Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. In Vitro Antioxidant Activity
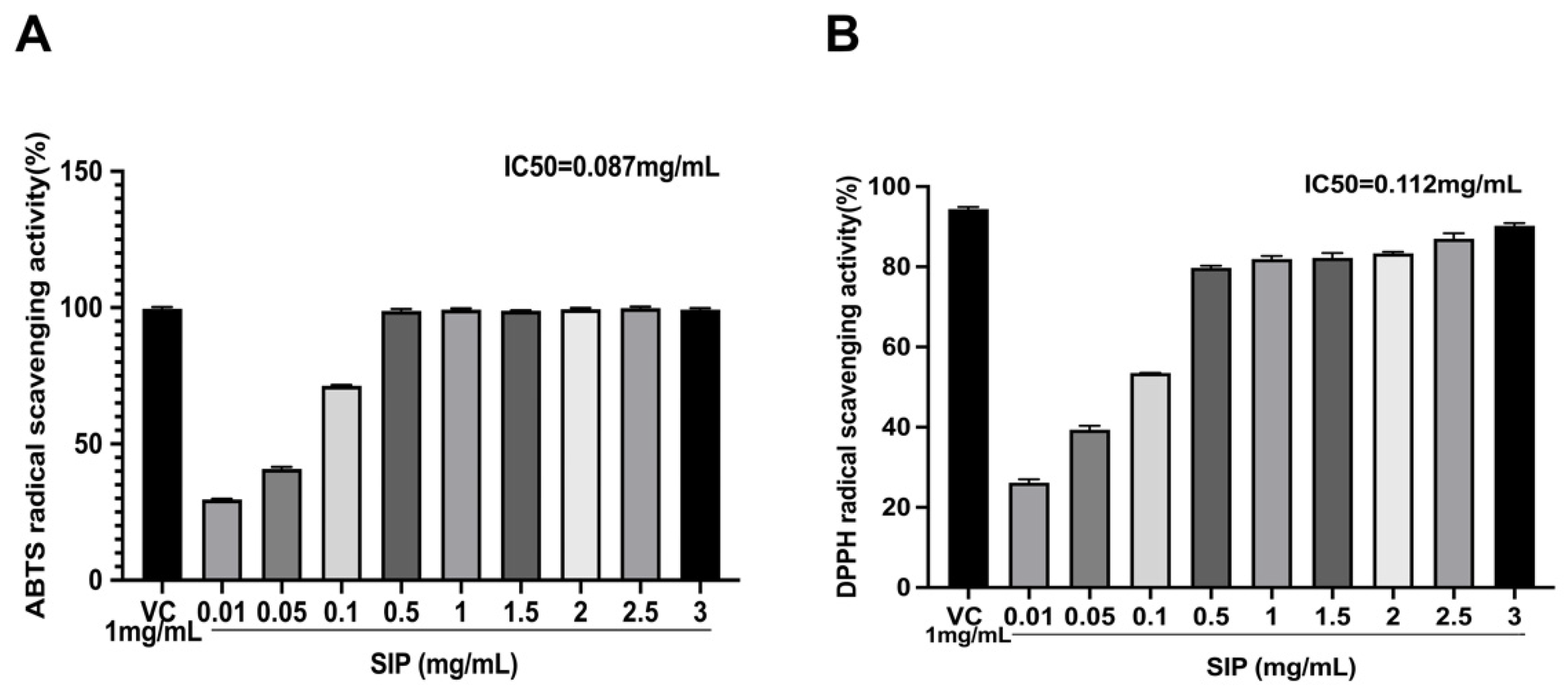
2.3.1. DPPH Radical Scavenging Assay
2.3.2. ABTS Cation Radical Scavenging Assay
2.4. Anti-Melanogenesis Activity Evaluation
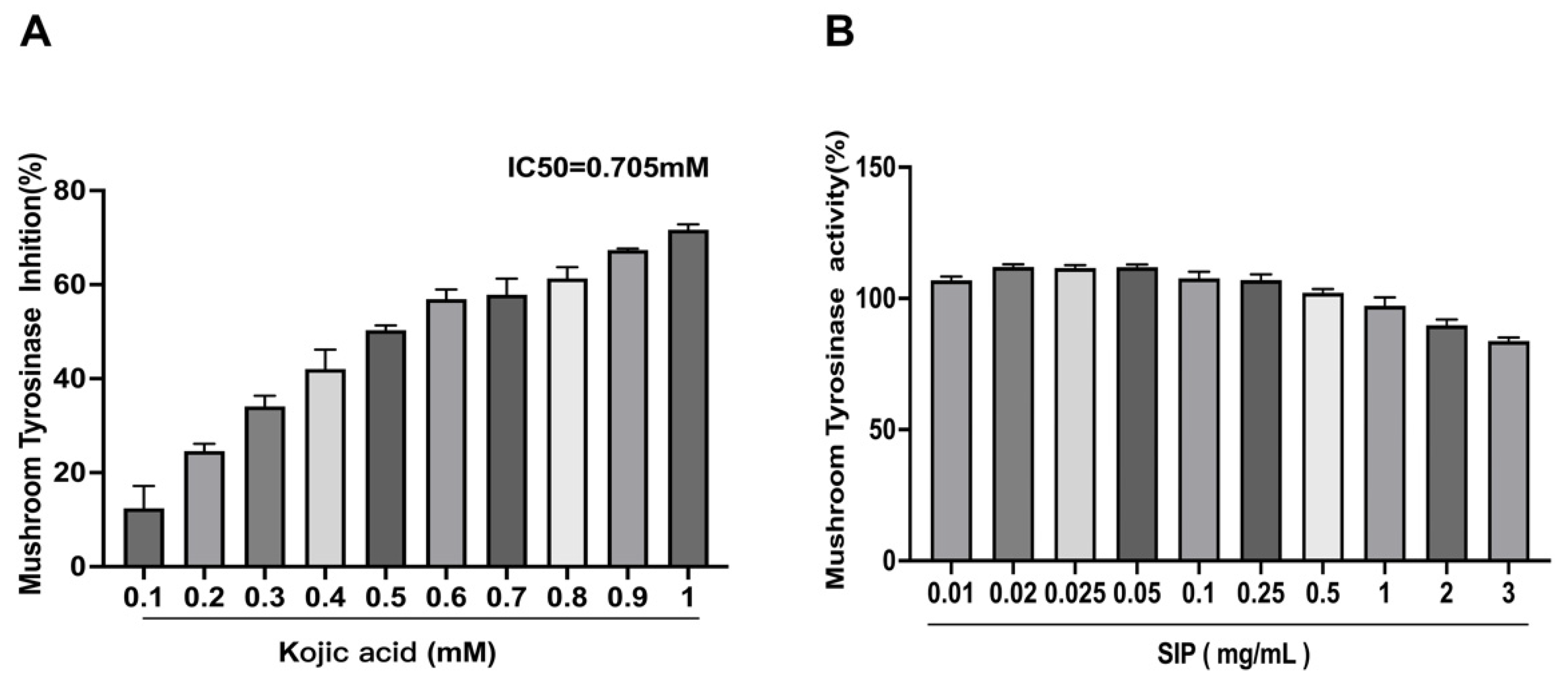
2.4.1. Tyrosinase Inhibitory Assay
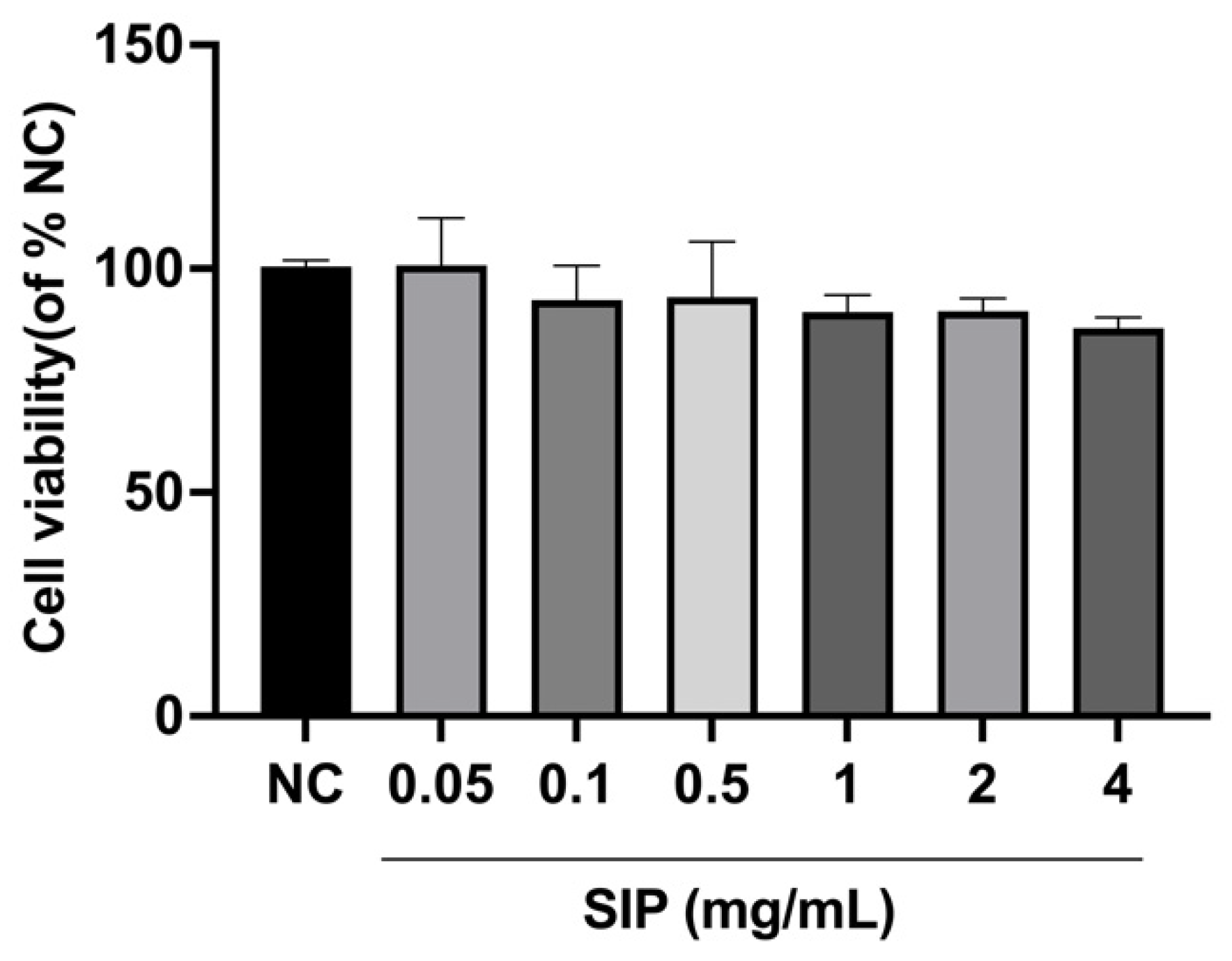
2.4.2. Cell Culture and Cell Viability Assay
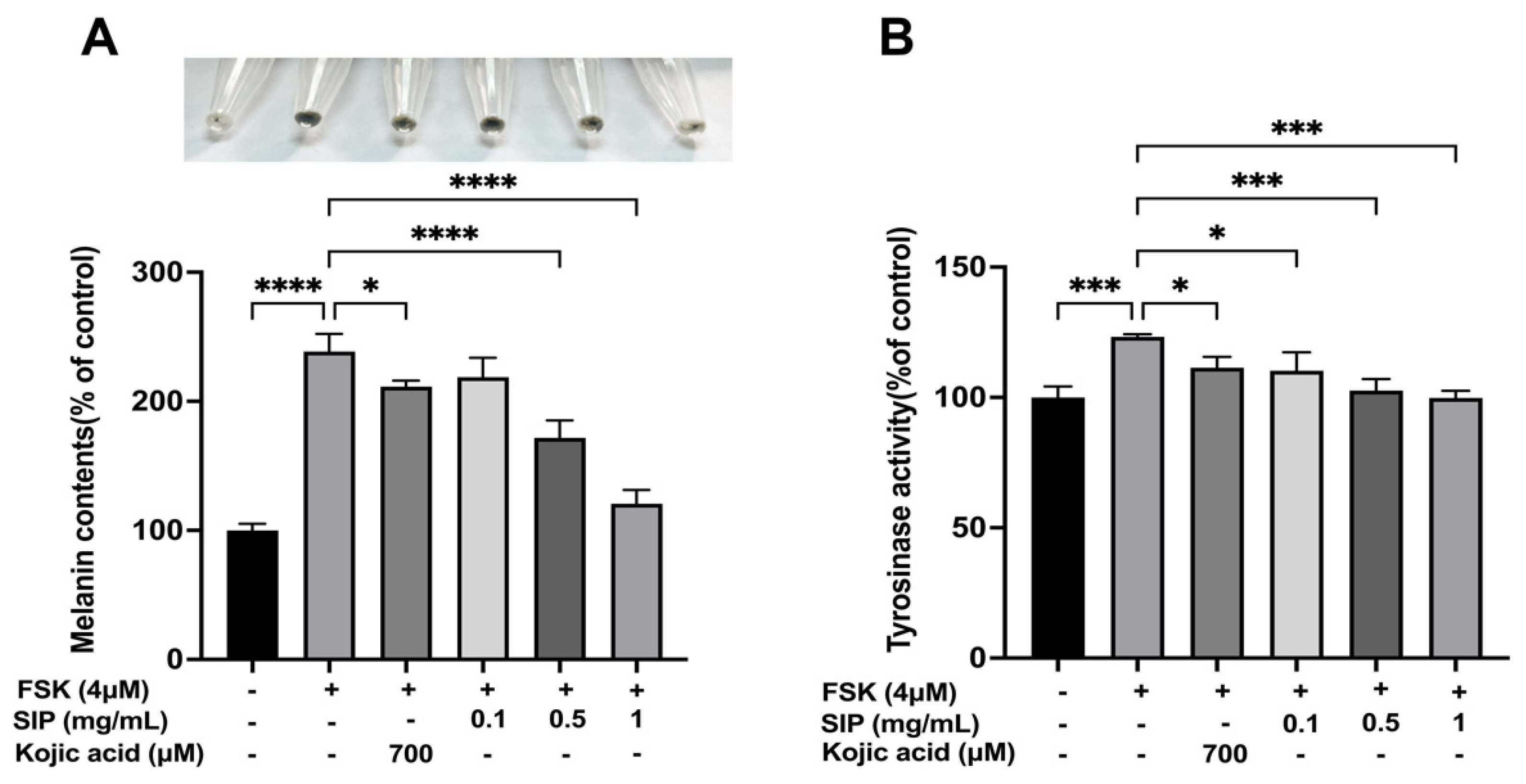
2.4.3. Measurement of Cellular Melanin Contents
2.4.4. Intracellular Tyrosinase Activity Assay
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Radical Scavenging Activity
3.2. Mushroom Tyrosinase Inhibitory Assay
3.3. Effect of SIP on B16F10 Melanoma Cell Viability
3.4. Effect of SIP on Melanin Content and Tyrosinase Activity
3.5. Effect of SIP on Tyrosinase and Tyrosinase-Related Protein Expressions in Forskolin Stimulated B16F10 Cells
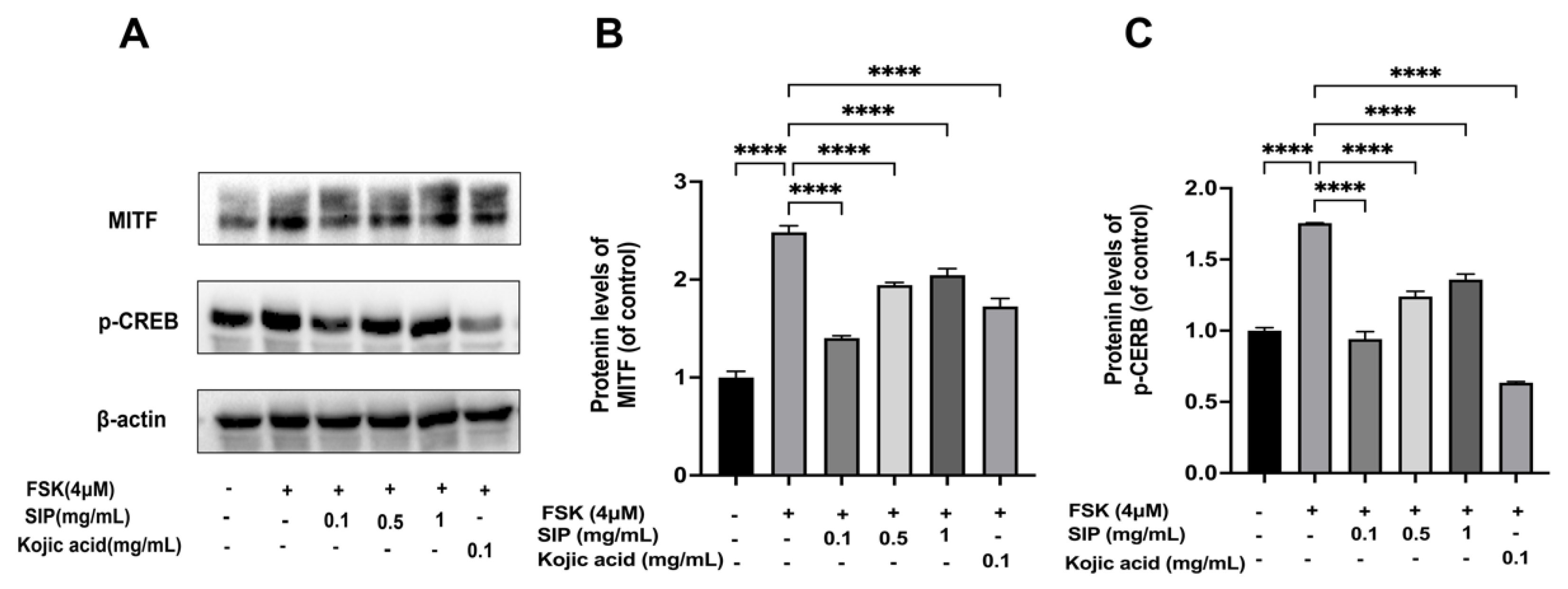
3.6. Effects of SIP MITF and Phosphorylation of CREB Protein Expressions in Forskolin-Stimulated B16F10 Cells
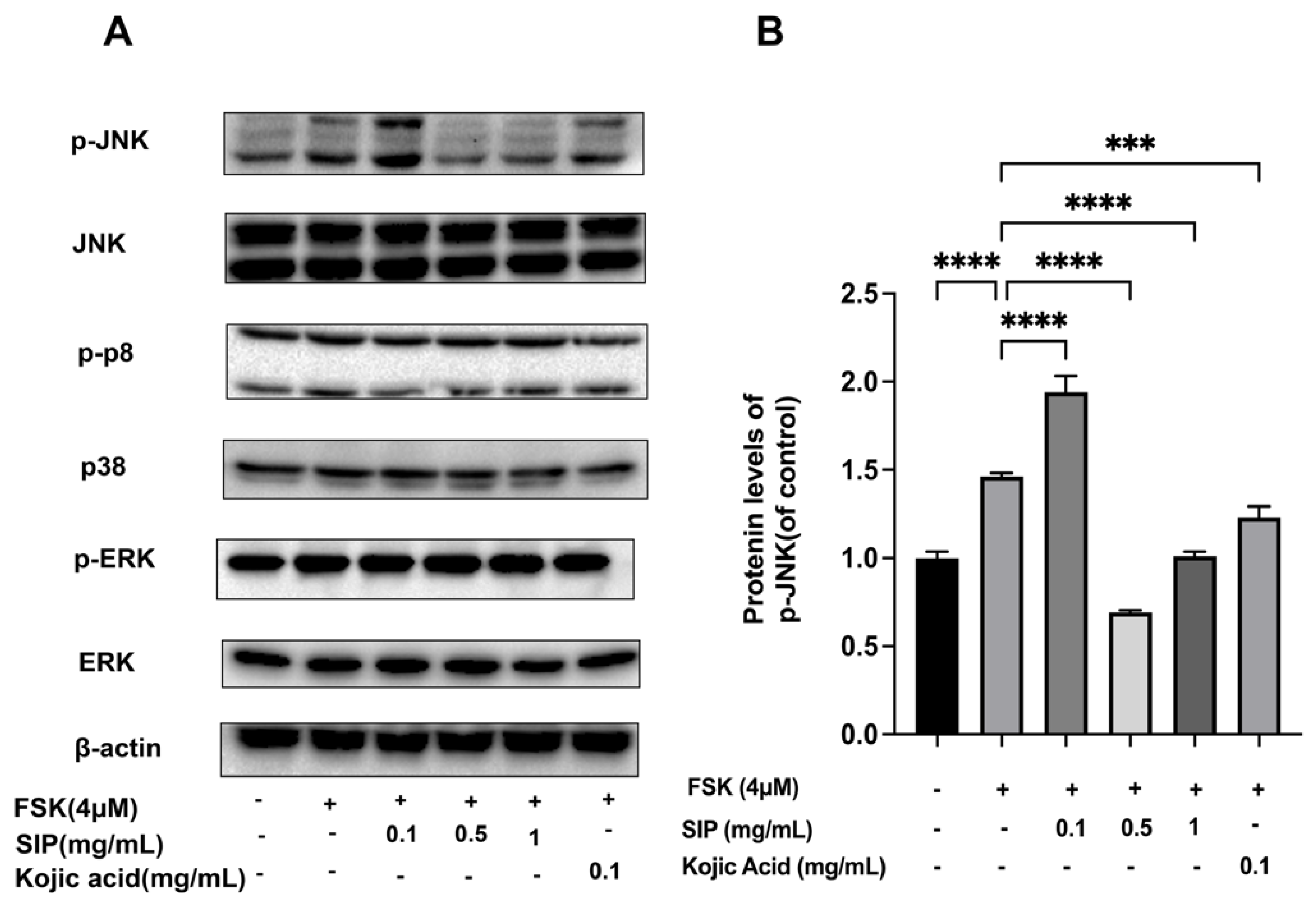
3.7. Effects of SIP on the Expression of MAPK Signaling Pathway in Forskolin-Stimulated B16F10 Cells
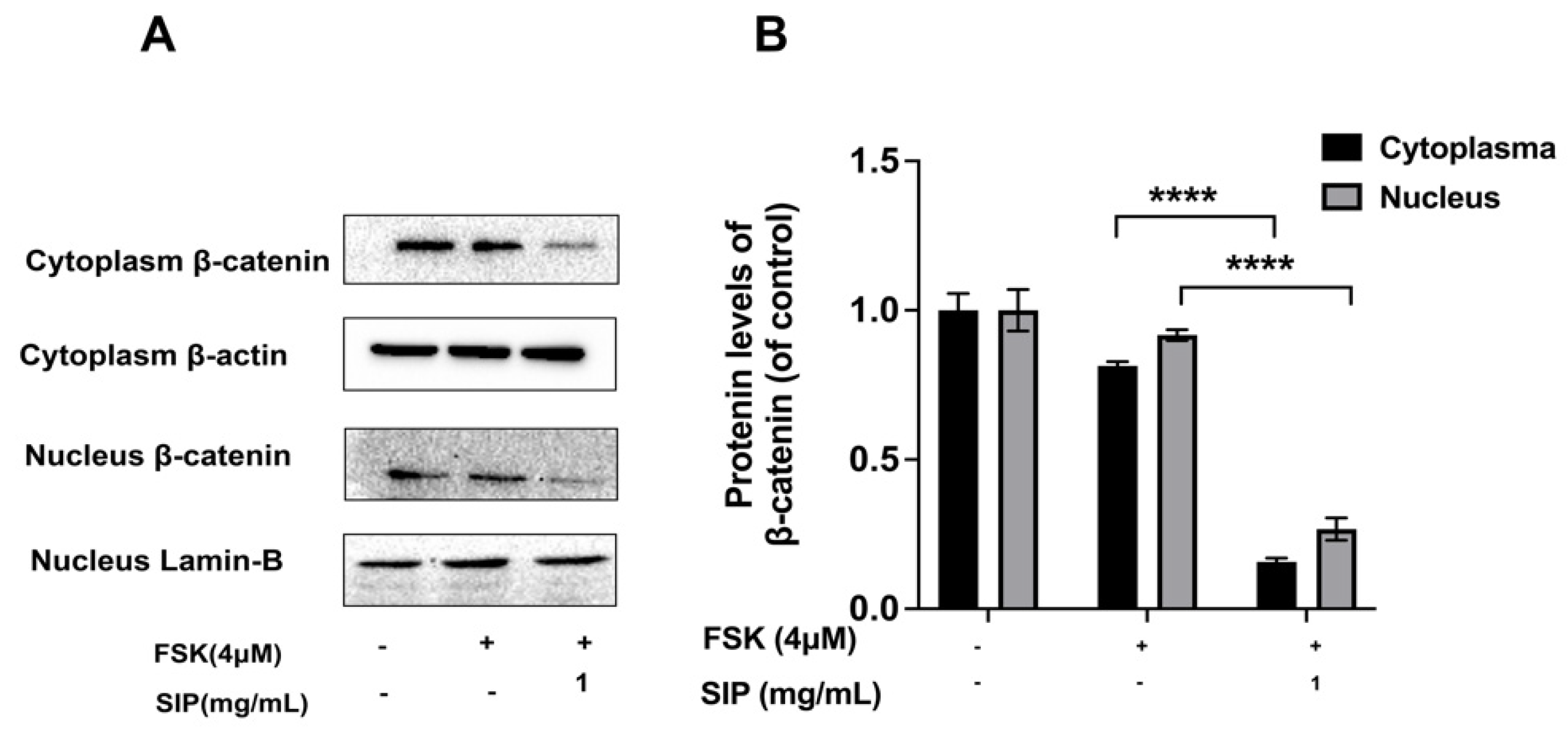
3.8. Effects of SIP on the β-Catenin Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, Y.C.; Hyun, C.-G. Inhibitory effects of pinostilbene hydrate on melanogenesis in B16F10 melanoma cells via ERK and p38 signaling pathways. Int. J. Mol. Sci. 2020, 21, 4732. [Google Scholar] [CrossRef] [PubMed]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef]
- Lerner, A.B.; Fitzpatrick, T.B. Biochemistry of melanin formation. Physiol. Rev. 1950, 30, 91–126. [Google Scholar] [CrossRef] [PubMed]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W.; et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. Embo J. 1992, 11, 519–526. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of quinone methides versus o-quinones in catecholamine metabolism and eumelanin biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef]
- Jimenezcervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garciaborron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18000. [Google Scholar] [CrossRef]
- Hearing, V.J.; Jimenez, M. Mammalian tyrosinase—The critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. 1987, 19, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. Faseb J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Balcos, M.C.; Kim, S.Y.; Jeong, H.-S.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Park, K.-C.; Kim, D.-S. Docosahexaenoic acid inhibits melanin synthesis in murine melanoma cells in vitro through increasing tyrosinase degradation. Acta Pharmacol. Sin. 2014, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Vance, K.W.; Goding, C.R. The transcription network regulating melanocyte development and melanoma. Pigment Cell Res. 2004, 17, 318–325. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Lin, H.-H.; Li, T.-S.; Tseng, C.-Y.; Wong, Y.; Chen, J.-H. Anti-melanogenesis effects of lotus seedpod in vitro and in vivo. Nutrients 2020, 12, 3535. [Google Scholar] [CrossRef]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Bae, J.-S.; Han, M.; Yao, C.; Chung, J.H. Chaetocin inhibits IBMX-induced melanogenesis in B16F10 mouse melanoma cells through activation of ERK. Chem.-Biol. Interact. 2016, 245, 66–71. [Google Scholar] [CrossRef]
- Azam, M.S.; Kwon, M.; Choi, J.; Kim, H.-R. Sargaquinoic acid ameliorates hyperpigmentation through cAMP and ERK-mediated downregulation of MITF in alpha-MSH-stimulated B16F10 cells. Biomed. Pharmacother. 2018, 104, 582–589. [Google Scholar] [CrossRef]
- Azam, M.S.; Joung, E.-J.; Choi, J.; Kim, H.-R. Ethanolic extract from Sargassum serratifolium attenuates hyperpigmentation through CREB/ERK signaling pathways in alpha-MSH-stimulated B16F10 melanoma cells. J. Appl. Phycol. 2017, 29, 2089–2096. [Google Scholar] [CrossRef]
- Bu, J.; Ma, P.-C.; Chen, Z.-Q.; Zhou, W.-Q.; Fu, Y.-J.; Li, L.-J.; Li, C.-R. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am. J. Chin. Med. 2008, 36, 245–263. [Google Scholar] [CrossRef]
- Wu, L.-C.; Lin, Y.-Y.; Yang, S.-Y.; Weng, Y.-T.; Tsai, Y.-T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef]
- Wang, Y.; Androlewicz, M.J. Oligosaccharide trimming plays a role in the endoplasmic reticulum-associated degradation of tyrosinase. Biochem. Biophys. Res. Commun. 2000, 271, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Svedine, S.; Wang, T.; Halaban, R.; Hebert, D.N. Carbohydrates act as sorting determinants in ER-associated degradation of tyrosinase. J. Cell Sci. 2004, 117, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Son, Y.H.; Lee, K.B.; Lee, J.H.; Kim, H.J.; Jeong, E.M.; Park, S.C.; Kim, I.G. 4-n-butylresorcinol enhances proteolytic degradation of tyrosinase in B16F10 melanoma cells. Int. J. Cosmet. Sci. 2017, 39, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-S.; Choi, H.-R.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Park, K.-C.; Kim, D.-S. Ceramide PC102 inhibits melanin synthesis via proteasomal degradation of microphthalmia-associated transcription factor and tyrosinase. Mol. Cell. Biochem. 2013, 375, 81–87. [Google Scholar] [CrossRef]
- Jeong, H.; Yu, S.-M.; Kim, S.J. Inhibitory effects on melanogenesis by thymoquinone are mediated through the beta-catenin pathway in B16F10 mouse melanoma cells. Int. J. Oncol. 2020, 56, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Jeong, Y.J.; Xuan, S.H.; Lee, J.-Y.; Park, J.; Park, S.N. Methyl-2-acetylamino-3-(4-hydroxyl-3,5-dimethoxybenzoylthio) propanoate suppresses melanogenesis through ERK signaling pathway mediated MITF proteasomal degradation. J. Dermatol. Sci. 2018, 91, 142–152. [Google Scholar] [CrossRef]
- Azam, M.S.; Kim, J.-I.; Choi, C.G.; Choi, J.; Lee, B.; Kim, H.-R. Sargahydroquinoic acid suppresses hyperpigmentation by cAMP and ERK1/2-mediated downregulation of MITF in alpha-MSH-stimulated B16F10 cells. Foods 2021, 10, 2254. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Draelos, Z.D. The latest cosmeceutical approaches for anti-aging. J. Cosmet. Dermatol. 2007, 6, 2–6. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Ariff, A.B. Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: Evidence from in vitro study. J. Cosmet. Dermatol. 2019, 18, 703–727. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chien, Y.-C.; Wu, C.-H.; Kuo, Y.-H.; Wu, W.-C.; Pan, Y.-Y.; Su, Y.-H.; Wen, K.-C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Pundir, M. Phytochemistry and pharmacology of Saussurea genus (Saussurea lappa, Saussurea costus, Saussurea obvallata, Saussurea involucrata). Mater. Today Proc. 2022, 56, 1173–1181. [Google Scholar] [CrossRef]
- Chik, W.-I.; Zhu, L.; Fan, L.-L.; Yi, T.; Zhu, G.-Y.; Gou, X.-J.; Tang, Y.-N.; Xu, J.; Yeung, W.-P.; Zhao, Z.-Z.; et al. Saussurea involucrata: A review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J. Ethnopharmacol. 2015, 172, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Su, D.; Xian, X.; Zhou, M.; Li, X.; Huang, J.; Wang, J.; Gao, H. Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch -Bip. on adjuvant arthritis in rats. J. Ethnopharmacol. 2016, 194, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, Q.; Xiao, J.; Sun, J.; Yuan, X.; Zhao, B.; Su, H.; Niu, S. Composition and antioxidant activity of the polysaccharides from cultivated Saussurea involucrata. Int. J. Biol. Macromol. 2012, 50, 849–853. [Google Scholar] [CrossRef]
- Xiao, W.; Li, X.; Li, N.; Bolati, M.; Wang, X.; Jia, X.; Zhao, Y. Sesquiterpene lactones from Saussurea involucrata. Fitoterapia 2011, 82, 983–987. [Google Scholar] [CrossRef]
- Wang, T.-M.; Wang, R.-F.; Chen, H.-B.; Shang, M.-Y.; Cai, S.-Q. Alkyl and phenolic glycosides from Saussurea stella. Fitoterapia 2013, 88, 38–43. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kao, J.-Y.; Kuo, D.-H.; Liao, C.-F.; Huang, C.-H.; Fan, L.-L.; Way, T.-D. Antifatigue and antioxidant activity of alcoholic extract from saussurea involucrata. J. Tradit. Complement. Med. 2011, 1, 64–68. [Google Scholar] [CrossRef]
- Gong, G.; Zheng, Y. The anti-UV properties of Saussurea involucrata Matsum. & Koidz. Via regulating PI3K/Akt pathway in B16F10 cells. J. Ethnopharmacol. 2021, 269, 113694. [Google Scholar] [CrossRef]
- Liu, G.; Kamilijiang, M.; Abuduwaili, A.; Zang, D.; Abudukelimu, N.; Liu, G.; Yili, A.; Aisa, H.A. Isolation, structure elucidation, and biological activity of polysaccharides from Saussurea involucrata. Int. J. Biol. Macromol. 2022, 222, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liao, X.; Zhang, Q.; Zhu, Y.; Mei, S.; Li, Q.; Zhou, X.; Li, X.; Luo, H.; Ye, H.; et al. Anti-melanogenesis effect from Wampee fruit pectin via alpha-MSH/TRY pathway in A375 cells. BMC Complement. Med. Ther. 2022, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-N.; Li, W.; Mehmood, S.; Pan, W.-J.; Wu, Q.-X.; Chen, Y.; Lu, Y.-M. Effect of polysaccharide FMP-1 from Morchella esculenta on melanogenesis in B16F10 cells and zebrafish. Food Funct. 2018, 9, 5007–5015. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The potential of sulfated polysaccharides isolated from the brown seaweedecklonia maximain cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef]
- Li, W.D.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, B.; Jeon, Y.-D.; Song, H.-W.; Lee, Y.-M.; Song, B.-J.; Kim, D.-K. Inhibitory effect of elaeagnus umbellata fractions on melanogenesis in alpha-MSH-stimulated B16-F10 melanoma cells. Molecules 2021, 26, 1308. [Google Scholar] [CrossRef]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3β/β-catenin signal pathway. Drug Des. Dev. Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Latres, E.; Chiaur, D.S.; Pagano, M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 1999, 18, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.H.; Kim, D.E.; Cho, H.S.; Kang, S.; Han, J.S.; Chang, M.Y.; Lee, C.K.; et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Hammer, J.A. Making sense of melanosome dynamics in mouse melanocytes. Pigment Cell Res. 2000, 13, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Coudrier, E. Myosins in melanocytes: To move or not to move? Pigment Cell Res. 2007, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Chen, X.-Z.; Gowrisankar, Y.V.; Yen, H.-R.; Chuang, J.-Y.; Yang, H.-L. The skin-whitening effects of ectoine via the suppression of alpha-MSH-stimulated melanogenesis and the activation of antioxidant Nrf2 pathways in UVA-irradiated keratinocytes. Antioxidants 2020, 9, 63. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Saad, H.M.; Tan, C.H.; Lim, S.H.; Manickam, S.; Sim, K.S. Evaluation of anti-melanogenesis and free radical scavenging activities of five Artocarpus species for cosmeceutical applications. Ind. Crops Prod. 2021, 161, 113184. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Sung, J.-H.; Park, S.-H.; Seo, D.-H.; Lee, J.-H.; Hong, S.-W.; Hong, S.-S. Antioxidative and skin-whitening effect of an aqueous extract of salicornia herbacea. Biosci. Biotechnol. Biochem. 2009, 73, 552–556. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Kim, D.-O.; Heo, H.J. Melanogenesis regulatory activity of the ethyl acetate fraction from Arctium lappa L. leaf on alpha-MSH-induced B16/F10 melanoma cells. Ind. Crops Prod. 2019, 138, 111581. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Kim, H.J.; Kim, J.-E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Wu, P.-C.; Lee, H.-J.; Tseng, Y.-H.; Wang, S.-Y. Essential oils of alpinia nantoensis retard forskolin-induced melanogenesis via ERK1/2-mediated proteasomal degradation of MITF. Plants 2020, 9, 1672. [Google Scholar] [CrossRef]
- Byun, E.-B.; Song, H.-Y.; Mushtaq, S.; Kim, H.-M.; Kang, J.A.; Yang, M.-S.; Sung, N.-Y.; Jang, B.-S.; Byun, E.-H. Gamma-Irradiated luteolin inhibits 3-isobutyl-1-methylxanthine-induced melanogenesis through the regulation of CREB/MITF, PI3K/Akt, and ERK pathways in B16BL6 melanoma cells. J. Med. Food 2017, 20, 812–819. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Lin, C.-C.; Wang, H.-Y.; Shih, Y.; Chou, S.-T. The melanogenesis alteration effects of achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, B.R.; Lee, E.K.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Lee, Y.J.; Ku, S.K. Inhibitory effect of dried pomegranate concentration powder on melanogenesis in B16F10 melanoma cells; involvement of p38 and PKA signaling pathways. Int. J. Mol. Sci. 2015, 16, 24219–24242. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef]
- Takeda, K.; Yasumoto, K.; Takada, R.; Takada, S.; Watanabe, K.; Udono, T.; Saito, H.; Takahashi, K.; Shibahara, S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000, 275, 14013–14016. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H. Getting in and out of the proteasome. Semin. Cell Dev. Biol. 2000, 11, 149–158. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamilijiang, M.; Zang, D.; Abudukelimu, N.; Aidarhan, N.; Liu, G.; Aisa, H.A. Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells. Nutrients 2022, 14, 5044. https://doi.org/10.3390/nu14235044
Kamilijiang M, Zang D, Abudukelimu N, Aidarhan N, Liu G, Aisa HA. Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells. Nutrients. 2022; 14(23):5044. https://doi.org/10.3390/nu14235044
Chicago/Turabian StyleKamilijiang, Mayila, Deng Zang, Nuermaimaiti Abudukelimu, Nurbolat Aidarhan, Geyu Liu, and Haji Akber Aisa. 2022. "Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells" Nutrients 14, no. 23: 5044. https://doi.org/10.3390/nu14235044
APA StyleKamilijiang, M., Zang, D., Abudukelimu, N., Aidarhan, N., Liu, G., & Aisa, H. A. (2022). Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells. Nutrients, 14(23), 5044. https://doi.org/10.3390/nu14235044






