The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016
Highlights
- The study utilized data from 12,156 adults aged 20 and older who participated in NHANES (2005–2016), with mortality follow-up through 2019.
- Circadian Syndrome (CircS) was defined based on components of the metabolic syndrome (MetS) plus short sleep and depression.
- CircS was found to be a stronger predictor of CVD than MetS, particularly for prevalent CVD in both men and women.
- Women with CircS alone had a higher relative risk for both prevalent CVD and CVD mortality compared with those with MetS alone.
Abstract
1. Introduction
2. Materials and Methods
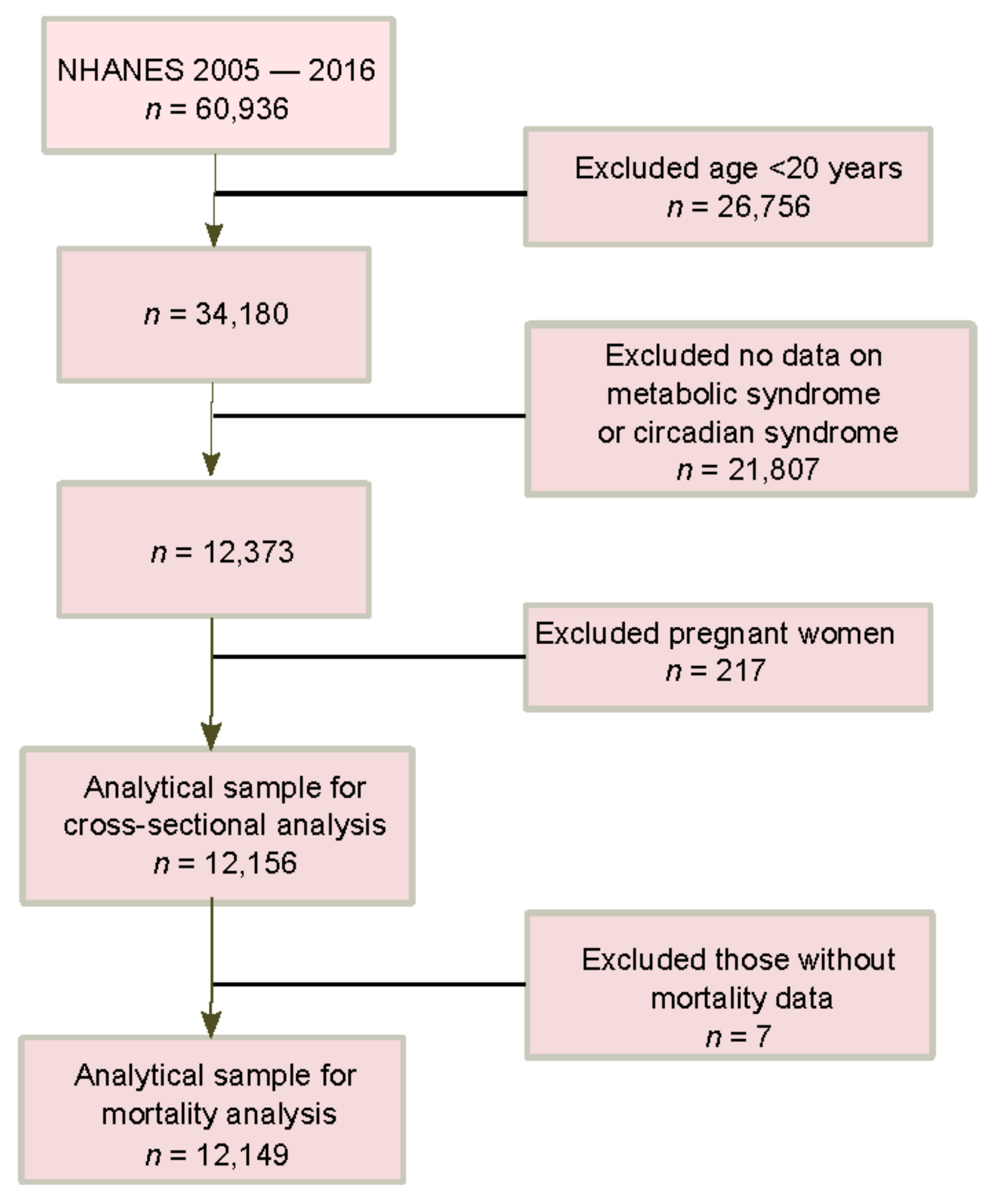
2.1. Study Design and Sample
2.2. Exposure measures: Metabolic Syndrome and Circadian Syndrome
2.3. Outcome Measure: Self-Reported History of CVD and CVD Mortality
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
Comparison with Other Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Qiao, Q.; Tuomilehto, J.; Balkau, B.; Borch-Johnsen, K.; Pyorala, K.; Group, D.S. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch. Intern. Med. 2004, 164, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; Group, I.D.F.E.T.F.C. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Groop, L. Genetics of the metabolic syndrome. Br. J. Nutr. 2000, 83 (Suppl. 1), S39–S48. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.K.; Stern, N.; El-Osta, A.; Bilu, C.; Einat, H.; Zimmet, P. The circadian syndrome predicts cardiovascular disease better than metabolic syndrome in Chinese adults. J. Intern. Med. 2021, 289, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, F.; Wu, C.; Zhang, Y.; Huang, X.; Qin, F.; Yuan, J. The Circadian Syndrome Predicts Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia Better Than Metabolic Syndrome in Aging Males: A 4-Year Follow-Up Study. Front. Med. 2021, 8, 715830. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2013, 2, 1–24. [Google Scholar]
- Heron, M. Deaths: Leading Causes for 2015. Natl. Vital Stat. Rep. 2017, 66, 1–76. [Google Scholar]
- Garcia, M.C.; Bastian, B.; Rossen, L.M.; Anderson, R.; Minino, A.; Yoon, P.W.; Faul, M.; Massetti, G.; Thomas, C.C.; Hong, Y.; et al. Potentially Preventable Deaths Among the Five Leading Causes of Death—United States, 2010 and 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Fakhouri, T.H.; Hales, C.M.; Fryar, C.D.; Li, X.; Freedman, D.S. Prevalence of Obesity Among Youths by Household Income and Education Level of Head of Household—United States 2011–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 186–189. [Google Scholar] [CrossRef]
- Hall, M.H.; Brindle, R.C.; Buysse, D.J. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am. Psychol. 2018, 73, 994–1006. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, Y.; Liu, J. Relationship between circadian syndrome and stroke: A cross-sectional study of the national health and nutrition examination survey. Front. Neurol. 2022, 13, 946172. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J. What Makes Us Tick?: Jeffrey C. Hall, Michael Rosbash and Michael W. Young all received a share of the 2017 Nobel Prize for Physiology or Medicine, for their discoveries of molecular mechanisms controlling the circadian rhythm. Eur. Heart J. 2020, 41, 4535–4537. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Meira, E.C.M.; Miyazawa, M.; Manfredini, R.; Cardinali, D.; Madrid, J.A.; Reiter, R.; Araujo, J.F.; Agostinho, R.; Acuna-Castroviejo, D. Impact of Daylight Saving Time on circadian timing system: An expert statement. Eur. J. Intern. Med. 2019, 60, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kantermann, T.; Juda, M.; Merrow, M.; Roenneberg, T. The human circadian clock's seasonal adjustment is disrupted by daylight saving time. Curr. Biol. 2007, 17, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, R.; Fabbian, F.; Cappadona, R.; Modesti, P.A. Daylight saving time, circadian rhythms, and cardiovascular health. Intern. Emerg. Med. 2018, 13, 641–646. [Google Scholar] [CrossRef]
- Khan, S.; Malik, B.H.; Gupta, D.; Rutkofsky, I. The Role of Circadian Misalignment due to Insomnia, Lack of Sleep, and Shift Work in Increasing the Risk of Cardiac Diseases: A Systematic Review. Cureus 2020, 12, e6616. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef]
- Huang, T.; Redline, S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity With Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2019, 42, 1422–1429. [Google Scholar] [CrossRef]
- Cao, Y.; Wittert, G.; Taylor, A.W.; Adams, R.; Shi, Z. Associations between Macronutrient Intake and Obstructive Sleep Apnoea as Well as Self-Reported Sleep Symptoms: Results from a Cohort of Community Dwelling Australian Men. Nutrients 2016, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Taylor, A.W.; Pan, X.; Adams, R.; Appleton, S.; Shi, Z. Dinner fat intake and sleep duration and self-reported sleep parameters over five years: Findings from the Jiangsu Nutrition Study of Chinese adults. Nutrition 2016, 32, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Ward Flemons, W.; Buysse, D.J.; Redline, S.; Oack, A.; Strohl, K.P.; Wheatley, J.R.; Young, T.; Douglas, N.J.; Levy, P.; McNicolas, W.; et al. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [Google Scholar]
- Rodsutti, J.; Hensley, M.; Thakkinstian, A.; D'Este, C.; Attia, J. A clinical decision rule to prioritize polysomnography in patients with suspected sleep apnea. Sleep 2004, 27, 694–699. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Le, M.H.; Devaki, P.; Ha, N.B.; Jun, D.W.; Te, H.S.; Cheung, R.C.; Nguyen, M.H. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS ONE 2017, 12, e0173499. [Google Scholar] [CrossRef]
- Barr, E.L.; Tonkin, A.M.; Welborn, T.A.; Shaw, J.E. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a statewide hospital morbidity database: The AusDiab study. Intern. Med. J. 2009, 39, 49–53. [Google Scholar] [CrossRef]
- Engstad, T.; Bonaa, K.H.; Viitanen, M. Validity of self-reported stroke: The Tromso Study. Stroke 2000, 31, 1602–1607. [Google Scholar] [CrossRef]
| Measure | Categorical Cut Points | Included in MetS * | Included in CircS |
|---|---|---|---|
| Elevated waist circumference | ≥102 cm in men, ≥88 in women | √ | √ |
| Elevated triglycerides (drug treatment for elevated triglycerides is an alternate indicator) | ≥150 mg/dL (1.7 mmol/L) | √ | √ |
| Low HDL-C (drug treatment for reduced HDL-C is an alternate indicator) | <40 mg/dL (1.0 mmol/L) in men; <50 mg/dL (1.3 mmol/L) in women | √ | √ |
| Elevated blood pressure (antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator) | Systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg | √ | √ |
| Elevated fasting glucose (drug treatment of elevated glucose is an alternate indicator) | ≥100 mg/dL | √ | √ |
| Short sleep | ≤6 h/day | √ | |
| Depression symptom | PHQ-9 score ≥5 | √ | |
| Definition criteria | ≥3 components | ≥4 components |
| Total | Normal | CircS Alone | MetS Alone | MetS and CircS | p-Value * | |
|---|---|---|---|---|---|---|
| n = 12,156 | n = 6060 (49.9%) | n = 259 (2.1%) | n = 1131 (9.3%) | n = 4706 (38.7%) | ||
| Age (years) (mean, SD) | 49.8 (17.7) | 42.5 (16.6) | 47.6 (15.8) | 54.0 (16.6) | 58.3 (15.1) | <0.001 |
| Age (years) | <0.001 | |||||
| 20–39 | 32.6% | 49.4% | 34.0% | 22.4% | 13.4% | |
| 40–59 | 33.6% | 32.4% | 41.7% | 35.5% | 34.1% | |
| 60+ | 33.8% | 18.2% | 24.3% | 42.1% | 52.5% | |
| Sex | <0.001 | |||||
| Men | 50.3% | 50.8% | 43.6% | 55.1% | 48.9% | |
| Women | 49.7% | 49.2% | 56.4% | 44.9% | 51.1% | |
| Education | <0.001 | |||||
| <11 grade | 25.3% | 21.4% | 33.2% | 24.6% | 30.1% | |
| High school | 22.9% | 21.1% | 25.5% | 24.0% | 24.7% | |
| Some college | 28.6% | 28.9% | 26.3% | 28.0% | 28.6% | |
| Higher than college | 23.2% | 28.6% | 15.1% | 23.3% | 16.7% | |
| Race | <0.001 | |||||
| Non-Hispanic White | 45.6% | 43.4% | 35.9% | 49.7% | 48.0% | |
| Non-Hispanic Black | 19.4% | 19.6% | 30.1% | 15.2% | 19.6% | |
| Mexican American/Hispanic | 15.7% | 15.9% | 15.8% | 17.0% | 15.2% | |
| Others | 19.3% | 21.1% | 18.1% | 18.1% | 17.3% | |
| Income to poverty ratio | <0.001 | |||||
| <1.30 | 31.2% | 29.5% | 43.9% | 27.3% | 33.7% | |
| 1.3–3.5 | 37.8% | 37.1% | 34.3% | 37.1% | 39.2% | |
| >3.5 | 31.0% | 33.5% | 21.8% | 35.6% | 27.1% | |
| Smoking | <0.001 | |||||
| Never | 53.8% | 58.1% | 49.8% | 53.3% | 48.5% | |
| Former | 25.1% | 20.1% | 15.8% | 30.1% | 30.9% | |
| Current smoker | 21.1% | 21.8% | 34.4% | 16.6% | 20.5% | |
| Drinking | <0.001 | |||||
| No | 18.0% | 12.9% | 22.8% | 17.9% | 24.5% | |
| Yes | 68.0% | 74.6% | 64.1% | 67.9% | 59.8% | |
| Missing | 13.9% | 12.5% | 13.1% | 14.1% | 15.7% | |
| Physical activity (METs minutes/week) | <0.001 | |||||
| <600 | 39.6% | 32.3% | 43.8% | 40.2% | 48.7% | |
| 600–1200 | 11.4% | 11.4% | 12.0% | 12.5% | 11.2% | |
| ≥1200 | 49.0% | 56.3% | 44.2% | 47.3% | 40.1% | |
| BMI (kg/m2) (mean, SD) | 29.0 (6.7) | 26.3 (5.5) | 29.8 (7.0) | 30.3 (6.1) | 32.0 (6.8) | <0.001 |
| CVD | 10.9% | 3.5% | 9.3% | 8.8% | 21.0% | <0.001 |
| Hypertension | 36.3% | 13.0% | 32.4% | 42.1% | 65.1% | <0.001 |
| Central obesity | 56.5% | 32.4% | 64.1% | 69.6% | 84.0% | <0.001 |
| Elevated glucose | 53.3% | 28.0% | 40.2% | 68.7% | 83.0% | <0.001 |
| Elevated triglycerides | 42.1% | 10.0% | 21.2% | 49.8% | 82.8% | <0.001 |
| Elevated blood pressure | 48.6% | 22.4% | 47.9% | 59.9% | 79.7% | <0.001 |
| Reduced HDL-C | 44.4% | 13.3% | 26.6% | 52.1% | 83.6% | <0.001 |
| Depression symptoms | 23.5% | 17.0% | 100.0% | 0.0% | 33.4% | <0.001 |
| Sleep ≤ 6 h/day | 35.2% | 32.2% | 100.0% | 0.0% | 44.0% | <0.001 |
| Circadian syndrome | 40.8% | 0.0% | 100.0% | 0.0% | 100.0% | <0.001 |
| Metabolic syndrome | 48.0% | 0.0% | 0.0% | 100.0% | 100.0% | <0.001 |
| Died during follow-up | 11.3% | 6.7% | 10.0% | 13.0% | 17.0% | <0.001 |
| Died from CVD during follow-up | 3.5% | 1.8% | 2.7% | 4.3% | 5.7% | <0.001 |
| MetS | CircS | ||||
|---|---|---|---|---|---|
| Per one of MetS components (continuous) | MetS (yes vs. no) | Per one of CircS components (continuous) | CircS (yes vs. no) | ||
| Prevalent CVD | |||||
| Men (n = 6113) | |||||
| Model 1 * | 1.54 (1.39–1.71) | 3.20 (2.38–4.30) | 1.53 (1.41–1.66) | 3.01 (2.28–3.97) | |
| Model 2 † | 1.56 (1.40–1.72) | 3.20 (2.38–4.30) | 1.51 (1.39–1.64) | 2.92 (2.21–3.86) | |
| Women (n = 6043) | |||||
| Model 1 * | 1.57 (1.43–1.72) | 3.62 (2.55–5.14) | 1.59 (1.47–1.71) | 4.02 (2.87–5.63) | |
| Model 2 † | 1.49 (1.36–1.64) | 3.04 (2.15–4.30) | 1.50 (1.39–1.63) | 3.27 (2.34–4.59) | |
| CVD mortality | |||||
| Men (n = 6108) | |||||
| Model 1 * | 1.11 (0.99–1.25) | 1.20 (0.81–1.77) | 1.16 (1.04–1.29) | 1.32 (0.93–1.88) | |
| Model 2 † | 1.10 (0.97–1.24) | 1.14 (0.77–1.71) | 1.12 (1.00–1.25) | 1.20 (0.85–1.70) | |
| Women (n = 6041) | |||||
| Model 1 * | 1.16 (1.05–1.27) | 1.52 (1.07–2.15) | 1.23 (1.12–1.36) | 1.76 (1.30–2.38) | |
| Model 2 † | 1.12 (1.00–1.25) | 1.36 (0.93–1.99) | 1.19 (1.07–1.33) | 1.55 (1.12–2.13) |
| Health Status | |||||
|---|---|---|---|---|---|
| Normal | CircS alone | MetS alone | CircS and MetS | ||
| Prevalent CVD | |||||
| Men (n = 6113) | |||||
| Model 1 * | 1.00 | 1.49 (0.62–3.56) | 1.83 (1.16–2.90) | 3.67 (2.69–5.02) | |
| Model 2 † | 1.00 | 1.16 (0.48–2.79) | 1.88 (1.18–2.98) | 3.61 (2.65–4.94) | |
| Women (n = 5908) | |||||
| Model 1 * | 1.00 | 4.15 (2.00–8.61) | 1.65 (0.81–3.35) | 4.64 (3.19–6.74) | |
| Model 2 † | 1.00 | 2.60 (1.25–5.42) | 1.54 (0.78–3.04) | 3.75 (2.59–5.42) | |
| CVD mortality | |||||
| Men (n = 6108) | |||||
| Model 1 * | 1.00 | 2.87 (0.69–11.89) | 1.09 (0.64–1.87) | 1.32 (0.89–1.95) | |
| Model 2 † | 1.00 | 1.83 (0.43–7.75) | 1.10 (0.63–1.92) | 1.22 (0.82–1.80) | |
| Women (n = 5906) | |||||
| Model 1 * | 1.00 | 3.16 (1.03–9.64) | 1.03 (0.47–2.23) | 1.74 (1.22–2.49) | |
| Model 2 † | 1.00 | 2.52 (0.82–7.76) | 1.01 (0.47–2.20) | 1.54 (1.04–2.26) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.; Stern, N.; El-Osta, A.; Chai, Z.; Bilu, C.; Einat, H.; Zimmet, P. The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016. Nutrients 2022, 14, 5317. https://doi.org/10.3390/nu14245317
Shi Z, Tuomilehto J, Kronfeld-Schor N, Alberti G, Stern N, El-Osta A, Chai Z, Bilu C, Einat H, Zimmet P. The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016. Nutrients. 2022; 14(24):5317. https://doi.org/10.3390/nu14245317
Chicago/Turabian StyleShi, Zumin, Jaakko Tuomilehto, Noga Kronfeld-Schor, George Alberti, Naftali Stern, Assam El-Osta, Zhonglin Chai, Carmel Bilu, Haim Einat, and Paul Zimmet. 2022. "The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016" Nutrients 14, no. 24: 5317. https://doi.org/10.3390/nu14245317
APA StyleShi, Z., Tuomilehto, J., Kronfeld-Schor, N., Alberti, G., Stern, N., El-Osta, A., Chai, Z., Bilu, C., Einat, H., & Zimmet, P. (2022). The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016. Nutrients, 14(24), 5317. https://doi.org/10.3390/nu14245317









