Probiotics’ Efficacy in Preventing Asthmatic Allergic Reaction Induced by Air Particles: An Animal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Drug Administration
2.1.1. Animals
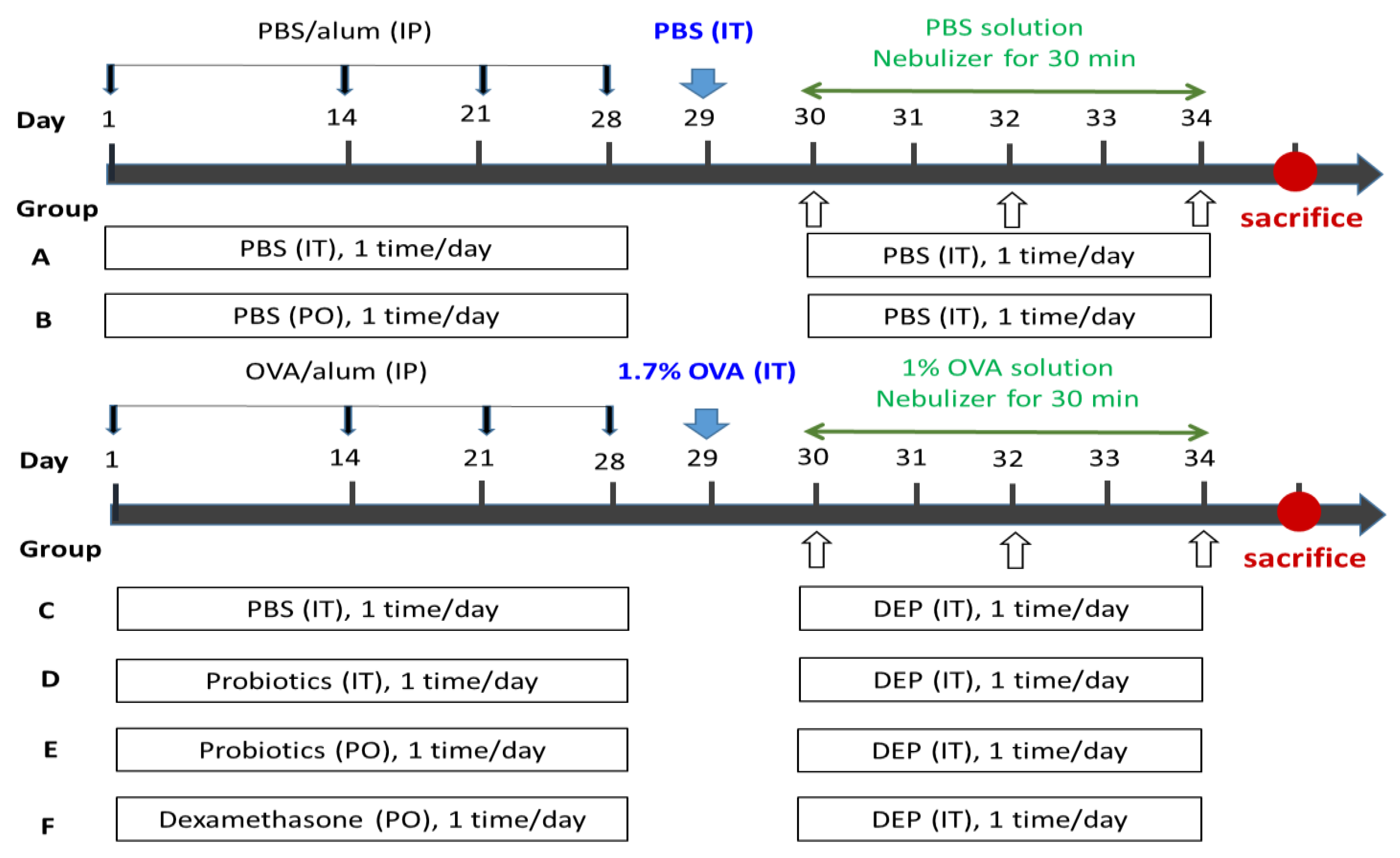
2.1.2. Drug Administration
- Group A (IT control group): IT-PBS + IP-challenged PBS + IT PBS,
- Group B (PO control group): PO-PBS + IP-challenged PBS + IT PBS,
- Group C (OVA-DEP group): IT-PBS + IP-challenged OVA + IT DEP,
- Group D (IT Probiotics group): IT-Probiotics (5 × 107 CFU/mL, 0.1 mL/rat) + IP-challenged OVA + IT DEP,
- Group E (PO Probiotics group): PO-Probiotics (2.0 × 107 CFU/mL, 10 mL/kg/rat) + IP-challenged OVA + IT DEP,
- Group F: PO-dexamethasone (0.2 mg/kg/rat) + IP-challenged OVA + IT DEP.
2.2. Reagents
2.3. Collection and Analysis of Blood Samples
2.4. Lung Histopathology
2.5. Statistical Analysis
3. Results
3.1. Mortality Rate
3.2. Body-Weight Changes
Weight Change Caused by OVA/DEP-Allergy Reduced by LP33 Intratracheal Administration
3.3. Levels of Inflammatory Cells in Bronchoalveolar-Lavage Fluid
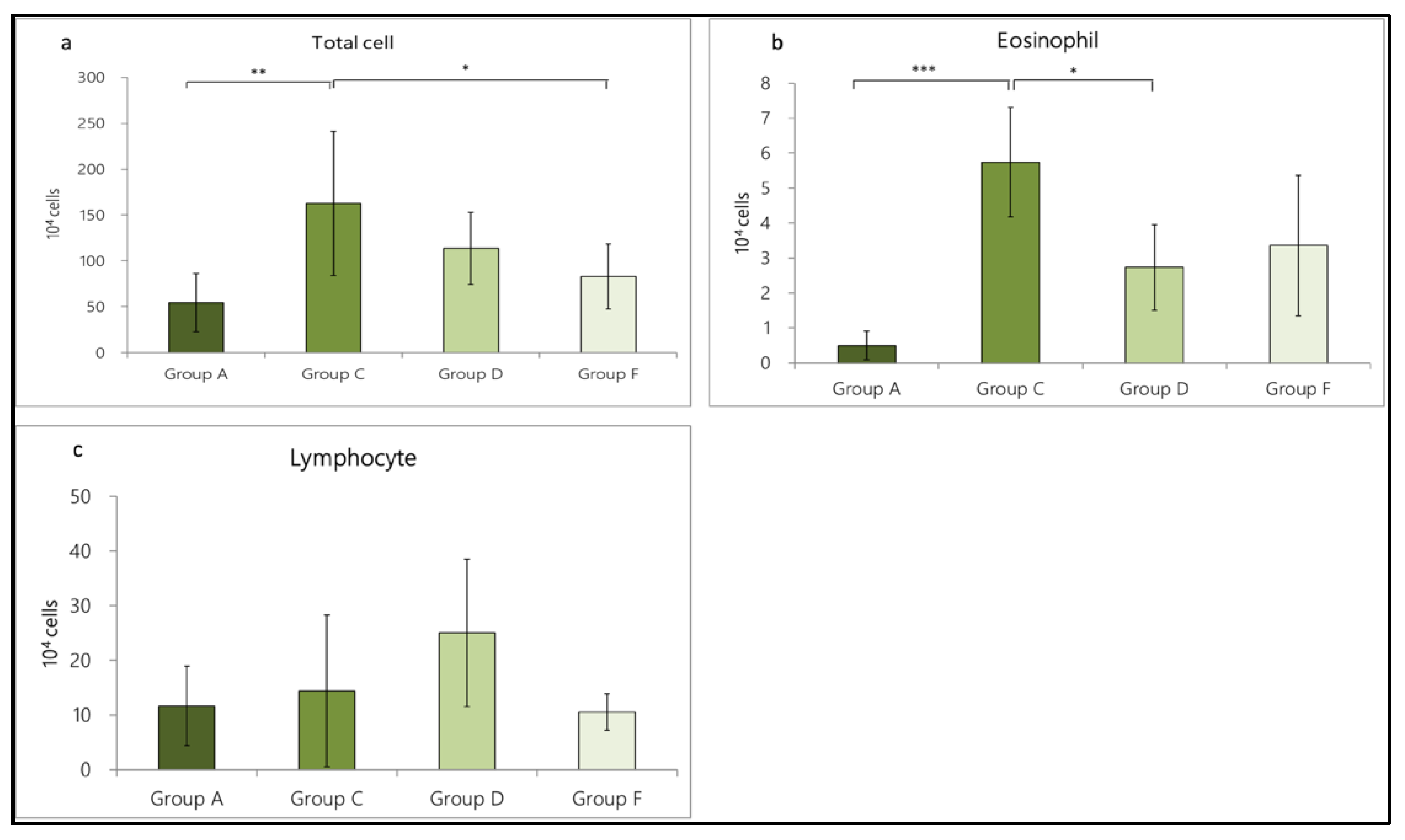
3.3.1. Change in Inflammatory Cell Expression in BALF Caused by OVA/DEP-Allergy Reduced by LP33 Intratracheal-Administration
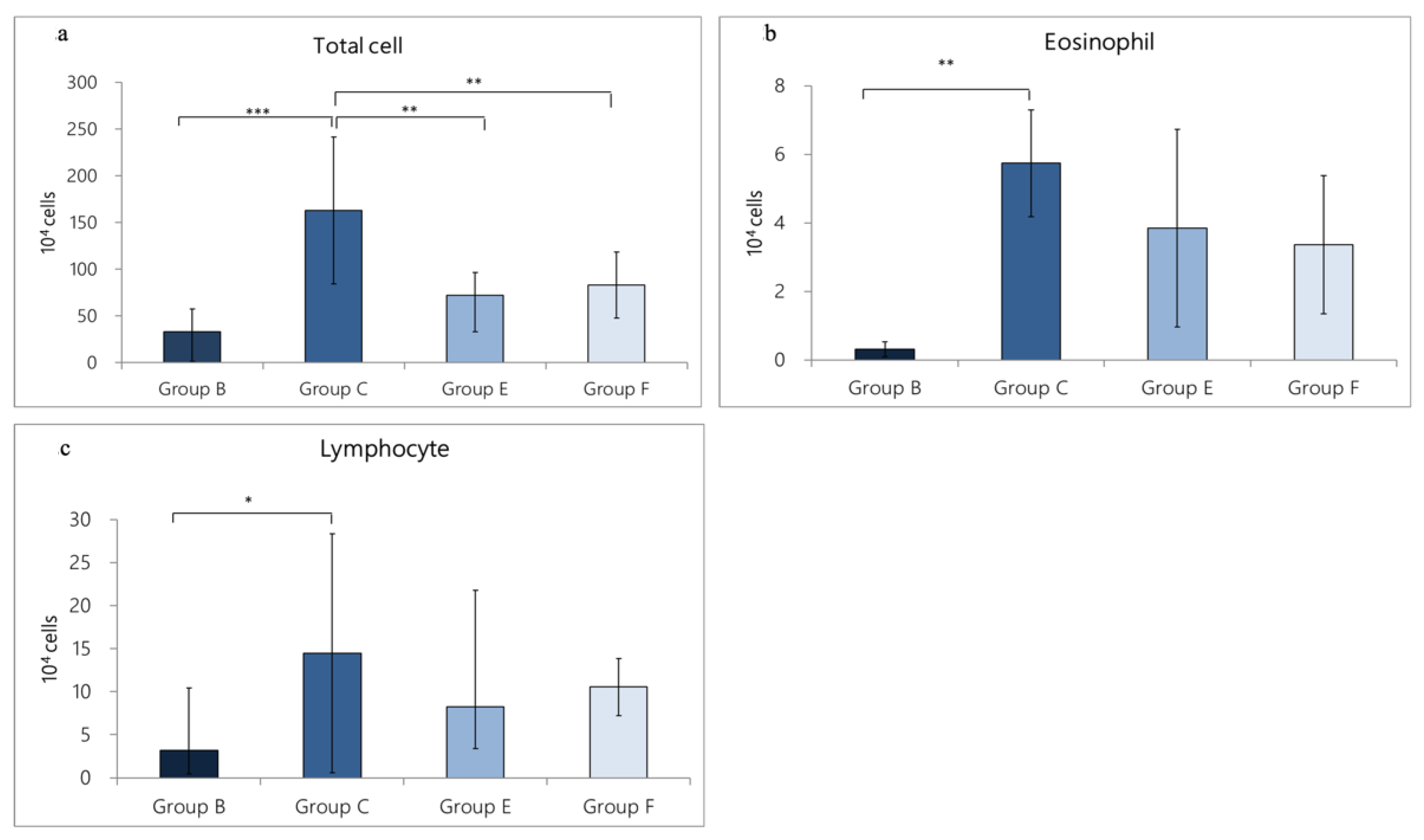
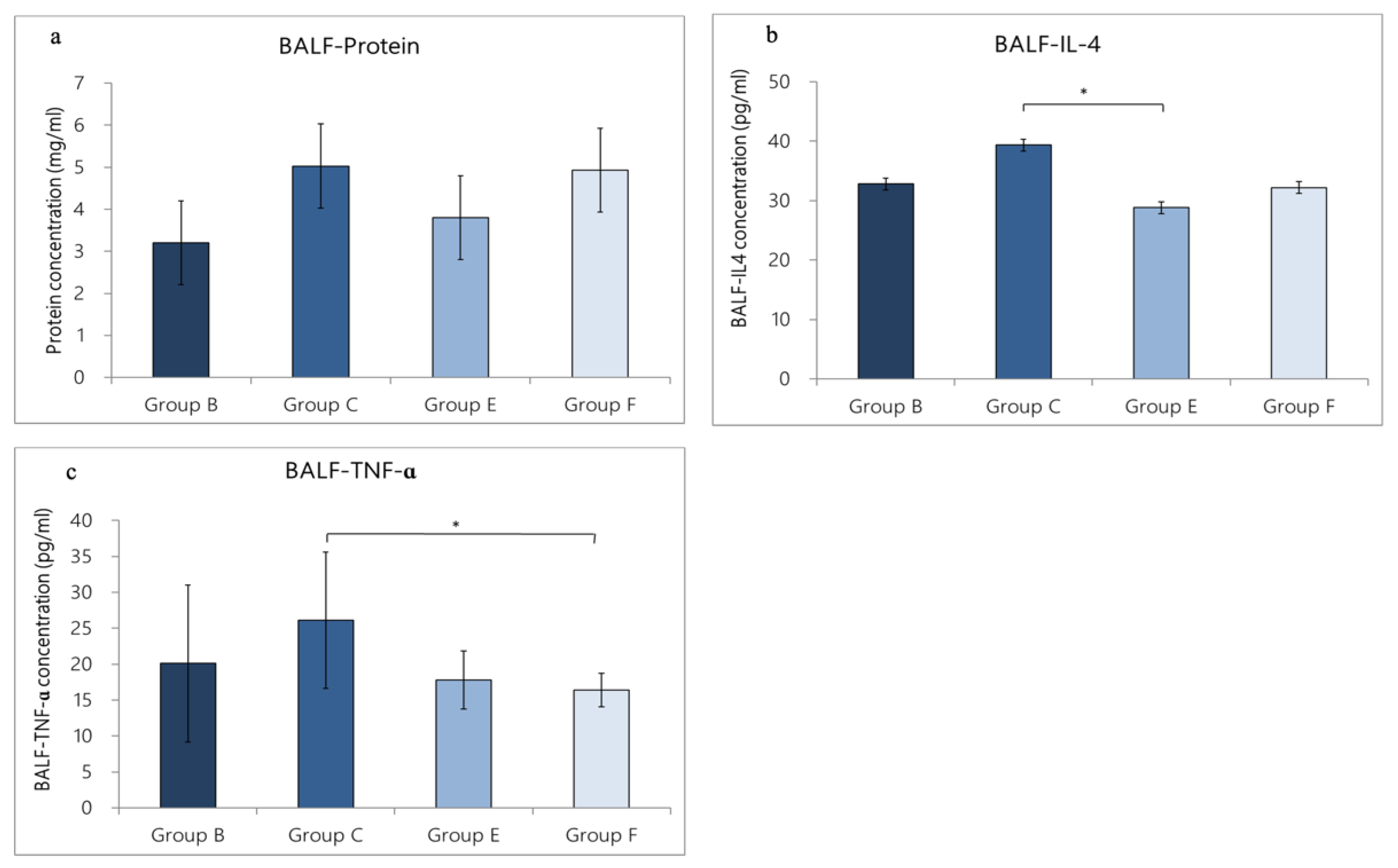
3.3.2. Change in Inflammatory-Cell Expression in BALF Caused by OVA/DEP-Allergy Reduced by LP33 Oral-Administration
3.4. Expression of Cytokines in BALF
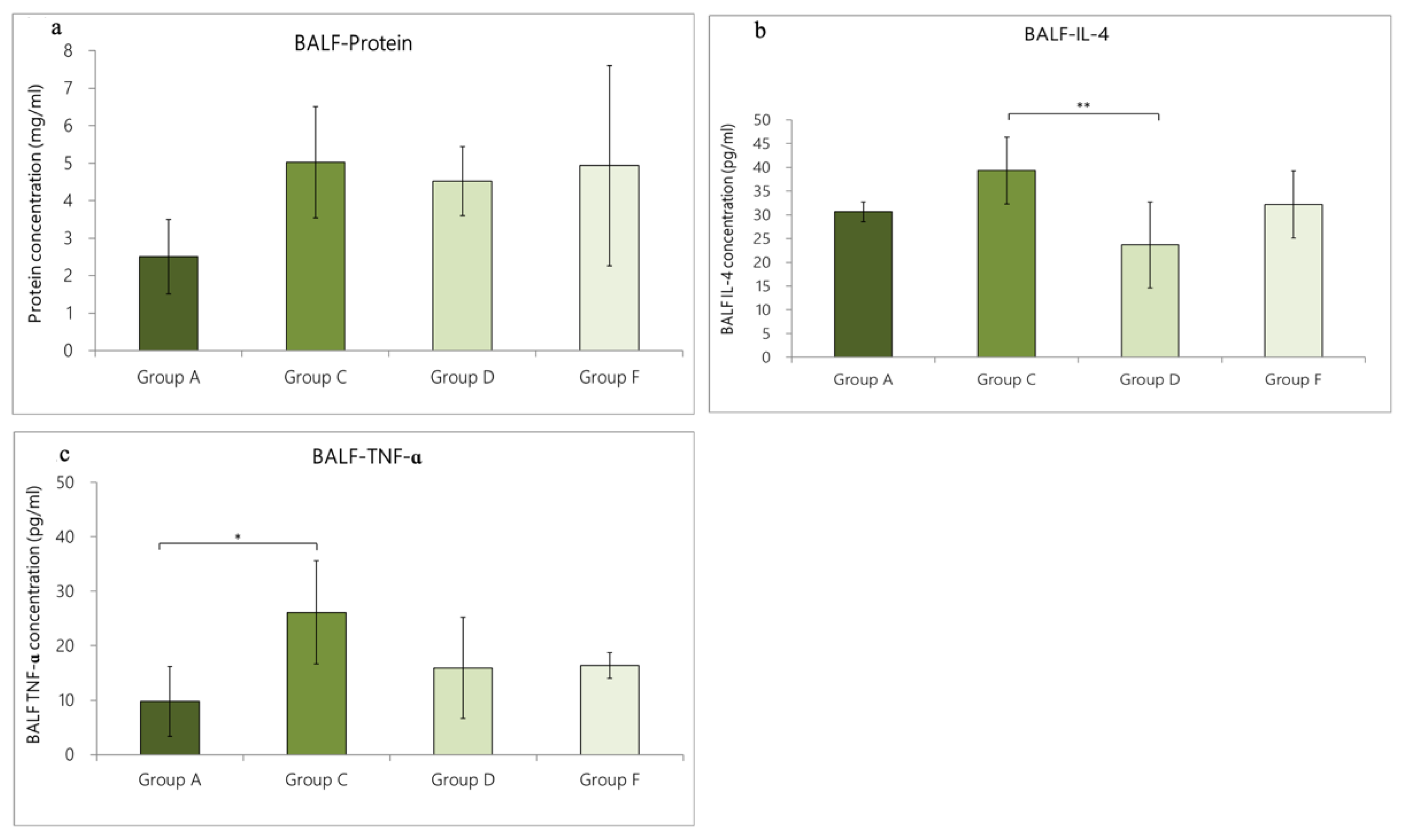
3.4.1. Change in Cytokine Expression in BALF Caused by OVA/DEP-Allergy Reduced by LP33 Intratracheal-Administration
3.4.2. Change in Cytokine Expression in BALF Caused by OVA/DEP-Allergy Reduced by LP33 Oral-Administration
3.5. Expression of Total IgE-Concentration in Rat Serum
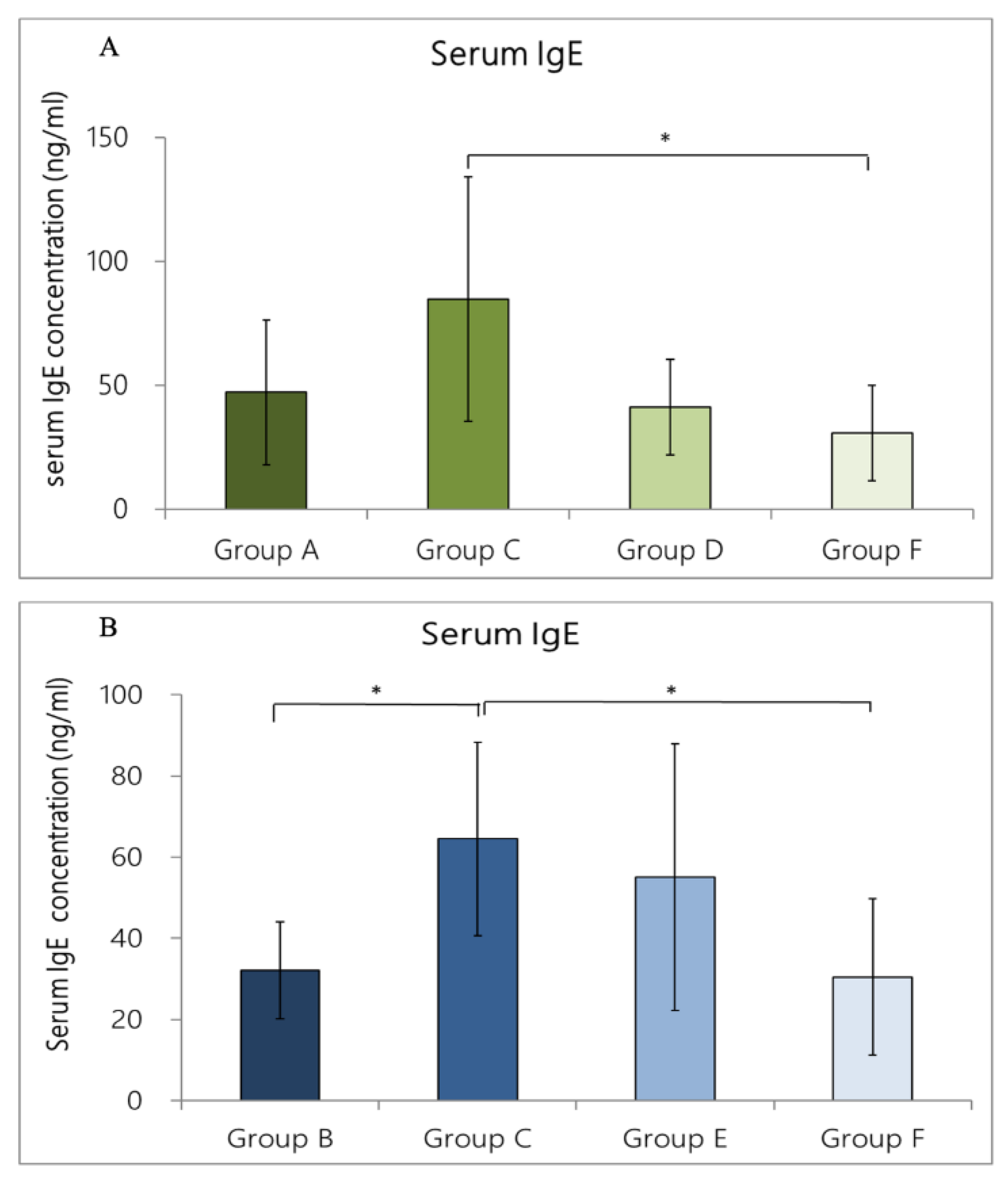
3.5.1. IgE Concentration in Rat Serum Caused by OVA/DEP-Allergy Reduced by LP33 Intratracheal-Administration
3.5.2. IgE Concentration in Rat Serum Caused by OVA/DEP-Allergy Reduced by LP33 Oral-Administration
3.6. Histopathology Examination in Rat Lungs
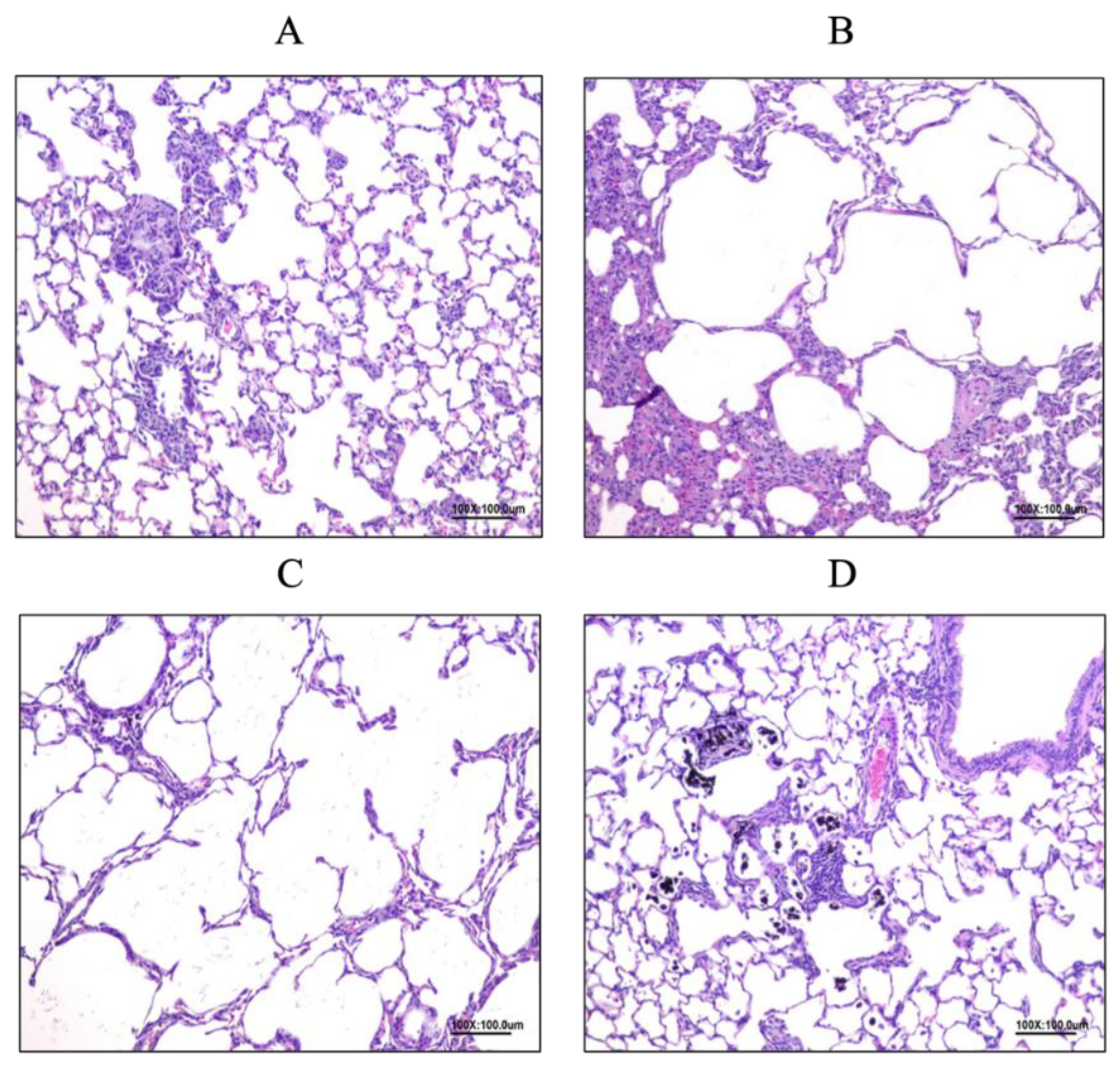
3.6.1. Allergy Reduced by LP33 Intratracheal-Administration
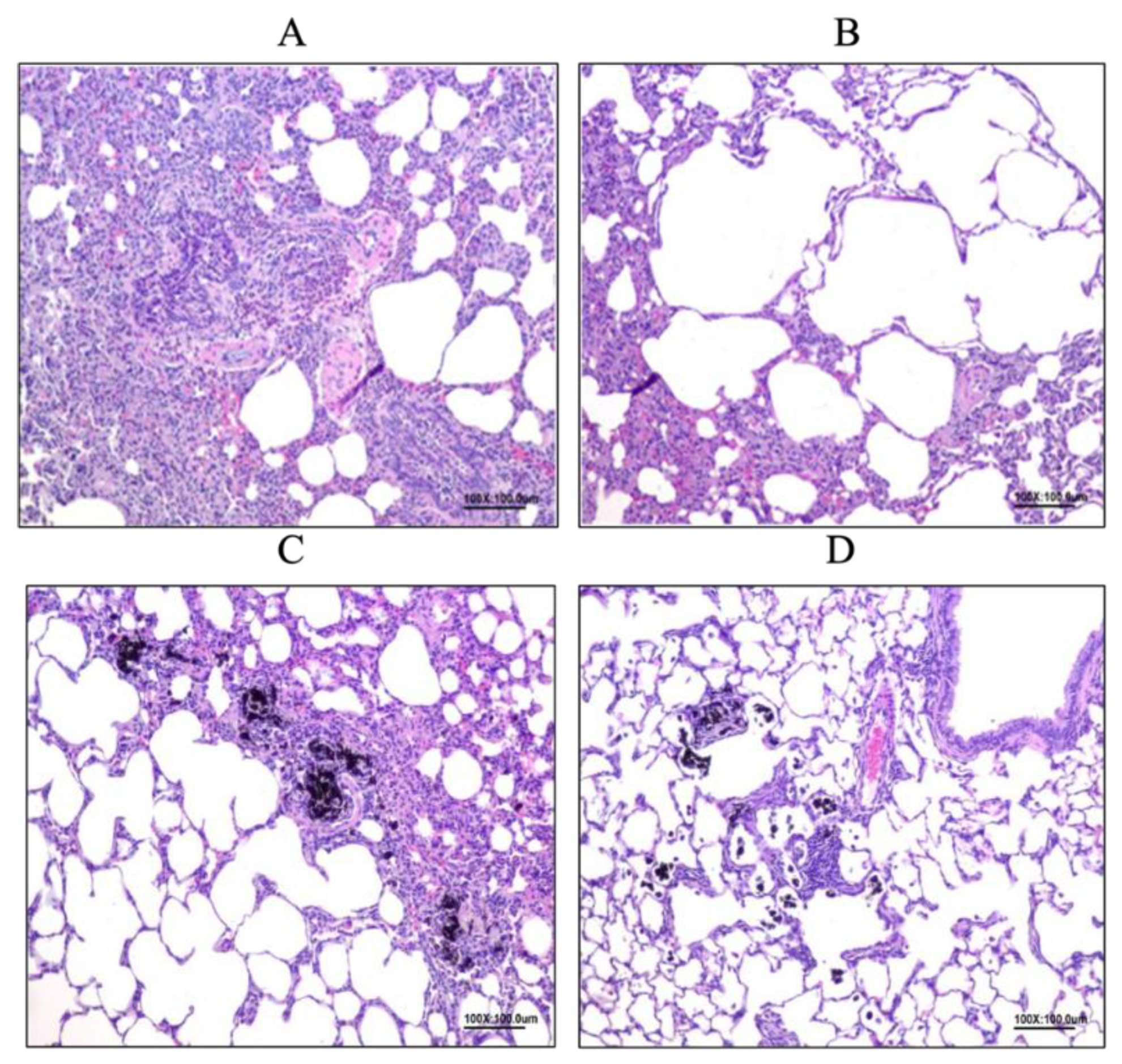
3.6.2. Allergy Reduced by LP33 Oral Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef] [PubMed]
- De Homdedeu, M.; Cruz, M.J.; Sánchez-Díez, S.; Gómez-Ollés, S.; Ojanguren, I.; Ma, D.; Muñoz, X. Role of diesel exhaust particles in the induction of allergic asthma to low doses of soybean. Environ. Res. 2021, 1, 196. [Google Scholar] [CrossRef] [PubMed]
- Balmes, J.R.; Earnest, G.; Katz, P.P.; Yelin, E.H.; Eisner, M.D.; Chen, H.; Trupin, L.; Lurmann, F.; Blanc, P.D. Exposure to traffic: Lung function and health status in adults with asthma. J. Allergy Clin. Immunol. 2009, 3, 626–631. [Google Scholar] [CrossRef]
- Robert, J.P.; Gina, S.; Amy, K.; John, R.B. Diesel Exhaust and Asthma: Hypotheses and Molecular Mechanisms of Action. Reviews 2002, 1, 103–112. [Google Scholar]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Kim, Y.E.; Shin, M.Y.; Kang, Y.; Bae, S.H.; Kim, M.J.; Rhim, T.; Park, C.S.; et al. Long-Term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse model. Allergy Asthma Immunol. Res. 2016, 3, 246–256. [Google Scholar] [CrossRef]
- Kwon, H.K.; Lee, C.G.; So, J.S.; Chae, C.S.; Hwang, J.S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.C.; Im, S.H. Generation of regulatory dendritic cells and CD4+Foxp3 + T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 5, 2159–2164. [Google Scholar] [CrossRef]
- Pellaton, C.; Nutten, S.; Thierry, A.C.; Boudousquié, C.; Barbier, N.; Blanchard, C.; Corthésy, B.; Mercenier, A.; Spertini, F. Intragastric and intranasal administration of lactobacillus paracasei NCC2461 modulates allergic airway inflammation in mice. Int. J. Inflam. 2012, 2012, 686739. [Google Scholar]
- Jan, R.L.; Yeh, K.C.; Hsieh, M.H.; Lin, Y.L.; Kao, H.F.; Li, P.H.; Chang, Y.S.; Wang, J.Y. Lactobacillus gasseri suppresses Th17 pro-inflammatory response and attenuates allergen-induced airway inflammation in a mouse model of allergic asthma. Br. J. Nutr. 2012, 108, 130–139. [Google Scholar] [CrossRef]
- Schabussova, I.; Hufnagl, K.; Tang, M.L.K.; Hoflehner, E.; Wagner, A.; Loupal, G.; Nutten, S.; Zuercher, A.; Mercenier, A.; Wiedermann, U. Perinatal maternal administration of lactobacillus paracasei NCC 2461 prevents allergic inflammation in a mouse model of birch pollen allergy. PLoS ONE 2012, 7, e40271. [Google Scholar] [CrossRef]
- Krishnan, J.A.; Lemanske, R.F.; Canino, G.J.; Elward, K.S.; Kattan, M.; Matsui, E.C.; Mitchell, H.; Sutherland, E.R.; Minnicozzi, M. Asthma outcomes: Symptoms. J. Allergy Clin. Immunol. 2012, 129, 124–135. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, S.; Kang, J.; Lin, J.; Lai, K.; Sun, Y.; Xiao, W.; Yang, L.; Yao, W.; Cai, S. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int. J. COPD 2018, 30, 399–407. [Google Scholar] [CrossRef]
- Barnes, P.J. Inhaled corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Jpn. Soc. Allergol. 2022, 71, 301–309. [Google Scholar] [CrossRef]
- Michail, S. The role of Probiotics in allergic diseases. Allergy 2009, 5, 5. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, P.; Su, H.; Sun, X. Effect of Probiotics on Respiratory Tract Allergic Disease and Gut Microbiota. Front. Nutr. 2022, 9, 821900. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Hessle, C.; Hanson, L.A.; Wold, A.E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin. Exp. Immunol. 1999, 116, 276–282. [Google Scholar] [CrossRef]
- Ichikawa, S.; Miyake, M.; Fujii, R.; Konishi, Y. Orally administered lactobacillus paracasei KW3110 induces in vivo IL-12 production. Biosci. Biotechnol. Biochem. 2009, 73, 1561–1565. [Google Scholar] [CrossRef]
- Peng, G.C.; Hsu, C.H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by the house-dust mite. Pediatr. Allergy Immunol. 2005, 5, 433–438. [Google Scholar] [CrossRef]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone administration in mice leads to less body weight gain over time, lower serum glucose, and higher insulin levels independently of nrf2. Antioxidants 2022, 11, 4. [Google Scholar] [CrossRef]
- Begueret, H.; Berger, P.; Vernejoux, J.M.; Dubuisson, L.; Marthan, R.; Tunon-De-Lara, J.M. Inflammation of bronchial smooth muscle in allergic asthma. Thorax 2007, 62, 8–15. [Google Scholar] [CrossRef]

| Group 1 | Total Incidence (N/N 2) | Cause |
|---|---|---|
| Group A | 1/8 | One rat died after intratracheal PBS administration |
| Group C | 2/8 | Two rats died after OVA induction |
| Group D | 2/8 | One rat died after OVA induction; one rat died after intratracheal-probiotic administration |
| Group F | 0/8 |
| Group 1 | Total Incidence (N/N 2) | Cause |
|---|---|---|
| Group B | 0/8 | |
| Group C | 2/8 | Two rats died after OVA induction |
| Group E | 1/8 | One rat died after OVA induction |
| Group F | 0/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-Y.; Zhang, F.-Y.; Wang, I.-J. Probiotics’ Efficacy in Preventing Asthmatic Allergic Reaction Induced by Air Particles: An Animal Study. Nutrients 2022, 14, 5219. https://doi.org/10.3390/nu14245219
Yang C-Y, Zhang F-Y, Wang I-J. Probiotics’ Efficacy in Preventing Asthmatic Allergic Reaction Induced by Air Particles: An Animal Study. Nutrients. 2022; 14(24):5219. https://doi.org/10.3390/nu14245219
Chicago/Turabian StyleYang, Chi-Yu, Fang-Yu Zhang, and I-Jen Wang. 2022. "Probiotics’ Efficacy in Preventing Asthmatic Allergic Reaction Induced by Air Particles: An Animal Study" Nutrients 14, no. 24: 5219. https://doi.org/10.3390/nu14245219
APA StyleYang, C.-Y., Zhang, F.-Y., & Wang, I.-J. (2022). Probiotics’ Efficacy in Preventing Asthmatic Allergic Reaction Induced by Air Particles: An Animal Study. Nutrients, 14(24), 5219. https://doi.org/10.3390/nu14245219







