, 25, 98–104. [Google Scholar] [CrossRef]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mork, S.; Berge, R.K. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA:cholesterol acyltransferase activity in liver of Zucker rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeon, J.E.; Jang, S.Y.; Jeong, Y.J.; Jeon, S.M.; Park, H.J.; Choi, M.S. Differential effects of powdered whole soy milk and its hydrolysate on antiobesity and antihyperlipidemic response to high-fat treatment in C57BL/6N mice. J. Agric. Food Chem. 2011, 59, 2584–2591. [Google Scholar] [CrossRef]
- Lee, Y.P.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Ackland, T.R.; Beilin, L.J.; Hodgson, J.M. Effects of lupin kernel flour-enriched bread on blood pressure: A controlled intervention study. Am. J. Clin. Nutr. 2009, 89, 766–772. [Google Scholar] [CrossRef]
- Morita, T.; Oh-hashi, A.; Takei, K.; Ikai, M.; Kasaoka, S.; Kiriyama, S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997, 127, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic effect of dietary pea proteins: Impact on genes regulating hepatic lipid metabolism. Mol. Nutr. Food Res. 2010, 54 (Suppl. 1), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, T.A.; Carvajal, A.K.; Slizyte, R.; Skorve, J.; Berge, R.K.; Bjørndal, B. Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kouguchi, T.; Shimizu, K.; Sato, M.; Takahata, Y.; Morimatsu, F. Chicken Collagen Hydrolysate Reduces Proinflammatory Cytokine Production in C57BL/6.KOR-ApoEshl Mice. J. Nutr. Sci. Vitaminol. 2010, 56, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef]
- Ramos-Roman, M.A.; Sweetman, L.; Valdez, M.J.; Parks, E.J. Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism 2012, 61, 202–212. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Sevostjanovs, E.; Vilks, K.; Volska, K.; Antone, U.; Kuka, J.; Makarova, E.; Pugovics, O.; Dambrova, M.; Liepinsh, E. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep. 2017, 7, 17528. [Google Scholar] [CrossRef]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., 3rd; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Hear. Assoc. 2016, 5, e003190. [Google Scholar] [CrossRef]
- Strand, E.; Pedersen, E.R.; Svingen, G.F.; Olsen, T.; Bjorndal, B.; Karlsson, T.; Dierkes, J.; Njolstad, P.R.; Mellgren, G.; Tell, G.S.; et al. Serum Acylcarnitines and Risk of Cardiovascular Death and Acute Myocardial Infarction in Patients With Stable Angina Pectoris. J. Am. Hear. Assoc. 2017, 6, e003620. [Google Scholar] [CrossRef]
- Ueland, T.; Svardal, A.; Oie, E.; Askevold, E.T.; Nymoen, S.H.; Bjorndal, B.; Dahl, C.P.; Gullestad, L.; Berge, R.K.; Aukrust, P. Disturbed carnitine regulation in chronic heart failure—Increased plasma levels of palmitoyl-carnitine are associated with poor prognosis. Int. J. Cardiol. 2013, 167, 1892–1899. [Google Scholar] [CrossRef]
- Mai, M.; Tonjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Chen, L.; Han, X.; Ji, L. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pr. 2014, 104, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjorndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Higgins, T.; Chaykin, S.; Hammond, K.B.; Humbert, J.R. Trimethylamine N-oxide synthesis: A human variant. Biochem. Med. 1972, 6, 392–396. [Google Scholar] [CrossRef]
- Hoseini Tavassol, Z.; Ejtahed, H.S.; Larijani, B.; Hasani-Ranjbar, S. Trimethylamine N-Oxide as a potential risk factor for non-communicable diseases: A systematic review. Endocr. Metab. Immune Disord. Drug Targets, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Lampousi, A.M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive oil in the prevention and management of type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies and intervention trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Hu, Q.; Li, G.; Zhang, L.; Qin, L.Q.; Zuo, H.; Xu, G. Dietary Intake and Biomarkers of alpha-Linolenic Acid and Mortality: A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2021, 8, 743852. [Google Scholar] [CrossRef]
- Wu, J.H.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: The Cardiovascular Health Study. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Iggman, D.; Arnlov, J.; Cederholm, T.; Riserus, U. Association of Adipose Tissue Fatty Acids With Cardiovascular and All-Cause Mortality in Elderly Men. JAMA Cardiol. 2016, 1, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Lewis, L.; Eckfeldt, J.H. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 1997, 65, 551–559. [Google Scholar] [CrossRef]
- Kawahara, S.; Takenoyama, S.; Takuma, K.; Muguruma, M.; Yamauchi, K. Effects of dietary supplementation with conjugated linoleic acid on fatty acid composition and lipid oxidation in chicken breast meat. Anim. Sci. J. 2009, 80, 468–474. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer. Available online: https://www.randomizer.org/ (accessed on 21 June 2017).
- Busserolles, J.; Mazur, A.; Gueux, E.; Rock, E.; Rayssiguier, Y. Metabolic syndrome in the rat: Females are protected against the pro-oxidant effect of a high sucrose diet. Exp. Biol. Med. 2002, 227, 837–842. [Google Scholar] [CrossRef]
- Cohen, S.A.; Strydom, D.J. Amino acid analysis utilizing phenylisothiocyanate derivatives. Anal. Biochem. 1988, 174, 1–16. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride--Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Kates, M. General analytical procedures. In Techniques in lipidology. Isolation, Analysis and Identification of Lipids, 2nd ed.; Dates, M., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1986; pp. 112–185. [Google Scholar]
- Parini, P.; Johansson, L.; Broijersen, A.; Angelin, B.; Rudling, M. Lipoprotein profiles in plasma and interstitial fluid analyzed with an automated gel-filtration system. Eur. J. Clin. Investig. 2006, 36, 98–104. [Google Scholar] [CrossRef]
- Pedrelli, M.; Davoodpour, P.; Degirolamo, C.; Gomaraschi, M.; Graham, M.; Ossoli, A.; Larsson, L.; Calabresi, L.; Gustafsson, J.A.; Steffensen, K.R.; et al. Hepatic ACAT2 knock down increases ABCA1 and modifies HDL metabolism in mice. PLoS ONE 2014, 9, e93552. [Google Scholar] [CrossRef] [PubMed]
- Galman, C.; Matasconi, M.; Persson, L.; Parini, P.; Angelin, B.; Rudling, M. Age-induced hypercholesterolemia in the rat relates to reduced elimination but not increased intestinal absorption of cholesterol. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E737–E742. [Google Scholar] [CrossRef] [PubMed]
- Vernez, L.; Wenk, M.; Krahenbuhl, S. Determination of carnitine and acylcarnitines in plasma by high-performance liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Bohov, P.; Bjorndal, B.; Seifert, R.; Nygard, O.; Svardal, A.; Glintborg, D.; Berge, R.K.; Gaster, M. Free carnitine and acylcarnitines in obese patients with polycystic ovary syndrome and effects of pioglitazone treatment. Fertil. Steril. 2012, 98, 1620–1626.e1. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Flatmark, T.; Osmundsen, H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur. J. Biochem. 1984, 141, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Hexeberg, S.; Skorve, J.; Lundquist, M.; Berge, R.K. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J. Lipid. Res. 1993, 34, 13–22. [Google Scholar] [CrossRef]
- Bremer, J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim. Biophys. Acta 1981, 665, 628–631. [Google Scholar] [CrossRef]
- Clinkenbeard, K.D.; Reed, W.D.; Mooney, R.A.; Lane, M.D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J. Biol. Chem. 1975, 250, 3108–3116. [Google Scholar] [CrossRef]
- Roncari, D.A. Fatty acid synthase from human liver. Methods Enzym. 1981, 71 Pt C, 73–79. [Google Scholar]
- Tanabe, T.; Nakanishi, S.; Hashimoto, T.; Ogiwara, H.; Nikawa, J.; Numa, S. Acetyl-CoA carboxylase from rat liver. Methods Enzym. 1981, 71 Pt C, 5–16. [Google Scholar]
- Bates, E.J.; Saggerson, D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977, 84, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Daae, L.N.; Bremer, J. The acylation of glycerophosphate in rat liver. A new assay procedure for glycerophosphate acylation, studies on its subcellular and submitochondrial localization and determination of the reaction products. Biochim. Biophys. Acta 1970, 210, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Raaka, B.M.; Lowenstein, J.M. Inhibition of fatty acid oxidation by 2-bromooctanoate. Evidence for the enzymatic formation of 2-bromo-3-ketooctanoyl coenzyme A and the inhibition of 3-ketothiolase. J. Biol. Chem. 1979, 254, 6755–6762. [Google Scholar] [CrossRef] [PubMed]
- Small, G.M.; Burdett, K.; Connock, M.J. A sensitive spectrophotometric assay for peroxisomal acyl-CoA oxidase. Biochem. J. 1985, 227, 205–210. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A Review on Health-Promoting, Biological, and Functional Aspects of Bioactive Peptides in Food Applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Chou, C.J.; Affolter, M.; Kussmann, M. A nutrigenomics view of protein intake: Macronutrient, bioactive peptides, and protein turnover. Prog. Mol. Biol. Transl. Sci. 2012, 108, 51–74. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.; Schroder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Khora, S.S. Marine fish-derived bioactive peptides and proteins for human therapeutics. Int. J. Pharm. Pharm. Sci. 2013, 5, 31–37. [Google Scholar]
- Bjorndal, B.; Aloysius, T.A.; Lund, A.; Slizyte, R.; Bohov, P.; Carvajal, A.K.; Berge, R.K. A chicken protein hydrolysate exerts anti-atherosclerotic effect beyond plasma cholesterol-lowering activity in Apoe(-/-) mice. Food Sci. Nutr. 2020, 8, 3052–3060. [Google Scholar] [CrossRef]
- Vik, R.; Tillander, V.; Skorve, J.; Vihervaara, T.; Ekroos, K.; Alexson, S.E.H.; Berge, R.K.; Bjorndal, B. Three differently generated salmon protein hydrolysates reveal opposite effects on hepatic lipid metabolism in mice fed a high-fat diet. Food Chem. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011, 76, H116–H121. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Madsen, L.; Hao, Q.; Criales, G.; Mellgren, G.; Marschall, H.U.; Hallenborg, P.; Espe, M.; Froyland, L.; Kristiansen, K. Fish protein hydrolysate elevates plasma bile acids and reduces visceral adipose tissue mass in rats. Biochim. Biophys. Acta 2009, 1791, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; DeBose-Boyd, R.A. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004754. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Cacabelos, D.; Burri, L.; Berge, K.; Wergedahl, H.; Christensen, B.; Portero-Otin, M.; Viste, A.; Pamplona, R.; Berge, R.K.; et al. Fish oil and 3-thia fatty acid have additive effects on lipid metabolism but antagonistic effects on oxidative damage when fed to rats for 50 weeks. J. Nutr. Biochem. 2012, 23, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Tondo, M.; Cedo, L.; Farras, M.; Escola-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- Manor, O.; Zubair, N.; Conomos, M.P.; Xu, X.; Rohwer, J.E.; Krafft, C.E.; Lovejoy, J.C.; Magis, A.T. A Multi-omic Association Study of Trimethylamine N-Oxide. Cell Rep. 2018, 24, 935–946. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 20, 279. [Google Scholar] [CrossRef]
- Kamei, Y.; Kamei, D.; Tsuchiya, K.; Mineshima, M.; Nitta, K. Association between 4-year all-cause mortality and carnitine profile in maintenance hemodialysis patients. PLoS ONE 2018, 13, e0201591. [Google Scholar] [CrossRef]
- Hryb, D.J.; Hogg, J.F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem. Biophys. Res. Commun. 1979, 87, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
| Casein | Chicken | Pork | CPH-50% | CPH | CO | CPH and CO | |
|---|---|---|---|---|---|---|---|
| Fat (47.5 E%) | |||||||
| Soy oil (g) | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 |
| Lard (g) | 225.0 | 202.6 | 189.5 | 204.4 | 184.1 | 127.2 | 83.3 |
| Chicken oil (g) | - | - | - | - | - | 100.8 | 100.8 |
| Fat from the protein source | 3.0 | 25.4 | 38.5 | 23.6 | 43.9 | 3.0 | 43.9 |
| Carbohydrate (32.4 E%) | |||||||
| Cornstarch (g) | 108.0 | 108.0 | 108.0 | 108.0 | 108.0 | 108.0 | 108.0 |
| Maltodextrin (g) | 158.6 | 158.6 | 158.6 | 158.6 | 158.6 | 158.6 | 158.6 |
| Sucrose (g) | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
| Fiber (g) | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Protein (20.1 E%) 1 | |||||||
| Casein (g) | 240 | - | - | 120 | - | 240 | - |
| Pork protein (g) | - | - | 240 | - | - | - | - |
| Chicken protein (g) | - | 240 | - | - | - | - | - |
| CPH (g) | - | - | - | 120 | 240 | - | 240 |
| Micronutrients 2 | |||||||
| AIN-93G-MX mineral mix (g) | 42.8 | 42.8 | 42.8 | 42.8 | 42.8 | 42.8 | 42.8 |
| AIN-93-VX vitamin mix (g) | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| L-Cysteine (g) | 3.67 | 3.67 | 3.67 | 3.67 | 3.67 | 3.67 | 3.67 |
| Choline bitartrate (g) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| tert-Butyl-hydroquinone (g) | 0.0171 | 0.0171 | 0.0171 | 0.0171 | 0.0171 | 0.0171 | 0.0171 |
| Total (g) | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Fatty Acid | % |
|---|---|
| 14:0 | 0.82 |
| 14:1 | 0.21 |
| 15:0 | 0.14 |
| 16:0 | 22.5 |
| 16:1n-9 | 0.42 |
| 16:1n-7 | 5.28 |
| 17:0 | 0.23 |
| 18:0 | 6.64 |
| 18:1n-11 + 18:1n-9 | 39.0 |
| 18:1n-7 | 1.93 |
| 18:2n-6 | 19.4 |
| 18:3n-6 | 0.17 |
| 18:3n-3 | 1.57 |
| 18:4n-3 | 0.18 |
| 20:0 | 0.081 |
| 20:1n-11 | 0.14 |
| 20:1n-9 | 0.45 |
| 20:2n-6 | 0.18 |
| 20:3n-6 | 0.11 |
| 20:4n-6 | 0.22 |
| 20:3n-3 | 0.043 |
| 20:4n-3 | 0.019 |
| 20:5n-3 | 0.070 |
| 22:0 | 0.035 |
| 22:1n-11 | 0.037 |
| 22:1n-9 | 0.021 |
| 22:5n-3 | 0.073 |
| 24:0 | 0.003 |
| 22:6n-3 | 0.060 |
| 24:1n-9 | 0.013 |
| Sum | 100.0 |
| Sum SFA | 30.4 |
| Sum MUFA | 47.5 |
| Sum PUFA | 22.1 |
| Sum omega-3 PUFA | 2.0 |
| Sum omega-6 PUFA | 19.9 |
| Sum EPA, DHA, DPA | 0.2 |
| Casein | Chic. | Pork | CPH-50% | CPH | CO | CPH and CO | |
|---|---|---|---|---|---|---|---|
| Ash (%) | 2.2 | 2.3 | 2.2 | 2.6 | 2.9 | 2.1 | 2.9 |
| Fat (%) | 13.2 | 11.7 | 11.8 | 12.9 | 12.9 | 13.3 | 12.9 |
| Protein (%) 1 | 13.1 | 12.6 | 13.2 | 14.6 | 13.3 | 13.1 | 14.1 |
| Moisture (%) | 46.0 | 48.7 | 47.2 | 47.5 | 45.8 | 46.2 | 45.4 |
| Combustion value (Kilojoule/g | 12.9 | 11.8 | 12.0 | 12.4 | 12.2 | 12.7 | 12.2 |
| Aspartic acid (%) 2 | 7.63 | 9.16 | 9.92 | 7.63 | 6.49 | 7.56 | 6.56 |
| Glutamic acid (%) 2 | 22.9 | 14.5 | 16.0 | 19.1 | 13.7 | 22.1 | 13.7 |
| Hydroksyproline (%) 2 | - | - | - | 4.12 | 6.18 | - | 6.49 |
| Serine (%) 2 | 6.03 | 3.97 | 4.35 | 4.89 | 3.36 | 5.88 | 3.44 |
| Glycine (%) 2 | 1.98 | 4.35 | 4.81 | 9.16 | 12.21 | 1.91 | 13.74 |
| Histidine (%) 2 | 2.98 | 2.82 | 4.05 | 2.44 | 1.76 | 2.90 | 1.76 |
| Arginine (%) 2 | 3.74 | 6.03 | 6.72 | 5.50 | 5.95 | 3.51 | 6.49 |
| Threonine (%) 2 | 4.58 | 4.27 | 4.96 | 3.82 | 2.75 | 4.35 | 2.90 |
| Alanine (%) 2 | 3.21 | 5.27 | 5.80 | 5.65 | 6.64 | 3.05 | 6.87 |
| Proline (%) 2 | 11.5 | 3.51 | 4.12 | 9.92 | 7.48 | 10.7 | 7.63 |
| Tyrosine (%) 2 | 3.97 | 2.44 | 2.75 | 2.75 | 1.22 | 4.35 | 1.30 |
| Valine (%) 2 | 6.87 | 5.04 | 5.57 | 4.81 | 2.75 | 6.49 | 2.98 |
| Methionine (%) 2 | 2.98 | 2.67 | 2.98 | 2.52 | 1.76 | 2.90 | 1.91 |
| Isoleucine (%) 2 | 5.65 | 4.96 | 5.42 | 4.05 | 2.29 | 5.34 | 2.52 |
| Leucine (%) 2 | 10.69 | 8.40 | 9.16 | 7.63 | 4.81 | 9.92 | 5.19 |
| Phenylalanine (%) 2 | 5.34 | 3.97 | 4.50 | 3.97 | 2.37 | 5.11 | 2.52 |
| Lysine (%) 2 | 8.40 | 9.16 | 9.92 | 7.25 | 5.34 | 8.40 | 5.80 |
| Cysteine/Cystine (%) 2 | 2.21 | 2.67 | 2.60 | 2.29 | 2.14 | 2.21 | 2.21 |
| Trypthophane (%) 2 | 1.15 | 1.07 | 1.30 | 0.84 | n.a. | 1.15 | n.a. |
| Casein | Chicken | CPH | CO | CPH and CO | |
|---|---|---|---|---|---|
| HDL cholesterol (mmol/L) | 1.91 ± 0.32 a | 1.62 ± 0.22 a,b | 1.32 ± 0.22 b | 1.77 ± 0.20 a | 1.31 ± 0.24 b |
| LDL cholesterol (mmol/L) | 0.49 ± 0.09 a | 0.38 ± 0.10 a | 0.23 ± 0.09 b | 0.43 ± 0.13 a | 0.15 ± 0.07 b |
| Triglycerides (mmol/L) | 1.02 ± 0.28 a | 1.26 ± 0.42 a | 1.10 ± 0.32 a | 1.06 ± 0.35 a | 1.12 ± 0.44 a |
| Phospholipids (mmol/L) | 2.01 ± 0.39 a | 1.90 ± 0.26 a,b | 1.67 ± 0.27 a,b | 1.87 ± 0.18 a,b | 1.56 ± 0.23 b |
| NEFA (mmol/L) | 0.31 ± 0.08 a | 0.28 ± 0.08 a | 0.34 ± 0.11 a | 0.30 ± 0.07 a | 0.28 ± 0.04 a |
| Casein | Chicken | CPH | CO | CPH and CO | |
|---|---|---|---|---|---|
| Carnitine, µM | 41.2 ± 6.5 a | 54.4 ± 4.0 b | 53.7 ± 8.3 b,c | 46.3 ± 5.2 a,b | 58.3 ± 9.2 c |
| Acetylcarnitine (AC2), µM | 17.3 ± 2.9 a | 23.49 ± 6.3 a,b | 28.4 ± 4.6 b,c | 19.3 ± 3.5 a | 31.5 ± 6.2 c |
| Propionoylcarnitine (AC3), µM | 0.56 ± 0.14 a | 0.86 ± 0.25 a,b | 0.90 ± 0.30 a,b | 0.68 ± 0.14 a | 1.12 ± 0.33 b |
| Valerylcarnitine (AC5), µM | 0.12 ± 0.02 a | 0.13 ± 0.03 a | 0.11 ± 0.03 a | 0.13 ± 0.03 a | 1.13 ± 0.04 a |
| Octanoyl-canitine (AC8), µM | 0.021 ± 0.003 a | 0.024 ± 0.004 a,b | 0.026 ± 0.004 a,b | 0.024 ± 0.007 a,b | 0.026 ± 0.002 b |
| Lauroylcarnitine (AC12), µM | 0.015 ± 0.002 a | 0.017 ± 0.002 a | 0.018 ± 0.002 a | 0.016 ± 0.002 a | 0.018 ± 0.001 a |
| Myristoylcarnitine (AC14), M | 0.026 ± 0.005 a | 0.028 ± 0.004 a | 0.030 ± 0.004 a | 0.026 ± 0.006 a | 0.028 ± 0.002 a |
| Palmitoylcarnitine (AC16), µM | 0.14 ± 0.02 a | 0.14 ± 0.02 a | 0.15 ± 0.01 a | 0.14 ± 0.02 a | 0.16 ± 0.01 a |
| Total carnitine, µM 2 | 59.3 ± 7.5 a | 79.0 ± 8.0 b,c | 83.3 ± 11.0 b,c | 66.6 ± 8.0 a,b | 91.3 ± 14.2 c |
| AC2/AC16 | 128.2 ± 23.4 a | 165.7 ± 33.2 a,b,c | 186.1 ± 33.6 b,c | 140.0 ± 19.1 a,b | 201.5 ± 45.5 c |
| Trimethyllysine, µM | 0.81 ± 0.13 a,b | 0.86 ± 0.10 a | 0.79 ± 0.05 a,b | 0.90 ± 0.06 a | 0.73 ± 0.07 b |
| γ-butyrobetaine, µM | 1.04 ± 0.16 a | 2.10 ± 0.28 b | 1.43 ± 0.27 c | 1.14 ± 0.13 a,c | 1.21 ± 0.18 c |
| TMAO, µM | 0.69 ± 0.13 a | 0.85 ± 0.22 a,c | 1.42 ± 0.57 b | 0.75 ± 0.25 a | 1.33 ± 0.49 b,c |
| Betaine, µM | 80.9 ± 10.5 a | 97.7 ± 12.1 a | 128.3 ± 11.0 b | 92.8 ± 20.8 a | 102.4 ± 17.0 a |
| Choline, µM | 12.1 ± 1.6 a | 12.5 ± 1.5 a | 13.2 ± 2.1 a | 12.9 ± 2.1 a | 13.6 ± 2.1 a |
| Metabolic Effect | CP | CPH | CO | CPH + CO |
|---|---|---|---|---|
| Plasma parameters: | ||||
| Total cholesterol conc. | = | ↓ | = | ↓ |
| Cytokines | ↑ | = | = | = |
| γ-butyrobetaine | ↑ | ↑ | = | = |
| L-carnitine | ↑ | ↑ | = | ↑ |
| Total carnitine | ↑ | ↑ | = | ↑ |
| AC2/AC16 ratio | ↑ | ↑ | = | ↑ |
| Betaine | = | ↑ | = | = |
| TMAO | = | ↑ | = | ↑ |
| Hepatic enzyme activity: | ||||
| Lipogenesis | = | ↓ | = | (↓) |
| β-oxidation | = | = | (↑) | ↑ |
| Anthropometry: | ||||
| Weight gain | = | = | = | ↓ |
| Adipose tissue weight | = | ↓ | = | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, T.A.; Tillander, V.; Pedrelli, M.; Dankel, S.N.; Berge, R.K.; Bjørndal, B. Plasma Cholesterol- and Body Fat-Lowering Effects of Chicken Protein Hydrolysate and Oil in High-Fat Fed Male Wistar Rats. Nutrients 2022, 14, 5364. https://doi.org/10.3390/nu14245364
Aloysius TA, Tillander V, Pedrelli M, Dankel SN, Berge RK, Bjørndal B. Plasma Cholesterol- and Body Fat-Lowering Effects of Chicken Protein Hydrolysate and Oil in High-Fat Fed Male Wistar Rats. Nutrients. 2022; 14(24):5364. https://doi.org/10.3390/nu14245364
Chicago/Turabian StyleAloysius, Thomas A., Veronika Tillander, Matteo Pedrelli, Simon N. Dankel, Rolf K. Berge, and Bodil Bjørndal. 2022. "Plasma Cholesterol- and Body Fat-Lowering Effects of Chicken Protein Hydrolysate and Oil in High-Fat Fed Male Wistar Rats" Nutrients 14, no. 24: 5364. https://doi.org/10.3390/nu14245364
APA StyleAloysius, T. A., Tillander, V., Pedrelli, M., Dankel, S. N., Berge, R. K., & Bjørndal, B. (2022). Plasma Cholesterol- and Body Fat-Lowering Effects of Chicken Protein Hydrolysate and Oil in High-Fat Fed Male Wistar Rats. Nutrients, 14(24), 5364. https://doi.org/10.3390/nu14245364






