Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
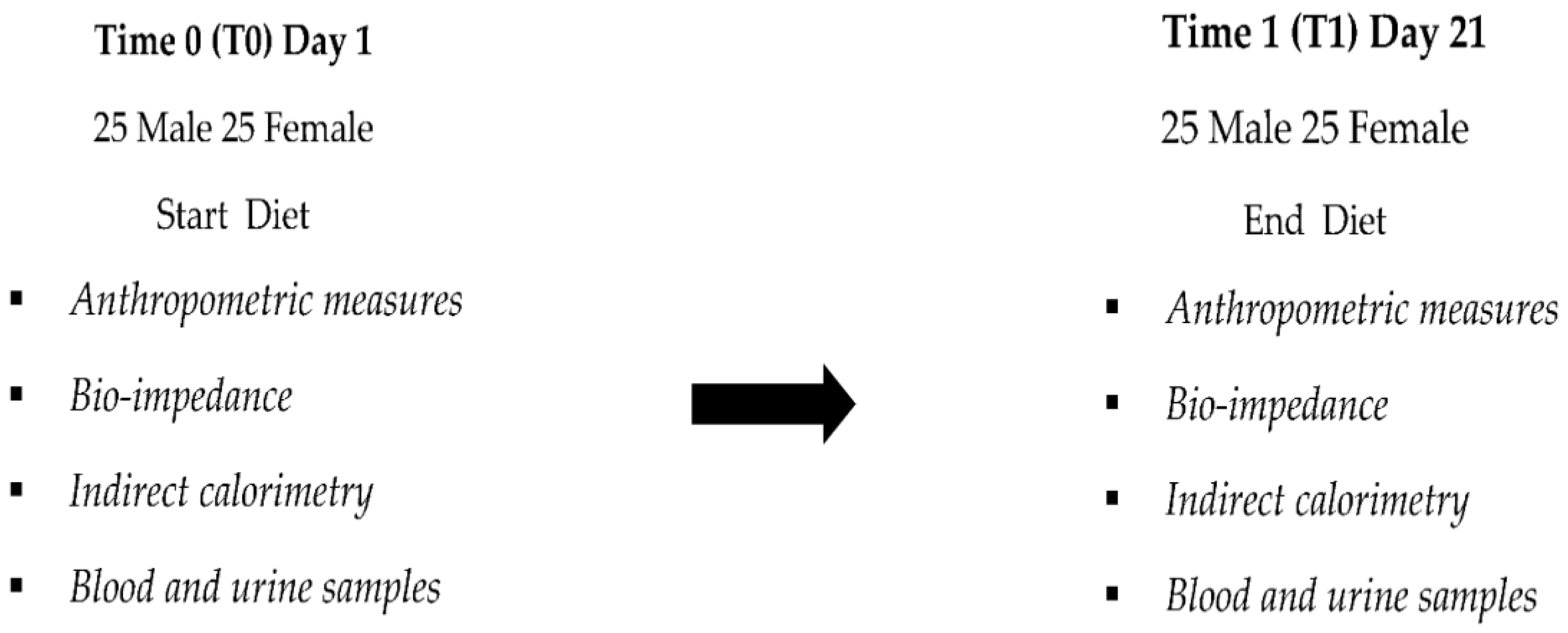
2.2. Clinical Experimental Design
2.3. Anthropometry
Indirect Calorimetry Measures
2.4. Body Composition
2.5. Indirect Calorimetry
2.6. Biochemical Analysis
2.7. The Revised Protein Sparing Diet Experimental Protocol: Dietary, Pharmacological, and Supplementation Program
2.8. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms Linking Obesity with Cardiovascular Disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Kaptoge, S.; Wormser, D.; Willeit, P.; Butterworth, A.S.; Bansal, N.; O’Keeffe, L.M.; Gao, P.; Wood, A.M.; et al. Association of Cardiometabolic Multimorbidity with Mortality. JAMA 2015, 314, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Tran, J.; Zottoli, M.; Ramakrishnan, R.; Hassaine, A.; Rao, S.; Li, Y.; Salimi-Khorshidi, G.; Norton, R.; Rahimi, K. Association between Cardiometabolic Disease Multimorbidity and All-Cause Mortality in 2 Million Women and Men Registered in UK General Practices. BMC Med. 2021, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Cernigliaro, A.; Cincione, R.I.; Buscemi, S.; Libra, M.; Galvano, F.; Grosso, G. Total Nut, Tree Nut, and Peanut Consumption and Metabolic Status in Southern Italian Adults. Int. J. Environ. Res. Public Health 2021, 18, 1847. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Cernigliaro, A.; Cincione, R.I.; Buscemi, S.; Libra, M.; Galvano, F.; Grosso, G. Polyphenol-Rich and Alcoholic Beverages and Metabolic Status in Adults Living in Sicily, Southern Italy. Foods 2021, 10, 383. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Buscemi, C.; Randazzo, C.; Borzì, A.M.; Barile, A.M.; Rosafio, G.; Ciaccio, M.; Caldarella, R.; Meli, F.; et al. Influence of Habitual Dairy Food Intake on LDL Cholesterol in a Population-Based Cohort. Nutrients 2021, 13, 593. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Alanazi, A.M.; Grosso, G.; Cincione, R.I.; La Vignera, S.; Buscemi, S.; Galvano, F. Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults. Nutrients 2022, 14, 4294. [Google Scholar] [CrossRef]
- Harrison, M.T.; Harden, R.M. The Long-Term Value of Fasting in the Treatment of Obesity. Lancet 1966, 2, 1340–1342. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-Term Effects of Lifestyle Intervention or Metformin on Diabetes Development and Microvascular Complications over 15-Year Follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Ma, C.; Avenell, A.; Bolland, M.; Hudson, J.; Stewart, F.; Robertson, C.; Sharma, P.; Fraser, C.; MacLennan, G. Effects of Weight Loss Interventions for Adults Who Are Obese on Mortality, Cardiovascular Disease, and Cancer: Systematic Review and Meta-Analysis. BMJ 2017, 359, j4849. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of Weight Reduction on Blood Lipids and Lipoproteins: A Meta-Analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, D.R.; Winterer, J.; Blackburn, G.L.; Young, V.; Sherman, M. Effect of a Protein-Sparing Diet and Brief Fast on Nitrogen Metabolism in Mildly Obese Subjects. J. Lab. Clin. Med. 1977, 89, 1030–1035. [Google Scholar] [PubMed]
- Barrea, L.; Verde, L.; Vetrani, C.; Marino, F.; Aprano, S.; Savastano, S.; Colao, A.; Muscogiuri, G. VLCKD: A Real Time Safety Study in Obesity. J. Transl. Med. 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; de Alteriis, G.; Muscogiuri, G.; Vetrani, C.; Verde, L.; Camajani, E.; Aprano, S.; Colao, A.; Savastano, S. Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients 2022, 14, 4213. [Google Scholar] [CrossRef]
- Frank, A. The Protein-Sparing Modified Fast for Obesity-Related Medical Problems. Cleve Clin. J. Med. 1997, 64, 444–445. [Google Scholar] [CrossRef]
- Currenti, W.; Buscemi, S.; Cincione, R.I.; Cernigliaro, A.; Godos, J.; Grosso, G.; Galvano, F. Time-Restricted Feeding and Metabolic Outcomes in a Cohort of Italian Adults. Nutrients 2021, 13, 1651. [Google Scholar] [CrossRef]
- Cincione, R.I.; Losavio, F.; Ciolli, F.; Valenzano, A.; Cibelli, G.; Messina, G.; Polito, R. Effects of Mixed of a Ketogenic Diet in Overweight and Obese Women with Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 12490. [Google Scholar] [CrossRef]
- Kleiner, A.; Cum, B.; Pisciotta, L.; Cincione, I.R.; Cogorno, L.; Prigione, A.; Tramacere, A.; Vignati, A.; Carmisciano, L.; Sukkar, S.G. Safety and Efficacy of Eucaloric Very Low-Carb Diet (EVLCD) in Type 1 Diabetes: A One-Year Real-Life Retrospective Experience. Nutrients 2022, 14, 3208. [Google Scholar] [CrossRef]
- Ivan, C.R.; Messina, A.; Cibelli, G.; Messina, G.; Polito, R.; Losavio, F.; Torre, E.L.; Monda, V.; Monda, M.; Quiete, S.; et al. Italian Ketogenic Mediterranean Diet in Overweight and Obese Patients with Prediabetes or Type 2 Diabetes. Nutrients 2022, 14, 4361. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Panza, F.; Castellana, M.; Lampignano, L.; Cincione, R.I.; Triggiani, V.; Giannelli, G.; Dibello, V.; Sardone, R.; et al. Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes. J. Clin. Med. 2021, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Defeudis, G.; Di Tommaso, A.M.; Di Rosa, C.; Cimadomo, D.; Khazrai, Y.M.; Faggiano, A.; Cincione, R.I.; Napoli, N.; Mazzilli, R. The Role of Antihyperglycemic Drugs and Diet on Erectile Function: Results from a Perspective Study on a Population with Prediabetes and Diabetes. J. Clin. Med. 2022, 11, 3382. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Sardone, R.; Sila, A.; Giagulli, V.A.; Triggiani, V.; Cincione, R.I.; Giannelli, G.; De Pergola, G. Preliminary Trajectories in Dietary Behaviors during the COVID-19 Pandemic: A Public Health Call to Action to Face Obesity. Int. J. Environ. Res. Public Health 2020, 17, 7073. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Snyders, D.; De Leeuw, I.H.; Bekaert, J.L. Anthropometric and Calorimetric Evidence for the Protein Sparing Effects of a New Protein Supplemented Low Calorie Preparation. Am. J. Clin. Nutr. 1985, 41, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Bakhach, M.; Shah, V.; Harwood, T.; Lappe, S.; Bhesania, N.; Mansoor, S.; Alkhouri, N. The Protein-Sparing Modified Fast Diet: An Effective and Safe Approach to Induce Rapid Weight Loss in Severely Obese Adolescents. Glob. Pediatr. Health 2016, 3, 2333794X15623245. [Google Scholar] [CrossRef]
- Steven, S.; Taylor, R. Restoring Normoglycaemia by Use of a Very Low Calorie Diet in Long- and Short-Duration Type 2 Diabetes. Diabet Med. 2015, 32, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a Primary Care-Led Weight-Management Intervention for Remission of Type 2 Diabetes: 2-Year Results of the DiRECT Open-Label, Cluster-Randomised Trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; Lorenzo, A.D.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Moonen, H.P.F.X.; Van Zanten, A.R.H. Bioelectric Impedance Analysis for Body Composition Measurement and Other Potential Clinical Applications in Critical Illness. Curr. Opin. Crit. Care 2021, 27, 344–353. [Google Scholar] [CrossRef]
- Ricciardi, R.; Talbot, L.A. Use of Bioelectrical Impedance Analysis in the Evaluation, Treatment, and Prevention of Overweight and Obesity. J. Am. Acad. Nurse Pract. 2007, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hurt, R.T.; Ebbert, J.O.; Croghan, I.; Nanda, S.; Schroeder, D.R.; Teigen, L.M.; Velapati, S.R.; Mundi, M.S. The Comparison of Segmental Multifrequency Bioelectrical Impedance Analysis and Dual-Energy X-Ray Absorptiometry for Estimating Fat Free Mass and Percentage Body Fat in an Ambulatory Population. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1231–1238. [Google Scholar] [CrossRef]
- Kim, D.; Sun, J.S.; Lee, Y.H.; Lee, J.H.; Hong, J.; Lee, J.-M. Comparative Assessment of Skeletal Muscle Mass Using Computerized Tomography and Bioelectrical Impedance Analysis in Critically Ill Patients. Clin. Nutr. 2019, 38, 2747–2755. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, D.-H.; Lee, J.; Kim, Y.J.; Jung, K.Y.; Kim, K.M.; Kwak, S.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; et al. Comparison between Two Methods of Bioelectrical Impedance Analyses for Accuracy in Measuring Abdominal Visceral Fat Area. J. Diabetes Complicat. 2016, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Fujitani, K.; Tsujinaka, T.; Imanishi, K.; Shirakata, H.; Kantani, A.; Hirao, M.; Kurokawa, Y.; Utsumi, S. InBody 720 as a New Method of Evaluating Visceral Obesity. Hepatogastroenterology 2011, 58, 42–44. [Google Scholar] [PubMed]
- De Lorenzo, A.; Di Renzo, L.; Morini, P.; de Miranda, R.C.; Romano, L.; Colica, C. New Equations to Estimate Resting Energy Expenditure in Obese Adults from Body Composition. Acta Diabetol. 2018, 55, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Lasaridis, A.N.; Nilsson, P.M.; Pikilidou, M.I.; Stafilas, P.C.; Kanaki, A.; Kazakos, K.; Yovos, J.; Bakris, G.L. Validity and Reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s Indices in Patients with Hypertension and Type II Diabetes. J. Hum. Hypertens. 2007, 21, 709–716. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which Is Best? J. Sports Sci Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Carey, P.E.; Small, P.K.; Taylor, R. Reversal of Type 2 Diabetes after Bariatric Surgery Is Determined by the Degree of Achieved Weight Loss in Both Short- and Long-Duration Diabetes. Diabet. Med. 2015, 32, 47–53. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of Dietary Composition on Energy Expenditure during Weight-Loss Maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Ilyas, Z.; Peroni, G.; Bazire, P.; Sajuox, I.; Maugeri, R.; Nichetti, M.; Gasparri, C. Effect of Very Low-Calorie Ketogenic Diet in Combination with Omega-3 on Inflammation, Satiety Hormones, Body Composition, and Metabolic Markers. A Pilot Study in Class I Obese Subjects. Endocrine 2022, 75, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy Expenditure and Body Composition Changes after an Isocaloric Ketogenic Diet in Overweight and Obese Men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Food Quotient, Respiratory Quotient, and Energy Balance. Am. J. Clin. Nutr. 1993, 57, 759S–764S, Discussion 764S–765S. [Google Scholar] [CrossRef]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: Executive Summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar] [CrossRef]
- Merra, G.; Miranda, R.; Barrucco, S.; Gualtieri, P.; Mazza, M.; Moriconi, E.; Marchetti, M.; Chang, T.F.M.; De Lorenzo, A.; Di Renzo, L. Very-Low-Calorie Ketogenic Diet with Aminoacid Supplement versus Very Low Restricted-Calorie Diet for Preserving Muscle Mass during Weight Loss: A Pilot Double-Blind Study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2613–2621. [Google Scholar]
- Di Rosa, C.; Lattanzi, G.; Taylor, S.F.; Manfrini, S.; Khazrai, Y.M. Very Low Calorie Ketogenic Diets in Overweight and Obesity Treatment: Effects on Anthropometric Parameters, Body Composition, Satiety, Lipid Profile and Microbiota. Obes. Res. Clin. Pract. 2020, 14, 491–503. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A Randomized Double-Blind, Cross-Over Trial of Very Low-Calorie Diet in Overweight Migraine Patients: A Possible Role for Ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the Mechanisms of Reversal of Type 2 Diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of Type 2 Diabetes: Normalisation of Beta Cell Function in Association with Decreased Pancreas and Liver Triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, J.; Bodson, A.; Desmecht, P.; Bemelmans, S.; Stein, F.; Crabbe, J. On the Relationship between Ketonuria and Natriuresis during Fasting and upon Refeeding in Obese Patients. Eur. J. Clin. Investig. 1978, 8, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-Carbohydrate Diet Review: Shifting the Paradigm. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Soares, J.; Caspersen, C.J.; McCurdy, T. Examining Variations of Resting Metabolic Rate of Adults: A Public Health Perspective. Med. Sci. Sports Exerc. 2014, 46, 1352–1358. [Google Scholar] [CrossRef]
- Müller, M.J.; Bosy-Westphal, A.; Kutzner, D.; Heller, M. Metabolically Active Components of Fat-Free Mass and Resting Energy Expenditure in Humans: Recent Lessons from Imaging Technologies. Obes. Rev. 2002, 3, 113–122. [Google Scholar] [CrossRef]
- Grattan, B.J.; Connolly-Schoonen, J. Addressing Weight Loss Recidivism: A Clinical Focus on Metabolic Rate and the Psychological Aspects of Obesity. ISRN Obes. 2012, 2012, 567530. [Google Scholar] [CrossRef]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Smith, F.E.; Thelwall, P.E.; Taylor, R. Inhibition of Lipolysis in Type 2 Diabetes Normalizes Glucose Disposal without Change in Muscle Glycogen Synthesis Rates. Clin. Sci. 2011, 121, 169–177. [Google Scholar] [CrossRef]
- Bajaj, M.; Suraamornkul, S.; Romanelli, A.; Cline, G.W.; Mandarino, L.J.; Shulman, G.I.; DeFronzo, R.A. Effect of a Sustained Reduction in Plasma Free Fatty Acid Concentration on Intramuscular Long-Chain Fatty Acyl-CoAs and Insulin Action in Type 2 Diabetic Patients. Diabetes 2005, 54, 3148–3153. [Google Scholar] [CrossRef]
- Giordani, I.; Malandrucco, I.; Donno, S.; Picconi, F.; Di Giacinto, P.; Di Flaviani, A.; Chioma, L.; Frontoni, S. Acute Caloric Restriction Improves Glomerular Filtration Rate in Patients with Morbid Obesity and Type 2 Diabetes. Diabetes Metab. 2014, 40, 158–160. [Google Scholar] [CrossRef]
- Cahill, G.F. Starvation in Man. Clin. Endocrinol. Metab. 1976, 5, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Ross, R.; Parsons, W.D.; Jones, P.J.H. Medium-Chain Triglycerides Increase Energy Expenditure and Decrease Adiposity in Overweight Men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef]
- Friedman, M.I.; Appel, S. Energy Expenditure and Body Composition Changes after an Isocaloric Ketogenic Diet in Overweight and Obese Men: A Secondary Analysis of Energy Expenditure and Physical Activity. PLoS ONE 2019, 14, e0222971. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Magno, S.; Santini, F.; Ceccarini, G. Ketogenic Diet and Weight Loss: Is There an Effect on Energy Expenditure? Nutrients 2022, 14, 1814. [Google Scholar] [CrossRef] [PubMed]
| Number of patients | 50 |
| Men/Women | 25/25 |
| Age (years) | 47 ± 10 |
| Parameters Mean ± SE | Baseline T0 Mean ± SD | T1 Day 21 Mean ± SD | p-Value | Δ% T0–T21 |
|---|---|---|---|---|
| Bw, Body Weight (kg) | 94.74 ± 3.03 | 87.45 ± 2.96 | p < 0.01 * | −7.83% |
| BMI, Body mass index (kg/m2) | 33.98 ± 0.94 | 31.31 ± 0.91 | p < 0.01 * | −7.96% |
| Whr, Waist-to-hip ratio | 0.88 ± 0.01 | 0.86 ± 0.01 | p < 0.01 * | −2.18% |
| Wc, Waist circumference (cm) | 106.58 ± 2.47 | 100.26 ± 2.46 | p < 0.01 * | −5.99% |
| Hc, Hip circumference (cm) | 120.43 ± 2.34 | 115.16 ± 2.52 | p < 0.01 * | −4.45% |
| Fm, Fat mass (kg) | 38.75 ± 2.04 | 33.57 ± 2.07 | p < 0.01 * | −14.38% |
| Vfa, Visceral fat area (cm2) | 232.56 ± 6.62 | 207.73 ± 6.80 | p < 0.01 * | −10.97% |
| Ffm, Free fat mass (kg) | 56.10 ± 1.95 | 53.70 ± 1.87 | p < 0.01 * | −4.23% |
| Mm, Muscle mass (kg) | 53.28 ± 1.85 | 51.09 ± 1.84 | p < 0.01 * | −4.16% |
| Ssm, Skeletal Muscle Mass | 31,84 ± 1.09 | 31.59 ± 0.97 | p > 0.05 | −0.79% |
| Tbw, Total body water (L) | 40.45 ± 1.45 | 38.39 ± 1.36 | p < 0.01 * | −5% |
| Ree, Resting energy expenditure (kcal) | 1721.83 ± 56.51 | 1620.43 ± 52.29 | p < 0.01 * | −5.77% |
| RQ, Respiratory quotient | 0.94 ± 0.004 | 0.75 ± 0.004 | p < 0.01 * | −19.96% |
| VO2 (mL/min) | 241.11 ± 47.70 | 215.16 ± 24.52 | p > 0.05 | −10.76% |
| VCO2 (mL/min) | 191.92 ± 52.44 | 143.58 ± 15.47 | p > 0.05 | −25.18% |
| VO2 (mL/min)/Ffm(kg) Ree/Kcal)/Ffm(kg) | 4.41 ± 1.340 30.10 ± 9.61 | 4.24 ± 0.68 27.98 ± 4.49 | p > 0.05 p > 0.05 | −6773% −9474% |
| Parameters | Baseline T0 Mean ± SD | T1 Day 21 Mean ± SD | p-Value | Δ% T0–T21 |
|---|---|---|---|---|
| Insulin (μU/mL) | 20.28 ± 2.57 | 10.65 ± 0.97 | <0.001 * | −36.07% |
| Glucose (mg/dL) | 183.03 ± 4.73 | 138.43 ± 2.62 | <0.001 * | −23.10% |
| HbA1c (%) | 7.98 ± 0.17 | 7.03 ± 0.16 | <0.001 * | −12.04% |
| HOMA-IR | 9.26 ± 6.38 | 3.65 ± 1.93 | <0.001 * | −85.4% |
| Total cholesterol (mg/dL) | 213.1 ± 7.09 | 169.43 ± 5.47 | <0.001 * | −19.74% |
| HDL cholesterol (mg/dL) | 54.1 ± 1.81 | 51.03 ± 1.55 | 0.016 | −4.51% |
| LDL cholesterol (mg/dL) | 136.93 ± 6.08 | 103.96 ± 4.43 | <0.001 * | −22.48% |
| Triglycerides (mg/dL) | 128.06 ± 6.83 | 98.4 ± 4.21 | <0.001 * | −20.50% |
| Uric acid (mg/dL) | 5 ± 0.15 | 5 ± 0.21 | 0.970 | 0.59% |
| Ketonemia (mmol/L) | 0 | 1.7 ± 0.06 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cincione, R.I.; Losavio, F.; Cibelli, G.; Messina, G.; Polito, R.; Casula, E.; Cincione, P.P.; Amatruda, M.; Limone, P. Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus. Nutrients 2022, 14, 5325. https://doi.org/10.3390/nu14245325
Cincione RI, Losavio F, Cibelli G, Messina G, Polito R, Casula E, Cincione PP, Amatruda M, Limone P. Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus. Nutrients. 2022; 14(24):5325. https://doi.org/10.3390/nu14245325
Chicago/Turabian StyleCincione, Raffaele Ivan, Francesca Losavio, Giuseppe Cibelli, Giovanni Messina, Rita Polito, Elias Casula, Pamela Pia Cincione, Marco Amatruda, and Pierpaolo Limone. 2022. "Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus" Nutrients 14, no. 24: 5325. https://doi.org/10.3390/nu14245325
APA StyleCincione, R. I., Losavio, F., Cibelli, G., Messina, G., Polito, R., Casula, E., Cincione, P. P., Amatruda, M., & Limone, P. (2022). Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus. Nutrients, 14(24), 5325. https://doi.org/10.3390/nu14245325









