Abstract
Bile acids (BA) are amphiphilic molecules synthesized in the liver (primary BA) starting from cholesterol. In the small intestine, BA act as strong detergents for emulsification, solubilization and absorption of dietary fat, cholesterol, and lipid-soluble vitamins. Primary BA escaping the active ileal re-absorption undergo the microbiota-dependent biotransformation to secondary BA in the colon, and passive diffusion into the portal vein towards the liver. BA also act as signaling molecules able to play a systemic role in a variety of metabolic functions, mainly through the activation of nuclear and membrane-associated receptors in the intestine, gallbladder, and liver. BA homeostasis is tightly controlled by a complex interplay with the nuclear receptor farnesoid X receptor (FXR), the enterokine hormone fibroblast growth factor 15 (FGF15) or the human ortholog FGF19 (FGF19). Circulating FGF19 to the FGFR4/β-Klotho receptor causes smooth muscle relaxation and refilling of the gallbladder. In the liver the binding activates the FXR-small heterodimer partner (SHP) pathway. This step suppresses the unnecessary BA synthesis and promotes the continuous enterohepatic circulation of BAs. Besides BA homeostasis, the BA-FXR-FGF19 axis governs several metabolic processes, hepatic protein, and glycogen synthesis, without inducing lipogenesis. These pathways can be disrupted in cholestasis, nonalcoholic fatty liver disease, and hepatocellular carcinoma. Thus, targeting FXR activity can represent a novel therapeutic approach for the prevention and the treatment of liver and metabolic diseases.
1. Introduction
Bile acids (BA) are components of human bile, a brownish or olive-green liquid made of water, organic solutes, and inorganic salt, at a pH of 7.6–8.6. Bile is the major excretion route of cholesterol which appears as solubilized cholesterol and as BA, i.e., degradation products. The three main lipids in bile are bile salts, phospholipids, and nonesterified cholesterol. Free cholesterol accounts for 97% of all sterols in bile, the rest are cholesterol precursors and dietary phytosterols. BA are amphipathic molecules synthesized as “primary” BA from cholesterol in the pericentral hepatocytes. Following their synthesis, primary BA are conjugated with the compound taurine (2-aminoethanesulfonic acid) and the amino acid glycine; this step increases BA solubility in aqueous solutions, such as bile. Primary-conjugated BA are actively secreted from the hepatocyte into bile and are mainly and actively absorbed in the terminal ileum. A small amount will travel to the colon and will undergo the microbiota-dependent biotransformation into “secondary” and “tertiary” BA, which will be passively absorbed. By this dynamic mechanism, BA undergo continuous enterohepatic circulation with 4–12 cycles/day. The fecal loss is minimal at every cycle (5%) and must be compensated by the de novo synthesis of primary BA in the liver (~200–600 mg/daily). The synthesis of BA is modulated by negative feedback mechanisms controlled by the farnesoid X receptor (FXR) in the liver and in the intestine [1,2]. This pathway is strictly linked with the enterohepatic circulation of BA [3], with the signaling role of BA [4], and with the composition and abundance of gut microbiota [5].
Because of this scenario, the role of BA is not simply limited in the emulsification and absorption of dietary fat and fat-soluble vitamins. BA also regulate the proliferation of epithelial cells [6,7], gene expression [8,9], epigenetic mechanisms [10,11], fibrogenesis [12], lipid [13] and glucose metabolism [14]. These effects derive from the role of BA as endogenous ligands and from their ability to activate specific receptors. Besides FXR, involved receptors also include the membrane-associated G-protein-coupled bile acid receptor-1 (GPBAR-1, also known as TGR5 or M-BAR) [6,15], and sphingosine-1-phosphate receptor 2 (S1PR2) [16,17] in the intestine, in the liver, in the muscle and in the brown adipose tissue [18,19]. In the latter decades, the recognition of BA as signaling molecules has shed new light on the complex pathophysiological mechanisms and potential therapeutic implications. Consequences of disrupted BA homeostasis include cholestasis [20], hepatic steatosis, liver fibrosis, and liver tumor [21,22]. Notably, therapeutic manipulation of the BA-FXR axis is paving the way to innovative and potent therapeutic tools [3,23,24], potentially able to act at multiple levels (i.e., BA composition, mitochondrial function, modulation of gut microbiota, glucose and lipid homeostasis, liver inflammation) [25,26,27,28,29,30,31,32,33]. The present review depicts the complex relationship linking BA and FXR, and how its modulation can become a valid therapeutic target for several liver diseases.
2. The Enterohepatic Circulation and Kinetics of BA and the FXR–FGF19 Dynamics
The cycling of BA between the liver and the intestine is defined enterohepatic circulation, while the total burden of BA in the enterohepatic circulation represents the circulating BA pool [34]. The complex process operating in the enterohepatic circulation of BA has been extensively described in previous papers [19,21]. Briefly, the enterohepatic circulation implies that BA reach the terminal ileum and the colon as well. In the ileum, BA act as signaling molecules and agonists to the nuclear receptor FXR. FXR has antimicrobial effects and protects the intestinal barrier. FXR activation is linked, in humans, with the transcription of the enterokine fibroblast growth factor 19 (FGF19) or the ortholog FGF15 in mice. FGF19 enters the portal flow and has effects on the gallbladder and liver [21]. BA activate FXR with the following rank order: chenodeoxycholic acid (CDCA) > lithocholic acid (LCA) = deoxycholic acid (DCA) > cholic acid (CA) in the conjugated and unconjugated forms [35].
FGF19 acts as agonist of the hepatic FGF receptor 4 (FGFR4)/β-Klotho and activates c-Jun N-terminal kinase/extracellular signal-regulated kinase (JNK/ERK). This pathway inhibits the expression of CYP7A1 and CYP8B1 and further hepatic BA synthesis, in concert with the FXR- small heterodimer partner (SHP) inhibitory pathway [36]. BA travelling from the intestine into the portal tract enter the liver via the sodium taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptide (OATP) transporters. FXR works with other nuclear receptors involved in BA homeostasis, namely retinoid X receptor (RXR), SHP, liver receptor homologous-1 (LRH-1), and liver X receptor (LXR) [18]. FXR is the main sensor of BA and regulates synthesis, secretion, and metabolism of BA in the liver, ileum and colon [36,37,38]. In the liver, FXR regulates target gene transcription by binding to RXRs as a heterodimer [39]. This binding leads to increased transcription of the SHP expression. SHP, in turn, inhibits CYP7A1 expression by blocking transactivation of the hepatic activators LRH-1 and hepatic nuclear factor 4 (HNF-4), at the promoter [39]. This pathway ultimately prevents the activation of target genes involved in the synthesis of fatty acids and BA. By contrast, at low concentrations of BA, LRH-1 operates together with LXR and stimulates the synthesis of BA [40,41,42]. FXR transcriptionally activates the enzymes involved in BA conjugation to glycine or taurine (bile acid-CoA synthetase [BACS] and bile acid-CoA: amino acid N-acetyltransferase [BAT]) [43], regulates hepatic BA secretion by bile salt export pump (BSEP), and hepatic phospholipid secretion by ABCB4. BA re-entering the liver also interact with GPBAR-1 on the macrophagic Kupffer cells, together with the pathway stimulated by the FGFR4/β-Klotho dimer. In addition, the activation of FXR induces expression of BA detoxification enzymes (i.e., cytosolic sulfotransferase 2A1 (SULT2A1 [44]), aldol-keto reductase 1 B7 (AKR1B7 [45]), cytochrome P450 3A4/3a11 (CYP3A4/Cyp3a11), and UDP-glycosyltransferase 2B4 (UTG2B4)) [46]. In the kidney (proximal tubule), circulating BA undergo uptake by the apical sodium/dependent bile acid transporter (ASBT). Glomerular filtration of BA are modulated by MRP 2, 3, 4 transporters [47].
Experimental models in animals show that the pathogenesis of NASH is likely linked with a markedly altered composition of BA in the enterohepatic- rather than in the systemic circulation, with specific reduction of secondary BAs (mainly DCA), and scarce presence of FXR and TGR5 ligands in the portal blood. In this case, the major role of enterohepatic BA in the pathogenesis of NASH is confirmed by the protection from NASH secondary to dietary correction (i.e., DCA supplementation) of the BA profile [48].
3. Regulation of BA Homeostasis: The Role of Gut Microbiota
The gut represents a dynamic interface between the internal and the external body environment [49], with several stimuli continuously interacting with the intestinal barrier [50]. The gut microbiota is sensitive to dietary habits and nutrients such as dietary fiber, proteins, fat, carbohydrates, [51]. They are also sensitive to external toxic agents, which include smoking [52], ethanol consumption [53], and environmental pollutants such as heavy metals and pesticides [54,55,56,57]. All these factors can affect the diversity and relative abundance of the gut microbiota [58] during the process of enterohepatic circulation, when the primary BA synthetized and secreted by the liver are transformed into secondary BA in the colon [58,59]. BA and gut microbes interact within a continuous bidirectional crosstalk in health but also in disease [50,60,61]. Gut dysbiosis can disrupt BA homeostasis and can change the composition of the BA pool, while the increased production of deoxycholic acid (DCA), a cytotoxic secondary BA, can damage the composition of gut microbiome [9]. BA bind to FXR and this step produces antimicrobial peptides (AMPs) (i.e., angiogenin 1 and RNase family member 4). These AMPs play an active role in the inhibition of gut microbial overgrowth and intestinal barrier dysfunction [62]. BA can also modulate the gut microbiome by stimulating the growth of BA-metabolizing bacteria or inhibiting other bile-sensitive bacteria. Gut Eubacterium lentum, Ruminococcus gnavus and Clostridium perfringens decrease the antimicrobial effect of BA via the iso-BA pathway by transforming DCA and LCA into iso-DCA and iso-LCA (3b-OH epimers). The secondary DCA has hydrophobic and cytotoxic profiles with detergent effects on the bacterial cell membranes and antimicrobial properties [63].
Changes of the microbiota and therefore BA pool can interfere with activation of signaling pathways [64] involved in intestinal, metabolic homeostasis and tumorigenesis. Dietary changes associated with low short-chain fatty acids have been linked with an high risk for cancer development [65,66]. Colorectal cancer represents another example since, in humans, dietary habits can vary BA conjugation. For example, diets enriched in animal protein favor taurine conjugation while vegetarian diets favor glycine conjugation. In this scenario, TCA can to stimulate gut microbes able to convert taurine and cholic acid to hydrogen sulfide and DCA, which act as genotoxin and tumor-promoter, respectively [65].
Dysbiosis and variations of the BA pool composition and size can play a role in alcohol associated liver disease (ALD) [67], in NAFLD, obesity, and type 2 diabetes [58,68,69,70]. Compared to healthy controls, higher levels of total faecal BA, primary CA and CDCA, and higher BA synthesis have been reported in patients with NASH, who also showed a higher ratio of primary to secondary BA, but a similar ratio of conjugated to unconjugated BA. Patients with NASH were also characterized by a decreased count of Bacteroidetes and Clostridium leptum. The count of C. leptum increased with fecal unconjugated LCA and decreased with unconjugated CA and CDCA. Taken together, these findings point to a link between NAFLD, dysbiosis and altered BA homeostasis, which puts patients at risk of further hepatic injury [71].
Dysbiosis can be also implicated in tumorigenesis via BA-induced changes. DCA increases on a western diet and becomes a predisposing factor to intestinal carcinogenesis. In DCA-treated APC (min/+) mice, a disrupted intestinal microbiota was associated with altered gut barrier, low-grade gut inflammation and tumor progression. Fecal microbiota transplantation from DCA-treated mice to Apc (min/+) mice increased tumor multiplicity, caused inflammation, recruited the M2 phenotype tumor-associated macrophages, and activated the tumor-associated Wnt/beta-catenin signaling pathway. Notably, the antibiotic-induced depletion of the microbiota blocked DCA-induced intestinal carcinogenesis [72]. Another important study found that a 2-week food exchanges profoundly affected the microbiota, metabolome profile, mucosal biomarkers of cancer risk when African Americans were fed a high-fibre, low-fat African-style diet and rural Africans were fed a high-fat, low-fibre western-style diet. In the African Americans the study found a protective profile by saccharolytic fermentation and butyrogenesis and suppressed secondary BA synthesis [66].
4. The Microbiota–BA–FXR Axis
The BA-FXR axis protects the liver for BA overload and potential harmful effects of BA upon accumulation in the hepato-biliary-intestinal tract [73]. Additional aspects of such interaction include systemic metabolic effects deriving from FXR activation and FGF19 secretion [19]. Thus, changes in the profile of the BA pool and primary/secondary BA ratio can produce multiple consequences, including altered metabolic pathways and liver damage [71,74,75]. In this context, a major role is played by microbiota-induced deconjugation of BA. This is the case when the murine primary BA Tauro-beta-muricholic Acid (TβMCA), a natural occurring FXR antagonist, will be deconjugated by the microbiota. This step will alleviate FXR signaling in ileum leading, in the liver, to inhibition of BA synthesis [60,76]. In normal conditions with a functioning enterohepatic circulation of a physiological BA pool, the FXR-induced gene expression including Ang1, iNos and Il18 in ileum, has antimicrobial action, enteral protection and inhibition of bacteria damage to the intestinal mucosa. In rodents, the biliary obstruction that causes small intestinal bacterial overgrowth (SIBO) can be reversed by administration of BA [77,78]. A study in the cholestatic model of mice also reported protection by a potent synthetic agonist of FXR [79]. Metabolic studies in mice show that the gut microbiota promotes diet-induced obesity via FXR signaling [62,75,76]. In an animal model of NAFLD induced by high-fat diet, the antibiotic treatment decreased BSH-encoding Lactobacillus, increased the synthesis of the FXR antagonist TβMCA, and improved insulin resistance and liver steatosis [80]. In humans with newly diagnosed type 2 diabetes, naïve treatment with metformin modified gut microbiota, decreasing Bacteroides fragilis and increasing the BA glycoursodeoxycholic acid (GUDCA) in the gut. These changes were paralleled by an inhibition of FXR signaling, pointing to GUDCA as an intestinal FXR antagonist able to improve metabolic dysfunction [81]. The inhibition of intestinal FXR is a key factor for the progression of NAFLD mediated by gut microbiome [75]. Mice fed a high milk- fat diet showed a shift in BA composition with increased TCA and expansion of Bilophila wadsworthia. This proteobacterium is recognized as a ‘‘bile-loving’’ microorganism associated to inflammatory bowel diseases [82,83].
In NAFLD rats, the administration of probiotics is able to significantly increase the expression of FXR, FGF15 mRNA, and protein in the liver, upregulating the diversity of gut microbiota, downregulating the abundance of pathogenic bacteria and finally alleviating NAFLD [26].
One note, changes in gut microbiota composition are possible following FXR/FGF19 modulation. In humans with biopsy-confirmed NASH, administration of the FGF19 analog aldafermin induced in the microbiota a dose-dependent enrichment in Veillonella, a rare commensal microbe which correlated with changes in serum bile acid profile, mainly in terms of decreased toxic BA [84].
Dietary lipids likely impact the gut microbial composition directly as a substrate or by shifting in BA composition [85]. The microbiota FXR signaling modulation was also assessed during the sub-ministration of the antioxidant Tempol, which resulted in a decrease of Lactobacillus and Clostridium (clusters IV and XIVa), accompanied by decreased BSH, accumulation of TβMCA and suppressed FXR signals [76]. Other nuclear receptors can also be involved during the interaction of microbiota with intestinal BA, such as the pregnane X receptor (PXR), vitamin D receptor (VDR), GPBAR-1 and Sphingosine-1-Phosphate Receptor 2 (S1PR2) [86,87,88].
5. Fibroblast Growth Factors FGF15 and Human Ortholog FGF19
Fibroblast growth factors FGF15 (rodent) and the human ortholog FGF19 are enterokines which play a major role in BA homeostasis and key metabolic functions, in concert with FXR [89]. The transcriptional regulation of FGF19 involves the FXR during the enterohepatic circulation of BA [90,91]. Additional players include vitamin D receptor (VDR), pregnane X receptor (PXR), drugs, vitamins and cholesterol [92]. The sterol regulatory element-binding protein 2 (SREBP2) is a negative transcriptional regulator of FGF19 [93]. FGFs belong to a family of at least 22 proteins with an effect on growth, development, and differentiation [90,94,95]. FGFs, currently grouped into six subfamilies, play an autocrine and paracrine role via activation of specific tyrosine kinase FGF receptors undergoing dimerization and activation of the cascade of intracellular signaling pathways [96]. The trio consisting of FGF15 (and the human ortholog FGF19), FGF21, and FGF23 act as circulating hormones [97]. FGF19 is the human protein encoded by the FGF19 gene and has endocrine hormonal functions at a systemic level [90,98]. FGF19 was originally identified in the fetal brain and, with FGF15 only shares ~50% amino acid identity [99]. FGF15/19 are predominantly expressed in the small intestine, gallbladder, kidney, skin, cartilage, and brain [36,100,101].
Previous studies confirmed that BA or FXR agonists induced FXR responsive element (FXRE) activation and FGF19-dependent repression of CYP7A1 (and therefore BA synthesis) in human hepatocytes [102]. A further proof was that Fgf15-knockout mice and intestine-specific Fxr-knockout mice stimulated by FXR agonists could not repress CYP7A1 [103].
FGF19 displays two daily peaks (about 3pm and 9pm). The serum peak of FGF15/19 follows the increase of BA in the small intestine and increases about 1.5–2.0 h after the rise of postprandial serum levels of BA [104,105]. FGF19 is a small 25 kDA molecule with fasting levels varying from 49 to 590 pg per mL. Following CDCA activation of the intestinal FXR, serum levels of FGF19 increase by +250%. FGF19 half-life is 30 min short, likely dependent on renal elimination [106]. FGF19 circulating levels can either decrease or increase in extrahepatic cholestasis, inflammatory bowel disease, kidney disease, BA malabsorption, obesity, and liver steatosis. Therapies involving FGF19 seem promising. However, the translational value of these attractive therapeutic tools should be carefully verified in terms of possible hepatic tumorigenesis, as a consequence of their mitogenic potential [107,108]. The effect likely involves the FGF19-FGFR4 interaction, since blocking the receptor can prevent, in rodents, the development of hepatocellular carcinoma [109].
The function of secreted FGFs requires the interaction with the transmembrane Klotho proteins found at the fibroblast growth factor receptors (FGFRs) [106]. FGF19 binds to the receptor complex composed of the FGF receptor 4 (FGFR4) and a co-receptor β-Klotho, both highly expressed in liver. Whereas the carboxy-terminal domain of FGF19 is the segment specifically recognized by β-Klotho, the amino-terminal region is specific for the FGF19–FGFR interaction [110].
Affinity of FGF15/19 is greater for the FGFR4, which is primarily expressed in the liver, than for FGFR1, primarily expressed in the white adipose tissue (WAT) [111,112]. FGFR4 is also expressed in other cell types as macrophages, HSCs and some central neurons [113]. The human FGF19 is not effective on mouse FGFR4 [114]. Binding of FGF19 to the FGFR4–β-Klotho complex activates a signaling pathway which includes the small guanosine triphosphatase Ras, the extracellular signal–regulated protein kinase 1, 2 (ERK1, ERK2), JUN N-terminal kinase (JNK), fibroblast growth factor receptor substrate 2α (FRS2α) [115], the growth factor receptor-bound protein 2 (GRB2), cAMP-response element-binding protein (CREB) which is de-phosphorylated and inactivated [89,106,116].
FGF19 is also able to modulate gallbladder volume, which contributes to the pulsatile and dynamic function of the enterohepatic circulation in the fasting and postprandial period [117,118]. During the postprandial period, the fat-stimulated CCK release from the upper gut enterocytes promotes smooth-muscle-mediated gallbladder contraction and ejection of concentrated bile into the duodenum. This phenomenon is well visible during the ultrasonographic functional study of time-dependent changes of gallbladder volume in response to a meal, by following the rhythmic alternation of emptying-refilling episodes [117,119,120,121]. Upon gallbladder contraction, BA in the duodenum inhibit further CCK production and, in turn, gallbladder contraction [122]. BA arriving in the distal ileum, moreover, will stimulate the release of FGF19 which, by activation of the gallbladder FGFR4-β-Klotho, promotes gallbladder relaxation ready for the next filling with dilute hepatic bile. FGF-19 interacts with the liver FGFR4-β-Klotho leading to suppression of the synthesis of hepatic BA [21].
6. FXR–FGF19 and BA Homeostasis
The hepatic and intestinal expression of FXR contributes to the regulation of BA synthesis and homeostasis [103], via a negative gut-liver feed-back sustained by the intestinal FGF15/19. In the liver, mild activation of FXR and SHP suppresses the CYP7A1/Cyp7a1 gene [40,123]. In parallel, the Cyp8b1 gene repression via FXR depends equally on both intestinal and liver FXR [103]. The key role of FXR in BA homeostasis is testified by the regulatory capacity of inducing the expression of key transporters active in the enterohepatic circulation of BA, i.e., BSEP, IBABP and OSTα/β, while suppressing NTCP and ASBT [124,125,126]. The BA-FXR-FGF15/19-BA sequence has a fundamental role in governing BA homeostasis. FGFR4 overexpression downregulates CYP7A1 and decreases the BA pool size [127].
By contrast, Knockout mice Fgf15-, Fgfr4- and β-klotho (Klb)- display impaired BA metabolism. Exogenous FGF19 fails to rescue this condition and fails to repress the function in CYP7A1 Fgfr4- and Klb-knockout animals [128,129]. The interaction between FGF15/19 and β-klotho-FGFR4 in the liver requires additional nuclear receptors to activate CYP7A1 repression. These nuclear receptors include the liver receptor homologue 1 (LRH-1) and hepatocyte nuclear factor 4α (HNF4α) [130] and SHP [131]. In addition to this complex picture, BA bind to ileal membrane-associated receptor GPBAR1. This activation is also associated with several extra-intestinal tissues to mediate host energy expenditure [132,133], glucose homeostasis [134], anti-inflammatory and immune responses [135,136].
7. FXR–FGF15/19 Pathway and Metabolic Effects
The axis FXR–FGF15/19 is also implicated in a number of metabolic functions which involve fat, glucose, glycogen, protein homeostasis, and energy expenditure [106].
7.1. Lipid Homeostasis
The pathways connecting cholesterol to BA synthesis are essential and contribute to the prevention of pathological amounts of cholesterol in the body [137], and also to metabolic homeostasis. Hepatic BA synthesis starts from cholesterol, and this step represents the main catabolic pathway of cholesterol metabolism in humans [21]. These pathways act through the “classic” neutral pathway (cholesterol 7α-hydroxylase, CYP7A1), contributing to about 75% of the total BA pool, and the “alternative” acidic pathway (sterol 27-hydroxylase, CYP27A1) contributing to about 25% of the total BA pool [21].
In this context, FXR has a relevant role in atherosclerotic risk factors, and strongly modulates the homeostasis of cholesterol due to the ability to inhibit CYP7A1.
Of note, increased serum levels of primary and secondary BA have been reported in patients with NAFLD, as compared with healthy controls [9]. This finding should suggest a role for the elevated BA production in the pathogenesis of NAFLD. This link, however, seems secondary to the increased proportion, in the BA pool, of the FXR antagonistic DCA, and to decreased levels of the FXR agonistic CDCA [9]. On the other hand, the cholesterol catabolism through BA synthesis has beneficial effects on metabolic homeostasis, and hepatic accumulation of cholesterol is a critical culprit for the development of NAFLD/NASH [138]. Transgenic mice overexpressing CYP7A1 in the liver are resistant to high-fat diet-induced obesity, fatty liver and insulin resistance, mainly through increased hepatic cholesterol catabolism and increased BA pool [139]. Further studies in FXR-deficient and CYP7A1-deficient mice confirmed the beneficial effects on hepatic inflammation of the increased CYP7A1 expression and BA synthesis secondary to activation of FXR [27].
FXR-deficient mice display increased content of hepatic lipids and increased plasma cholesterol and triglycerides [140]. By contrast the activation of FXR by the synthetic agonist GW4064 or by BA decrease liver steatosis and serum triglycerides [141,142]. Mechanisms include suppression of de novo fatty acid synthesis [141]. Primary hepatocytes incubated with the recombinant FGF19 protein display suppression of insulin-dependent stimulation of fatty acid synthesis with or without insulin [143]. FXR activation induces the SHP-mediated suppression of sterol regulatory element-binding protein 1c (SREBP1c), a step decreasing triglyceride load in hepatocytes [141]. This pathway also occurs with FGF19 via increased expression of SHP, increased signal transducer and activator of transcription 3 (STAT3) and decreased peroxisome proliferator-activated receptor γ coactivator-1β (PGC-1 β) [143]. An additional mechanism is the FXR-mediated activation of ApoCII gene transcription in the liver. This step, in turn, activates the lipoprotein lipase and decreases ApoCIII, a lipoprotein lipase inhibitor. The net effect is increased lipolysis of triglycerides in the vasculature [30,144]. Additional effects of FXR activation include increased triglyceride hydrolysis and clearance, increased fatty acid oxidation [28,29,30], and decreased hepatic export of very low-density lipoprotein (VLDL) [145].
Negative-correlations have been recently shown between stimulated intestinal FXR-FGF15 secretion and pathways linking hepatic cAMP regulatory element-binding protein (CREB) and peroxisome proliferator-activated receptor gamma, coactivator 1α (PGC1A). This effect indicates a possible downregulation of hepatic PGC1α by inactivation of CREB, finally resulting in suppression of fatty acid oxidation [146].
FXR contributes to the control of cholesterol homeostasis. Human cells lack enzymes able to degrade the ring structure of cholesterol and excess cholesterol must be excreted to avoid a harmful body accumulation. The excretion of cholesterol is mainly achieved in bile via the physiological carriers BA and phospholipids assembled with cholesterol as micelles and vesicles [147]. In addition, the concept of reverse cholesterol transport (RCT) encompasses the pathway transporting excess cholesterol accumulating within peripheral tissues back to the liver for biliary excretion into the feces [148]. FXR stimulates the RCT, as well as the trans-intestinal cholesterol excretion (TICE), the latter representing a significant alternative route to the biliary pathway of RCT. Mechanisms linking FXR to RCT include the changes in the BA pool hydrophobicity and their micellar function with cholesterol [148,149].
A critical role in the relationships between FXR, glucose, fatty acid metabolism and homeostasis seems to be played by the key enzyme pyruvate dehydrogenase kinase 4 (PDK4). In fact, an increased expression of this enzyme has been reported in FXR-null mice, and the inhibition of PDK4 expression alleviated lipid accumulation in hepatocytes both in vivo and in vitro [150].
7.2. Glucose Homeostasis and Gluconeogenesis
In both fed and fasted states, the liver is essential to maintain physiological blood glucose levels. Postprandially, exogenous glucose is used in liver to synthesize glycogen and triglycerides. During fasting, glucose is produced after gluconeogenesis and glycogenolysis. FGF15/19 release mediates the effects of FXR on glucose and lipid regulation. FGF15-deficient mice fail to maintain blood concentrations of glucose and normal postprandial amounts of liver glycogen [151]. Activation of the FXR-FGF15/19 axis is involved in glucose metabolism resulting in reduced hepatic gluconeogenesis and glycolysis, and paralleled by increased glycogen synthesis [152]. The mechanisms involve the downstream signaling via SHP-mediated suppression of transcription factors critically involved in gluconeogenesis [153]. Mechanisms might differ between species [154,155] or experimental models, and the role of FXR in glucose metabolism, although evident, is somewhat difficult to interpret. FXR-deficient mice show increased serum glucose levels and impaired glucose and insulin tolerance. By contrast, hepatic gluconeogenesis decreases, and insulin sensitivity improves if FXR is stimulated by feeding the primary BA cholate, treating with the agonist GW4064 or if FXR is overexpressed [142,156]. These results must be interpreted in the context of diet-induced obesity. In aged mice, hepatic loss of FXR and the FXR target SHP improves lipid and glucose homeostasis [157], likely due to an increase in autophagic gene expression (normally repressed by FXR postprandially) [157,158].
Additional sets of results originate from the manipulation of intestinal FXR. Mice with selective genetic deletion of intestinal FXR displayed protection against diet-induced diabetes and obesity [75,76].
Here, additional complex pathways might play a role. FXR might inhibit the secretion of the intestinal incretin Glucagon-like peptide 1 (GLP-1), as shown by administering the FXR agonist GW4064 [159]. The lack of intestinal FXR would therefore promote the intestinal L-cell release of GLP-1, which improves glycemic control and promotes weight loss.
In type 2 diabetic mice, the FXR antagonist Mebhydrolin improved glucose homeostasis by suppressing hepatic gluconeogenesis via FXR/miR-22-3p/PI3K/AKT/FoxO1 pathway. An additional effect was the promotion of glycogen synthesis through FXR/miR-22-3p/PI3K/AKT/GSK3β pathway [31].
Fexaramine is the intestine-specific FXR agonist that increases FGF15 signaling, leading to altered BA pool and increasing the level of the secondary tauro-conjugated BA tauro-litocholic acid (TLCA) [160]. TLCA is a strong agonist of the membrane-associated receptor GPBAR-1 in the intestinal (ileum, colon) L-cell, and this step induces the secretion of Glucagon-like peptide 1 (GLP-1). This step appears to explain the improved glucose tolerance and insulin resistance, as well as the stimulation of browning of white adipocytes and energy expenditure i.e., produced heat through uncoupled electron transport in the mitochondria [161,162] by fexaramine in mice [163]. Additional pathways are likely active, since fexaramine treatment in GPBAR-1-deficient mice was also associated with increased energy expenditure [162].
The enterokine FGF15/19 also plays a role in glucose metabolism. FGF19 acts independently from the activity of insulin or the protein kinase Akt. FGF19 acts through a mitogen-activated protein kinase signaling pathway that activates components of the protein translation machinery and stimulates glycogen synthase activity. The processes are tightly regulated to maintain glucose homeostasis. As insulin, FGF15/19 inhibits gluconeogenic gene expression [105]. However, insulin acts through Akt-dependent phosphorylation and subsequent degradation of FOXO1, a transcription factor involved in fasting-mediated induction of gluconeogenic gene expression. Conversely, FGF19 operates promoting dephosphorylation and inactivation of the transcription factor cAMP regulatory element-binding protein (CREB). This effect blunts the expression of peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) and other genes involved in hepatic metabolism, such as glucose-6-phosphatase (G6pase). In human and rat hepatocytes and in mouse livers, activation of FXR increased glucose levels via expression of phosphoenolpyruvate carboxykinase (PEPCK) [105,164]. In turn, the overexpression of PGC-1α blocks the inhibitory effect of FGF15/19 on the expression of gluconeogenic gene. In support of this function, mice lacking FGF15 are not able to maintain proper blood concentrations of glucose [151].
Notably, mice treated with FGF19 or overexpressing FGF19 have a lower body weight despite elevated food intake. Mice had repression of acetyl-CoA carboxylase 2 (ACC2) and stearoyl-CoA desaturase 1 (SCD1), enhanced energy expenditure and were protected against diet-induced obesity. Decreased ACC2, in turn, decreases mitochondrial Malonyl-CoA levels, and is associated with the upregulation of Carnitine palmitoyltransferase I (CPT1), more availability of fatty acids for oxidation [165].
In addition, FGF19 inhibits the synthesis of hepatic fatty acid after suppressing SREBP1c activity [143]. The translational meaning of the above-mentioned studies requires extensive validation [166]. A role for FGF19 at the level of the central nervous system in improving glycemia and peripheral insulin signaling is also possible [167], and points to novel therapeutic targets with antidiabetic drugs. FGF15/19 works in tune with insulin (serum peak of 15 min), as a postprandial regulator of hepatic carbohydrate homeostasis.
7.3. FGF19 and Role in Glycogen Synthesis
Hepatic glycogen synthesis is also under the regulation of FGF15/19. In line with this function and compared to control wild-type mice, Fgf15 knockout mice become glucose intolerant and store half as much glycogen in the liver. The defect is rescued by administration of FGF19 [151]. Diabetic mice lacking insulin have defective glycogen storage but FGF19 treatment rescues hepatic glycogen concentrations to normal levels. These models suggest that FGF19 activates insulin-independent pathways in regulating glycogen metabolism [151]. To promote the synthesis of glycogen and proteins, FGF19 activates the Ras/ERK pathway, whereas insulin activates the PI3K/Akt pathway [151].
Glycogen synthesis in the liver is regulated negatively by glycogen synthase kinase (GSK) 3α and GSK3β. The mechanism is modulated by the effects of BA on phosphorylation and inhibition of glycogen synthase (GS). Phosphorylation also inactivates GSK3 kinases, thus preventing the inhibition of GS and increasing glycogen synthesis. FGF19 acts directly on the liver and induces the phosphorylation of both GSK3α (Ser21) and GSK3β (Ser9), in parallel with a reduced phosphorylation of Ser641 and Ser645. In this scenario, GS activity increases, liver glycogen content increases by about 30% without effect on liver cholesterol or triglycerides, as well as insulin or glucagon level. In accord with a direct function of FGF19 on the hepatocyte and glycogen synthesis, it is apparent that fed Fgf15−/− mice had >50% less hepatic glycogen and impaired glucose uptake from the circulation, as compared with wild-type animals. FGF19 administration rescued this phenotype completely [151].
Notably, maximal serum insulin levels occur within 1 h of a meal, serum FGF19 levels peak about 3 h after a meal [104] and, in human subjects, liver glycogen levels peak ~4 h after a meal [168]. The conclusive picture which arises from this experimental scenario is that insulin and FGF19 work in a coordinated temporal fashion, and that this synergy facilitates the postprandial storage of nutrients. FGF19, as compared to other anabolic enterokines, namely incretins, GLP-1 and GIP, appears to mimic insulin action independently and without stimulating its release.
7.4. FGF19 and Role in Protein Synthesis
In mice, the acute administration of FGF19 increases total protein synthesis by 18% and synthesis of albumin by 40%, while prolonged FGF19 administration increases levels of plasma albumin by 10% [151]. FGF19 stimulates hepatic protein synthesis with the phosphorylation of the ERK1/2, phosphorylation of eIF4B, eIF4E and the ribosomal protein S6 kinase (S6K1), an mTOR- dependent master regulator of muscle cell growth and therefore muscle weight [169]. In primary hepatocytes, the activation of FXR by the agonist INT-747 stimulates amino acids catabolism. In vivo, FXR activation increased ammonium clearance through induction of ureagenesis and glutamine synthesis [170]. In humans, the links between FGF19 and protein synthesis might play a critical role in muscle homeostasis. Lower FGF-19 levels and higher FGF-21 levels have been reported in elderly patients with- as compared to those without sarcopenia, impacting muscle strength [171]. This finding is paralleled by results deriving from animal models, in which antibiotic therapy induced skeletal muscle atrophy secondary to microbial dysbiosis, aberrant BA metabolism and inhibition of the FXR-FGF15 signaling. Of note, in this model, skeletal muscle loss was partly reversed by administration of FGF19 [172].
7.5. FGF19 and Role in Energy Expenditure
FGF19 has profound effects on overall metabolism and energy expenditure. The key role of FGF19 in maintaining the physiological homeostasis is testified at various levels and by different models. In the animal models using transgenic mice which express human FGF19 and have decreased fat mass. Mice did not become obese or diabetic when fed a high fat diet. This outcome appears to be the consequence of increased energy expenditure. Likely, FGF19 may increase energy expenditure via increase in brown adipose tissue (BAT) mass. In addition, reduced liver triglyceride levels derive from decreased liver expression of acetyl coenzyme A carboxylase 2 (ACC2) and, in turn, decreased levels of mitochondrially associated malonyl CoA levels and increased activity of carnitine palmitoyl transferase 1 (CPT1), with increased availability of fatty acids for β oxidation [173]. FGF19 increased metabolic rate in mice fed a high fat diet, and this effect was paralleled by reduced body weight and diabetes in leptin-deficient mice [165]. In the central nervous system FGF19 plays an additional role since it improves insulin sensitivity by reducing the activity of hypothalamic agouti-related peptide (AGRP)/neuropeptide Y (NPY) [174]. Thus, FGF15 and FGF19 appear to have several metabolic effects which can be summarized as weight loss, increased insulin sensitivity and thermogenesis. Serum concentrations of cholesterol and triglyceride also decrease with stimulation of FGF15/19. The translational value of such findings, however, requires additional evidence in humans. Postprandially, FGF19 and insulin promote protein and glycogen synthesis and suppresses hepatic gluconeogenesis [89]. At variance with insulin, however, lipogenesis is not stimulated by FGF19, due to different cellular signaling pathways. Taken together, this evidence suggests that FGF15/19 brings beneficial effects on metabolic syndrome and treatment of NASH. Modified FGF19 is beneficial in mouse models of NASH and cholestasis [175]. To harmonize somewhat conflicting results concerning FXR regulation of lipid/glucose metabolism, and possibly metabolic syndrome, it should be noted that FXR knockout mice develop fatty liver, increased serum concentrations of free fatty acids (FFAs) and glucose, and display insulin resistance [156]. In addition, overexpression or activation of hepatic FXR in diabetic db/db and wild-type mice was associated with decreased serum concentrations of FFAs and glucose, and with increased insulin sensitivity [142].
8. The Role of FXR in Liver Disease
8.1. Inflammation and Fibrosis
Experimental evidence show that FXR can have an anti-inflammatory function in the liver by reducing cholestasis and levels and accumulation of toxic BA [32,176]. The migration and infiltration of monocytes/macrophages is regulated by the chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) [177]. The synthetic FXR agonist WAY-362450 reduced MCP-1 expression and inflammatory cell infiltration in the liver of mice put on methionine-choline deficient (MCD) diet, which induces NASH. The reduction of hepatic fibrosis by WAY-362450 treatment was paralleled by a reduction in hepatic gene expression of fibrosis markers and was specifically linked to the presence of FXR [32]. The expression of various pro-inflammatory genes is induced by the transcription factor Nuclear factor kappa-light chain enhancer of activated B cell (NF-κB) [178]. Treatment with lipopolysaccharide (LPS) induced in FXR KO mice a strong hepatic inflammation, with massive liver necrosis and marked increase in hepatic cytokine signaling molecules inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and interferon-γ (IFN-γ) [176]. The use of FXR agonists in HepG2 cells and mouse primary hepatocytes suppressed NF-κB mediated inflammation in a FXR-dependent manner [176]. Notably, FXR activation mitigates the development of liver fibrosis due to an increased anti-fibrotic gene expression in hepatic stellate cells (HSCs). The pathway is based on the activation of FXR, induction of SHP, increased expression of peroxisomal proliferator activated receptor γ (PPARγ) with inactivation of HSCs [179,180].
In an animal model of cholestasis induced by parenteral nutrition, the administration of the FXR agonist GW4064 prevented hepatic injury and cholestasis. Treated animals showed a normalization of serum BA levels which were associated with increased expression of canalicular bile, of sterol and phospholipid transporters, and with suppression of macrophage recruitment and activation. These effects were secondary to the restoration of hepatic FXR signaling [181].
FGF15/19 can also play a role in inflammation. FGF15 deficiency is associated with the loss of FGF15-mediated suppression of BA synthesis, increased FXR activation, and reduced hepatic fibrosis [182]. In a human HSC cell line, LX2, FGF19 suppresses inflammation through modulating inhibitor of nuclear factor kappa B (IκB) activity without suppression of fibrogenic gene expression [182].
8.2. Cholestasis
The BA pool is made of amphipathic BA with either protective or toxic effects depending on the tight maintenance of the hydrophilic-hydrophobic balance [183]. Notably, BA structure is responsible for their double signaling function as protective molecules (i.e., proliferation in hepatocytes) or toxic molecules [184]. The process of cholestasis starts with decreased or abolished bile flow. Several conditions may be responsible for cholestasis, but the ultimate step leading to cholestatic liver injury is the intrahepatic accumulation of BA, a situation also defined as BA overload. Chronic BA overload will inevitably cause a progressive BA-induced damage, with the aid of additional toxic components [20,185]. Excess BA retention generates hepatocyte damage, steatosis, fibrosis and even liver tumorigenesis [21,22].
BA overload can develop at the hepatic and/or systemic level [186] when trans-hepatocyte BA flow is scarce because of decreased sinusoidal and/or canalicular BA transport [184,187], or in the presence of bile duct obstruction. Both extended (>70%) partial hepatectomy and massive hepatocyte loss [184,188,189,190,191] are a predisposing condition to BA overload [191,192]. Therefore, BA spillover will be evident into the systemic circulation, as confirmed in both animal and human models [19,187,193,194,195,196].
In the model where mice are put on a LCA-enriched diet [197] or to develop impaired pathways of SIRT1 [44], FGF receptors [198,199], SHP [200], FGF15 [201] studies show the expansion of a hydrophobic BA pool which interferes with the liver repairment capacity. Another example is that Cyp2c70-/- mice develop a more human-like hydrophobic BA pool with liver inflammation [202] and altered FXR signaling [203]. This is due to the presence of a more hydrophilic bile in mice, as compared to humans, since the enzyme CYP2c70 (missing in humans) converts CDCA to the more hydrophilic muricholic acid [204].
In line with such evidence, BSEP/abcb11-/- mice develop a mild non-progressive cholestasis [197], likely due to an enrichment of the BA pool with hyper-hydroxylated, less hydrophobic, and less cytotoxic BA [205]. In humans, progressive familial intrahepatic cholestasis (PFIC) is an autosomal recessive disease causing 15% of cases of neonatal cholestasis. The PFIC2 form has a mutation in the ABCB 11 gene encoding BSEP. PFIC patients develop a disrupted secretion of BA from the hepatocytes, progressive hepatic fibrosis, liver cirrhosis and end stage liver disease requiring liver transplantation [206]. Thus, beneficial effects during cholestatic liver injury might derive from an increased hydrophilic profile of the BA pool, either in the animal model [207] and in PFIC children (i.e., by increasing the content of tetrahydroxy BA) [208]. BA overload will cause damage [209] via deranged mitochondrial function [210,211]. This step involves the release of cytochrome c and release of excessive reactive oxygen species (ROS) [212], plasma membrane damage, necrosis, apoptosis and cell death [213]. The indirect damage of excess BA retention involves inflammatory changes associated with cytokine release, neutrophils recruitment and macrophages activation [209,214].
Up to a certain limit, FXR-dependent adaptive responses will try to counteract the cholestatic liver damage. A first preventive mechanism includes the intrahepatic activation of the physiological BA sensor FXR by BA. Both basolateral and canalicular BA transporters and enzymes governing BA synthesis and conjugation are involved [193,215] and contribute to counteracting BA overload in the hepatocytes [216]. FXR-dependent mechanisms can modulate hepatocyte cell cycle progression [192], with a possible regulation of BA homeostasis [114], alcohol-related liver injury [217] and liver regeneration after partial hepatectomy [218,219]. FXR pathways can involve cholangiocyte cell cycle progression [219,220] during BA synthesis suppression [221,222]. The pro-inflammatory effect of BA is mediated by the intracellular assembly of the inflammasome, but FXR exhibits anti-inflammatory effects, because of the interaction with the NLRP3 protein machinery [223]. The modulatory effects of FXR on BA homeostasis and hepatocyte/cholangiocyte cell cycle progression are important to decrease liver BA uptake (inhibition of BA transporters), BA synthesis (suppression of CYP7A1/Cyp7a1 gene [36,123] and Cyp8b1 gene [103]), while stimulating BA excretion (activation of BA transporters [124,125]).
FXR is also expressed by mast cells infiltrating the liver during cholestasis, and promoting hepatic fibrosis. As shown by an animal model, FXR expressed by mast cells has a critical role in hepatic damage and ductular reaction, acting on BA homeostasis through disrupted intestinal and biliary FXR/FGF15 signaling [224].
9. FXR as a Therapeutic Target?
The tight interaction between BA-FXR-FGF19 and reflections on metabolism and inflammation in health and disease, has boosted the interest of research on the role of potential therapeutic approaches focusing on FGF19 and FXR stimulation.
9.1. FGF19 and FGF21 Variants
Scarce studies have focused on the role of FGF19 variants devoid of stimulatory effect on FGFR4 (because of potential tumorigenic activity). Mimetic molecules include chimeric molecules FGF19-4, 5 and 6 (mutagenesis in the N-terminus and in the heparin binding domains in amino acids 38–42), FGF19v (Conjugation of amino acids 1–20 of FGF21 with amino acids 25–194 of FGF19), M70 (3 amino acid substitutions and 5 amino acid deletions in the N-terminus). Such molecules are still able to modulate glucose metabolism but studies are restricted to animal models [225,226,227] with scarce studies in humans [226,228]. The translational value of FGF19 variants is still under evaluation and further evidence is awaited in this field.
FGF21 belong to the FGF19 subfamily of endocrine FGFs and, as FGF19, require the co-receptor β Klotho for binding and signaling through the FGF receptors [229]. As shown in experimental models, FGF21 is a negative regulator of BA synthesis, being able to decrease BA levels in the liver and in the small intestine, with a significant reduction of the BA pool size. These findings are paralleled by decreased colonic and fecal BA, with a concomitant increase in fecal cholesterol and fatty acid excretions [230]. The modulatory effect of FGF21 on BA synthesis seems independent of the FXR/FGF15 pathway [231]. Due to beneficial metabolic effects, FGF21 variants are emerging as promising therapeutic tools in metabolic diseases. The FGF21 variant LY2405319 has been tested in patients with obesity and type 2 diabetes, with beneficial effects on lipid metabolism, body weight, fasting insulin and adiponectin levels, but no significant reduction in fasting glucose levels [228]. Another long-acting FGF21 variant, PF-05231023, induced a body weight loss, an improvement in plasma lipoprotein profile and in adiponectin levels in overweight/obese subjects with type 2 diabetes, without effects on blood glucose. In the treated cohort, however, possible effects on bone formation and resorption were noticed [232]. In obese patients with hypertriglyceridaemia on atorvastatin, with or without type 2 diabetes, the same molecule reduced triglycerides in the absence of weight loss. In the treated group, however, serious adverse effects were noticed, causing the discontinuation of therapy in some participants [233]. Pegbelfermin (BMS-986036), a PEGylated FGF21 analog has been used in obese patients with type 2 diabetes predisposed to fatty liver, resulting in an improvement of the lipid profile, of fibrosis biomarkers and adiponectin levels, in the presence of mild adverse events [234]. Pegbelfermin has been also used in a phase 2a study in obese/overweight subjects and in NASH patients. In this trial, treatment significantly reduced hepatic fat fraction, in the presence of mild side effects (mainly diarrhea and nausea) [235]. Of note, Pegbelfermin promotes a significant reduction from baseline in serum concentrations of DCA and conjugates in patients with NASH and in overweight/obese adults, with a possible modulatory role on the synthesis of secondary BA, also acting on gut microbiome [236]. The extent of total BA decrease recorded in the cited study (about 20–30%) [236] is comparable to that obtained following treatment with FXR agonists [237], with the advantage to be selective for secondary BA [236].
9.2. FXR Agonists
Clinical trials are on the way using FXR modulators in chronic liver diseases such as primary biliary cholangitis, in cholestasis, nonalcoholic steatohepatitis (NASH), obesity, metabolic syndrome, hypertriglyceridemia, lipodystrophy. Additional trials include bile acid diarrhea, hepatitis B or association with reactivation of latent pro-virus (clinical trials.gov). Table 1 lists the main FXR agonists/modulators explored, at the moment, in clinical trials or experimental studies.

Table 1.
FXR agonists/modulators mainly evaluated in clinical or experimental studies.
Table 1.
FXR agonists/modulators mainly evaluated in clinical or experimental studies.
| Obeticholic acid (approved for the treatment of primary biliary cholangitis) | [238,239,240,241] |
| Tropifexor (LJN452) | [242,243,244,245,246,247,248,249] |
| Cilofexor (GS-9674) | [250,251] |
| Vonafexor | [252] |
| Nidufexor | [253] |
| GW4064 | [254] |
| MET409 | [255] |
| TC-100 | [256] |
| BMS-986339 | [257] |
| HEC96719 | [258] |
| WAY-450 | [259] |
| WAY-362450 | [260] |
| Px-102 | [261] |
| Px-104 | [262] |
| TERN-101 | [263] |
| EDP-305 | [264] |
| INT-767 (FXR-TGR5 dual agonist) | [25,265,266] |
Most solid studies are reporting results with the steroidal molecule obeticholic acid (OCA), the 6α-Ethyl-Chenodeoxycholic Acid (6-ECDCA) and the non-steroidal Tropifexor (LJN452). OCA is modified from CDCA and is about 100 times more potent [238].
In male Wistar rats with cholestasis receiving i.v. infusion of LCA to impair bile flow, OCA alone did not induce cholestasis and during co-infusion with LCA, reversed the impairment of bile flow and protected hepatocytes from necrosis [238]. OCA is approved for the treatment of primary biliary cholangitis (PBC) especially in the subgroup of patients who fail to respond to UDCA [239,240,241].
Zucker (fa/fa) rats have a loss of function mutation in the hunger hormone leptin receptor [267], and suffer from hyperphagia and hyperleptinaemia resulting in obesity, insulin resistance, diabetes and fatty liver resembling NAFLD [267]. OCA treatment (10 mg/kg/day) over 7 weeks–meaning FXR activation -reversed insulin resistance, prevented body weight gain and fat deposition in the liver, reduced serum levels of triglycerides and aminotransferases, and improved liver histology [267]. Evidence suggests that the activation of FXR by OCA improves hyperglycemia through enhanced insulin secretion and glucose uptake by the liver. OCA increases insulin secretion in mouse β-TC6 cells and human pancreatic islets, while in β-TC6 cells OCA induces AKT (Protein Kinase B)-dependent translocation of glucose transporter 2 (GLUT2). This step increases glucose uptake by these cells [268]. The anti-inflammatory and anti-fibrotic properties of OCA became evident while investigating the NF-κB signaling pathway. In HepG2 cells stimulated with LPS or tumor necrosis factor alpha (TNFα), pretreatment with OCA at 3 uM inhibited the expression of cytokine-inducible enzymes COX-2 and iNOS [176]. OCA inhibited iNOS in LPS-treated primary mouse hepatocytes [176].
The agonist activity of OCA on FXR operates also in humans and trials are ongoing [33]. In NASH, OCA showed promising results, with an improvement of liver blood tests, a decreased extent of liver fibrosis and with no worsening of NASH [241]. The efficacy and safety of OCA have been evaluated in patients with type 2 diabetes and NAFLD by a phase IIa study using placebo (n = 23), 25 mg OCA (n = 20), or 50 mg OCA (n = 21) for 6 weeks (ClinicalTrials.gov, Number: NCT00501592). Treatment was well tolerated, and beneficial effects of OCA were observed in both groups with reduced γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT) levels, dose-related weight loss, improved insulin sensitivity, elevated FGF19 serum levels, decreased BA precursor 7a-hydroxy-4-cholesten-3-one (C4) and endogenous BA. Results confirmed the OCA-dependent activation of FXR in human [269].
In the phase IIb trial Farnesoid X Receptor Ligand OCA in NASH Treatment (FLINT), OCA was tested in a multicenter, double-blind, randomized fashion. Non-cirrhotic NASH received 25 mg OCA (n = 141) daily or placebo (n = 142) for 72 weeks. OCA, compared with placebo, improved biochemical and histological features of NASH. NAFLD activity score improved by two points or greater (without worsening of fibrosis) in 45% of OCA patients vs. 21% in the placebo group. Side effects with OCA included pruritus and dyslipidemia [237].
The use of OCA in NASH patients awaits further safety and efficacy data [270]. The phase III trial REGENERATE (by Intercept) was designed to assess the effects of OCA on liver histology and clinical outcomes in 2065 biopsy- confirmed NASH patients. Groups included OCA 10 mg, 25 mg, or placebo for a total duration of six years. The interim analysis was performed by liver biopsy at 18 months in February 2019, and OCA achieved the primary endpoint of improving liver fibrosis without worsening of NASH [271].
Results from a REGENERATE 18-Month Interim Analysis in patients with NASH showed improvement of NASH and in health-related quality of life, despite the occurrence of mild pruritus early after the start of OCA therapy [272].
Tropifexor (LJN452), a non-steroidal FXR agonists reduced oxidative stress, steatosis, inflammation and fibrosis in the mouse models of NASH [242]. Tropifexor was safe and well-tolerated following single oral doses ranging from 10 μg to 3 mg. Circulating levels of FGF19 protein increased transiently in a dose-dependent fashion, pointing to a potent on-target FXR agonist activity [273]. Tropifexor evidenced ed a favourable tolerability and pharmacokinetic profile and induced a dose-dependent increase of FGF19 level, with no change in serum lipids in healthy volunteers [243]. In patients with primary bile acid diarrhoea, treatment with tropifexor 60 µg once daily showed an acceptable safety and tolerability profile. Tropifexor treatment decreased 7α-hydroxy-4-cholesten-3-one and bile acid concentration while increased FGF19 level [244]. Ongoing listed studies are focusing on efficacy and tolerability of tropifexor in patients with mild, moderate, severe hepatic impairment or NASH and fibrosis, and primary biliary cholangitis [274]. Recently, in patients with primary biliary cholangitis and inadequate response to UDCA, tropifexor induced a significant improvement in cholestatic markers, in the presence of mild to moderate adverse effects (mainly pruritus) [249].
Thus, further studies are needed with this agonist [245,246,247,248], also with respect to side effects, as compared with OCA. Furthermore, the safety, efficacy, tolerability, pharmacokinetics, pharmacodynamics of novel non-bile acid FXR agonist were intensively examined [275].
Cilofexor (GS-9674), another non-steroidal FXR agonist, evidenced safety and efficacy in non-cirrhotic NASH patients [250]. In detail, a double-blind, placebo-controlled, phase 2 trial was conducted on 140 NASH patients receiving orally cilofexor 100 mg, 30 mg, or placebo once daily for 24 weeks (ClinicalTrials.gov No. NCT02854605.). The study showed that cilofexor was safe and associated with significant attenuation of hepatic steatosis (lipid accumulation), liver enzymes (AST, ALT, and GGT), and bile acids synthesis without any significant alteration of fasting plasma levels of FGF19. In addition, cilofexor treatment was able to reduce different markers of fibrosis and liver stiffness which indicate that cilofexor may have potential antifibrotic effects. In noncirrhotic subjects with large-duct primary sclerosing cholangitis who underwent a 96-week open-label extension of a phase II trial, cilofexor treatment was safe and led to a significant improvement of liver biochemistry and biomarkers of cholestasis and cellular injury [251].
Despite promising results, the side effects and major obstacles of FXR agonist (mainly atherogenic risk, pruritogenic potency) and FGF19 variants (mainly increased appetite, diarrhea and nausea [232,235,276], altered bone homeostasis [232], increased blood pressure and heart rate [233]) still represent a significant matter of concern. Research needs to develop FXR agonists or modulators with beneficial anti-inflammatory effects and minimal metabolic actions. From this point of view, according to available results, the risk-benefit profile of FXR agonists can be modulated by structural optimization of FXR agonist. Harrison et al. [255] evaluated the effect of structurally optimized FXR agonist MET409 in NASH patients. At 12-week post treatment, MET409 reduced fat liver content and bile acid with no significant increase in FGF19 level. The low dose (50 mg) treatment of MET409 showed a 16% pruritus rate and a 9% LDL-C increase but with favorable reduction of fat liver content. The study suggested that the improvements in efficacy/tolerability of FXR agonist treatment could be achieved.
At the molecular level, one oral dose of Px-102, a nonsteroidal FXR agonist, decreased the synthesis of BA in healthy volunteers independently of increases in FGF19 [261] suggesting that that activation of hepatic FXR suppress BA synthesis, independently of FGF19.
The safety and efficacy of FXR agonist Vonafexor for the treatment of chronic hepatits B were also evaluated by a double-blind, placebo-controlled trial. Vonafexor was well tolerated overall with moderate gastrointestinal adverse effects (pruritus occurred in ~60% of subjects with twice-daily treatment compared with 16% in subjects with once-daily treatment). Vonafexor alone or combined with interferon-α2a showed an anti-viral effect by reducing HBV markers [252]. In non-diabetic NAFLD patients, a 4-weeks of treatment with the non-steroidal FXR agonist PX-104 improved liver enzymes and insulin sensitivity with no serious adverse events. Interestingly, PX-104 influenced gut microbiota by reducing Coriobacteriaceae abundance and total fecal BAs [262]. TERN-101 (FXR agonist) treatment in healthy volunteer was well-tolerated and showed a reduction in bile acid synthesis (decreases in serum 7α-hydroxy-4-cholesten-3-one) [263].
In an animal model (mice with obstructed BA flow), the novel FXR agonist TC-100 reduced BA pool size in serum and bile, with a shift to a more hydrophilic composition. The changes in BA pool were paralleled by a prevention of intestinal mucosal damage and by a progressive increase in Firmicutes:Bacteroidotes ratio, with increased Akkermansia muciniphila abundance [256].
In a recent preclinical study, the BMS-986339, a novel non-bile acid FXR agonist showed potent in vitro and in vivo activation of FXR, with anti-fibrotic efficacy and tissue-selective effects in vivo. The safety of this molecule, however, still requires to be adequately tested in humans [257].
HEC96719, another novel tricyclic FXR agonist, exhibits a potency of FXR activation superior to obeticholic acid, higher FXR selectivity and more favorable tissue distribution in liver and intestine. Although HEC96719 seems a promising tool in NASH treatment, its efficacy and safety profiles need further confirmations [258].
Interestingly, in a rat model of NAFLD/NASH, the administration of dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, alleviated NASH also through a reduced de novo lipogenesis mediated by an upregulation of FXR/SHP and a downregulation of LXRα/SREBP-1c in the liver [277]. These findings parallel a recent observation in patients with T2D and NAFLD, in whom treatment with SGLT2-inhibitors was linked with improvement of liver steatosis and fibrosis markers and circulating pro-inflammatory and redox status [278].
The oral FXR agonist EDP-305 has been proposed for the treatment of NASH. Recent results from a double-blind phase II study in patients with fibrotic NASH showed a significant decrease in ALT levels and liver fat content in 12 weeks, with adverse effects (mainly pruritus) leading to drug discontinuation in about 20% of patients [264].
Interesting perspectives derive from experimental studies using FXR-TGR5 dual agonists [25,265,266]. Recently, in a mouse model of NASH, treatment with the FXR-TGR5 dual agonist INT-767 was able to prevent the progression of disease, with beneficial effects on liver mitochondrial function, lipid homeostasis, BA composition (i.e., decreased hydrophobicity index), liver inflammation and gut dysbiosis [25].
10. Conclusions and Future Perspectives
Cholesterol is eliminated from human body via biliary secretion and synthesis of primary BA, which are essential for digestion of fat and signaling functions on important receptors. BA homeostasis is a complex scenario which requires several pathways and dynamic events to maintain BA qualitative/quantitative profiles and function in health (Figure 1).
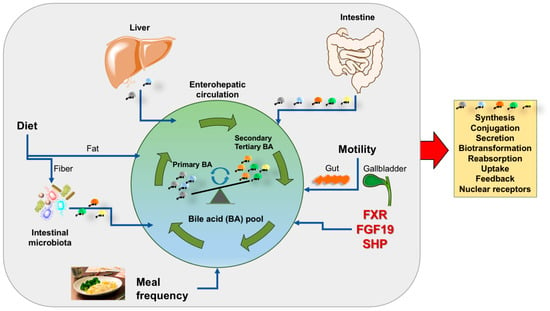
Figure 1.
Summary of key factors involved in the composition of the bile acid pool. Several dynamic events are active daily either in the fasting and postprandial period (grey area) and contribute to shape the qualitative/quantitative profile of the bile acid pool, its maintenance and expansion. Such events work in concert with bile acid synthesis and related steps (orange box). Abbreviations: FGF-19, fibroblast growth factor 19; FXR, farnesoid X receptor; SHP, small heterodimer partner; primary bile acids are cholic and chenodeoxycholic acid (grey); secondary bile acids are deoxycholic acid (orange) and lithocholic acid (green); tertiary bile acid is ursodeoxycholic acid (yellow).
The effect of BA works in concert with the activation of the nuclear receptor FXR. This step, in turn, controls BA synthesis, excretion, and reabsorption. These pathways minimize over-accumulation of potentially toxic BA in the liver. Activation of FXR also brings several metabolic effects by regulating lipid metabolism, reducing hepatic gluconeogenesis, glycolysis, and increasing glycogen synthesis. In parallel, FXR activation has anti- inflammatory properties during liver injury. The FXR-FGF15/19 axis increases energy expenditure and glycogen synthesis and decreases gluconeogenesis and fatty acid synthesis (Figure 2). FXR and FGF19 have become promising targets for the treatment of NASH. Further studies are required in this important field.
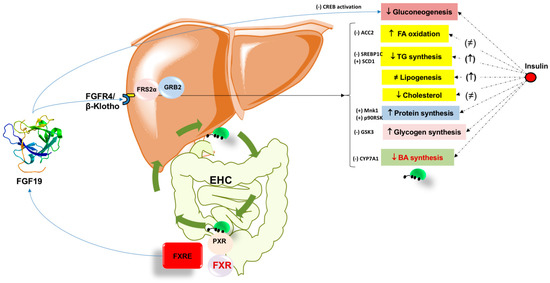
Figure 2.
Summary of the metabolic effects of Fibroblast growth factor 19 (FGF19) in the liver. The role of insulin is shown for comparison. Bile acids during the enterohepatic circulation (EHC) activate intestinal FXR promoting FGF19 secretion. Circulating FGF19 signaling requires the presence of β-Klotho [279] which is the fibroblast growth factor receptor 4 (FGFR4) co-receptor required for liver-specific FGF19 actions [112]. The tissue-specific expression pattern of β-klotho and FGFR isoforms determines FGF19 metabolic activity [116]. In the hepatocyte, the signaling events allow the recruitment of cytosolic adaptors such as fibroblast growth factor receptor substrate 2α (FRS2α) and growth factor receptor-bound protein 2 (GRB2). The ultimate metabolic effects of FGF19 are depicted on the right within the boxes, with major regulatory elements listed. Legend; ACC2, acetyl-CoA carboxylase 2; CYP7A1, cholesterol-7α-hydroxylase; CREB cAMP-response element-binding protein; FXR, farnesoid X receptor; FXRE, FXR responsive element; GSK3, glycogen synthase kinase 3; Mnk1, protein kinase; p90 ribosomal S6 kinase; PXR, pregnane X receptor; PXRE, PXR response element; SCD1; stearoyl-CoA desaturase 1; SREBP1C, sterol regulatory element-binding protein 1C. (+) activation; (-) inhibition; ≠, unchanged.
Author Contributions
Conceptualization, A.D.C. and P.P.; literature review, A.D.C., L.B., J.B., M.K., G.G., F.S.; writing—original draft preparation, A.D.C., J.B., G.G.; writing—review and editing; supervision, H.H.W., D.Q.-H.W., F.S., P.P.; funding acquisition, P.P.; F.S. has particularly participated to the paragraph on bile acid kinetics. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has been partly supported by funding to P.P. from the European Union’s Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie Grant Agreement No. 722619 (FOIE GRAS), Grant Agreement No. 734719 (mtFOIE GRAS), Grant Regione Puglia, CUP H99C20000340002 (Fever Apulia), and Grant EUROSEEDS Uniba-S56-By-products Sustainable Recovery 4 Health (BSR-4H): University of Bari Aldo Moro, 2022. The project SYSTEMIC “an integrated approach to the challenge of sustainable food systems: adaptive and mitigatory strategies to address climate change and malnutrition”, Knowledge hub on Nutrition and Food Security, has received funding from national research funding parties in Belgium (FWO), France (INRA), Germany (BLE), Italy (MIPAAF), Latvia (IZM), Norway (RCN), Portugal (FCT), and Spain (AEI) in a joint action of JPI HDHL, JPI-OCEANS and FACCE-JPI launched in 2019 under the ERA-NET ERA-HDHL (n° 696295).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are indebted to Gerard van Berge-Henegouwen, Dan Dumitrascu, Gustav Paumgartner, Thomas LaMont, Giuseppe Palasciano, Karel van Erpecum for continuous and restless scientific discussion. The authors thank Rosa De Venuto, Paola De Benedictis, Domenica Di Palo, Ilaria Farella, Elisa Lanza for their longstanding and skilful professional technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 3β-HSD | 3β-hydroxy-D5-C27-steroid dehydroxylase |
| 6-ECDCA | 6a-ethyl-chenodeoxycholic acid |
| ACC2 | Acetyl CoA carboxylase |
| AGRP | Agouti-related peptide |
| AKR1C4 | 3a-hydroxysteroid dehydrogenase |
| AKR1D1 | D4-3-oxosteroid 5b-reductase |
| AKT | Protein kinase B |
| ALT | Alanine aminotransferase |
| AMPs | Antimicrobial peptides |
| ASBT | Apical sodium-dependent bile salt transporter |
| BA | Bile acids |
| BAAT | Bile acid-CoA:amino acid N-acyltransferase |
| BACS | Bile acid CoA synthase |
| ABCB11 | Bile acid export pump |
| BAT | Brown adipose tissue |
| BSEP | Bile salt export pump |
| BSH | Bile salt hydrolase |
| C4 | 7a-hydroxy-4-cholesten-3-one |
| CA | Cholic acid |
| CCK | Cholecystokinin |
| CCl4 | Carbon tetrachloride |
| CDCA | Chenodeoxycholic acid |
| CF | Cycling frequency |
| COX-2 | Cylcooxygenase-2 |
| CREB | cAMP regulatory element binding protein |
| CYP7A1 | Cholesterol-7α-hydroxylase |
| CYP27A1 | Cytochrome P450 27A1 |
| CYP2A12 | Cytochrome P450 2A12 |
| CYP2C70 | Cytochrome P450 2C70 |
| CYP7A1 | Cytochrome P450 7A1 |
| CYP7B1 | Cytochrome P450 7B1 |
| CYP8B1 | Cytochrome P450 8B1 |
| DBD | DNA binding domain |
| DCA | Deoxycholic acid |
| eIF4B | Eukaryotic initiation factor 4B |
| eIF4E | Eukaryotic initiation factor 4E |
| ER2 | Everted hexanucleotide repeat separated by 2 nucleotides |
| ERK | Extracellular-signal-regulated protein kinase |
| FAR | Fractional absorption rate |
| FFA | Free fatty acid |
| FGF | Fibroblast growth factor |
| FGF15 | Fibroblast growth factor 15 |
| FGF19 | Fibroblast growth factor 19 |
| FGFR | Fibroblast growth factor receptor |
| FGFR1 | Fibroblast growth factor receptor 1 |
| FGFR4 | Fibroblast growth factor receptor 4 |
| FLINT | Farnesoid X receptor ligand obeticholic acid in NASH treatment |
| FXR | Farnesoid X receptor |
| FXRE | Farnesoid X receptor response element |
| FTR | Fractional turnover rate |
| GGT | Gamma-glutamyltransferase |
| GLP-1 | Glucagon-like peptide 1 |
| GLUT2 | Glucose transporter 2 |
| GPBAR-1 | G-protein-coupled bile acid receptor-1 |
| GS | Glycogen synthase |
| GSK3a | Glycogen synthase kinase 3a |
| HREs | Hormone response elements |
| HSC | Hepatic stellate cell |
| IkB | Inhibitor of nuclear factor kappa B |
| IBABP | Intestinal bile acid binding protein |
| IFN-g | Interferon-gamma |
| iNOS | Inducible nitric oxide synthase |
| IR1 | Inverted hexanucleotide repeat separated by 1 nucleotide |
| KO | Knockout |
| LBD | Ligand-binding domain |
| LCA | Lithocholic acid |
| LRH-1 | Liver receptor homologue 1 |
| LPS | Lipopolysaccharide |
| JNK | JUN N-terminal kinase |
| JUN | Jun proto-oncogene |
| MAGs | Metagenome-assembled genomes |
| MCA | Muricholic acid |
| MCD | Methionine-choline deficient |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MRP2 | Multidrug resistance-associated protein 2 |
| mTOR | Mammalian target of rapamycin |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NF-kB | Nuclear factor kappa-light chain enhancer of activated B cell |
| NPY | Neuropeptide Y |
| NR s | Nuclear receptors |
| NLRP3 | NACHT LRR and PYD domains-containing protein 3 |
| NTCP | Sodium taurocholate co-transporting polypeptide |
| OATP | Organic anion transporting polypeptide |
| OCA | Obeticholic acid |
| OSTα | Organic solute transporter alpha |
| OSTb | Organic solute transporter beta |
| P90RSK | p90 ribosomal S6 kinase |
| PBC | Primary biliary cirrhosis |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PFIC | Progressive familial intrahepatic cholestasis |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PGC-1b | Peroxisome proliferator-activated receptor gamma coactivator 1-beta |
| PPARγ | Peroxisomal proliferator activated receptor gamma |
| PXR | Pregnane X receptor |
| RCT | Reverse cholesterol transport |
| RXR | Retinoid X receptor |
| S1PR2 | Sphingosine-1-phosphate receptor 2 |
| S6K1 | S6 kinase beta-1 |
| SCD1 | Stearoyl-CoA desaturase 1 |
| SIBO | Small intestinal bacterial overgrowth |
| SHP | Small heterodimer partner |
| SREBP1c | Sterol regulatory element-binding protein 1c |
| STAT3 | Signal transducer and activator of transcription 3 |
| SULT2A1 | Sulfotransferase 2A1 |
| TCA | Taurocholic acid |
| TDCA | Taurodeoxycholic acid |
| TGR5 | Takeda G-protein receptor 5 |
| TLCA | Taurolithocholic acid |
| TNFα | Tumor necrosis factor alpha |
| VDR | Vitamin D receptor |
| VLDL | Very low-density lipoprotein |
| WAT | White adipose tissue |
References
- Gonzalez, F.J. Nuclear receptor control of enterohepatic circulation. Compr. Physiol. 2012, 2, 2811–2828. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR signaling in the enterohepatic system. Mol. Cell Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Shaw, R.P.H.; Anakk, S. Bile Acids—A Peek Into Their History and Signaling. Endocrinology 2022, 163, bqac155. [Google Scholar] [CrossRef]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Guillot, A.; Guerri, L.; Feng, D.; Kim, S.J.; Ahmed, Y.A.; Paloczi, J.; He, Y.; Schuebel, K.; Dai, S.; Liu, F.; et al. Bile acid-activated macrophages promote biliary epithelial cell proliferation through integrin alphavbeta6 upregulation following liver injury. J. Clin. Investig. 2021, 131, e132305. [Google Scholar] [CrossRef] [PubMed]
- Hylemon, P.B.; Takabe, K.; Dozmorov, M.; Nagahashi, M.; Zhou, H. Bile acids as global regulators of hepatic nutrient metabolism. Liver Res. 2017, 1, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Pavlovic, N.; Stanimirov, B.; Mikov, M. Bile Acids as Novel Pharmacological Agents: The Interplay Between Gene Polymorphisms, Epigenetic Factors and Drug Response. Curr. Pharm. Des. 2017, 23, 187–215. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jung, H.; Seok, S.; Zhang, Y.; Ma, J.; Li, T.; Kemper, B.; Kemper, J.K. MicroRNA-210 Promotes Bile Acid-Induced Cholestatic Liver Injury by Targeting Mixed-Lineage Leukemia-4 Methyltransferase in Mice. Hepatology 2020, 71, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Zimny, S.; Koob, D.; Li, J.; Wimmer, R.; Schiergens, T.; Nagel, J.; Reiter, F.P.; Denk, G.; Hohenester, S. Hydrophobic Bile Salts Induce Pro-Fibrogenic Proliferation of Hepatic Stellate Cells through PI3K p110 Alpha Signaling. Cells 2022, 11, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Pal Pathak, D.; Asthana, S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism. Int. J. Biol. Sci. 2020, 16, 2308–2322. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R. Bile acids and the metabolic syndrome. Ann. Clin. Biochem. 2019, 56, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Tang, D.; Yu, C.; Zhao, L. Progress in research on the roles of TGR5 receptor in liver diseases. Scand. J. Gastroenterol. 2021, 56, 717–726. [Google Scholar] [CrossRef]
- Wang, Y.; Aoki, H.; Yang, J.; Peng, K.; Liu, R.; Li, X.; Qiang, X.; Sun, L.; Gurley, E.C.; Lai, G.; et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 2017, 65, 2005–2018. [Google Scholar] [CrossRef]
- Nagahashi, M.; Yuza, K.; Hirose, Y.; Nakajima, M.; Ramanathan, R.; Hait, N.C.; Hylemon, P.B.; Zhou, H.; Takabe, K.; Wakai, T. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J. Lipid Res. 2016, 57, 1636–1643. [Google Scholar] [CrossRef]
- Garruti, G.; Wang, H.H.; Bonfrate, L.; de Bari, O.; Wang, D.Q.; Portincasa, P. A pleiotropic role for the orphan nuclear receptor small heterodimer partner in lipid homeostasis and metabolic pathways. J. Lipids 2012, 2012, 304292. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Garruti, G.; Vacca, M.; De Angelis, M.; Wang, D.Q. Bile Acids and GPBAR-1: Dynamic Interaction Involving Genes, Environment and Gut Microbiome. Nutrients 2020, 12, 3709. [Google Scholar] [CrossRef]
- Zollner, G.; Trauner, M. Mechanisms of cholestasis. Clin. Liver Dis. 2008, 12, 1–26. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Molina-Molina, E.; Lunardi Baccetto, R.; Calamita, G.; Palmieri, V.O.; Portincasa, P. Bile Acids and Cancer: Direct and Environmental-Dependent Effects. Ann. Hepatol. 2017, 16, s87–s105. [Google Scholar] [CrossRef] [PubMed]
- Panzitt, K.; Zollner, G.; Marschall, H.U.; Wagner, M. Recent advances on FXR-targeting therapeutics. Mol. Cell. Endocrinol. 2022, 552, 111678. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Shi, A.; Wei, Y.; Luo, X.; Xi, L. Farnesoid X Receptor as a Promising Therapeutic Target for Nonalcoholic Fatty Liver Disease (NAFLD) and the Current Development of Its Agonists. Discov. Med. 2021, 32, 113–121. [Google Scholar]
- Wang, X.X.; Xie, C.; Libby, A.E.; Ranjit, S.; Levi, J.; Myakala, K.; Bhasin, K.; Jones, B.A.; Orlicky, D.J.; Takahashi, S.; et al. The Role of FXR and TGR5 in Reversing and Preventing Progression of Western Diet-induced Hepatic Steatosis, Inflammation, and Fibrosis in Mice. J. Biol. Chem. 2022, 298, 102530. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yan, J.; Wu, L.; Wu, J.; Chen, Z.; Jiang, J.; Chen, Z.; He, B. Probiotics Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats via Gut Microbiota/FXR/FGF15 Signaling Pathway. J. Immunol. Res. 2021, 2021, 2264737. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pathak, P.; Boehme, S.; Chiang, J.L. Cholesterol 7alpha-hydroxylase protects the liver from inflammation and fibrosis by maintaining cholesterol homeostasis. J. Lipid Res. 2016, 57, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Pineda Torra, I.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.C.; Staels, B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, A.; Claudel, T.; Martin, G.; Brozek, J.; Kosykh, V.; Darteil, R.; Hum, D.W.; Fruchart, J.C.; Staels, B. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004, 566, 173–177. [Google Scholar] [CrossRef]
- Claudel, T.; Inoue, Y.; Barbier, O.; Duran-Sandoval, D.; Kosykh, V.; Fruchart, J.; Fruchart, J.C.; Gonzalez, F.J.; Staels, B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology 2003, 125, 544–555. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; He, A.; Wang, S.; Chen, Y.; Lu, J.; Lv, J.; Li, S.; Wang, J.; Qian, M.; et al. Mebhydrolin ameliorates glucose homeostasis in type 2 diabetic mice by functioning as a selective FXR antagonist. Metabolism 2021, 119, 154771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, Q.; Harnish, D.C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 2009, 51, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.D.; Veidal, S.S.; Fensholdt, L.K.D.; Rigbolt, K.T.G.; Papazyan, R.; Nielsen, J.C.; Feigh, M.; Vrang, N.; Young, M.; Jelsing, J.; et al. Combined obeticholic acid and elafibranor treatment promotes additive liver histological improvements in a diet-induced ob/ob mouse model of biopsy-confirmed NASH. Sci. Rep. 2019, 9, 9046. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F.; Lutjohann, D. Dynamics of the enterohepatic circulation of bile acids in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G55–G66. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Gofflot, F.; Murzilli, S.; D’Orazio, A.; Salvatore, L.; Pellegrini, F.; Nicolucci, A.; Tognoni, G.; Copetti, M.; Valanzano, R.; et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology 2010, 138, 636–648, 648.e1-12. [Google Scholar] [CrossRef]
- Liu, H.; Hu, C.; Zhang, X.; Jia, W. Role of gut microbiota, bile acids and their cross-talk in the effects of bariatric surgery on obesity and type 2 diabetes. J. Diabetes Investig. 2018, 9, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.K. Regulation of FXR transcriptional activity in health and disease: Emerging roles of FXR cofactors and post-translational modifications. Biochim. Biophys. Acta 2011, 1812, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef]
- Nishimaki-Mogami, T.; Une, M.; Fujino, T.; Sato, Y.; Tamehiro, N.; Kawahara, Y.; Shudo, K.; Inoue, K. Identification of intermediates in the bile acid synthetic pathway as ligands for the farnesoid X receptor. J. Lipid Res. 2004, 45, 1538–1545. [Google Scholar] [CrossRef]
- Pircher, P.C.; Kitto, J.L.; Petrowski, M.L.; Tangirala, R.K.; Bischoff, E.D.; Schulman, I.G.; Westin, S.K. Farnesoid X receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 2003, 278, 27703–27711. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, J.L.; Barbier-Torres, L.; Fernandez-Alvarez, S.; Gutierrez-de Juan, V.; Monte, M.J.; Halilbasic, E.; Herranz, D.; Alvarez, L.; Aspichueta, P.; Marin, J.J.; et al. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology 2014, 59, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Schmidt, S.; Holmstrom, S.R.; Makishima, M.; Yu, R.T.; Cummins, C.L.; Mangelsdorf, D.J.; Kliewer, S.A. AKR1B7 is induced by the farnesoid X receptor and metabolizes bile acids. J. Biol. Chem. 2011, 286, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Bellafante, E.; Moschetta, A. Master regulation of bile acid and xenobiotic metabolism via the FXR, PXR and CAR trio. Front. Biosci. (Landmark Ed.) 2008, 14, 4719–4745. [Google Scholar] [CrossRef]
- Wagner, M.; Zollner, G.; Trauner, M. New molecular insights into the mechanisms of cholestasis. J. Hepatol. 2009, 51, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.; Clerbaux, L.A.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. 2022, 4, 100387. [Google Scholar] [CrossRef] [PubMed]
- Dommett, R.; Zilbauer, M.; George, J.T.; Bajaj-Elliott, M. Innate immune defence in the human gastrointestinal tract. Mol. Immunol. 2005, 42, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 2013, 8, e59260. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Gao, B.; Chi, L.; Mahbub, R.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017, 30, 996–1005. [Google Scholar] [CrossRef]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef]
- Joly, C.; Gay-Queheillard, J.; Leke, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and in the rat. Environ. Sci. Pollut. Res. Int. 2013, 20, 2726–2734. [Google Scholar] [CrossRef]
- Joly Condette, C.; Bach, V.; Mayeur, C.; Gay-Queheillard, J.; Khorsi-Cauet, H. Chlorpyrifos Exposure During Perinatal Period Affects Intestinal Microbiota Associated With Delay of Maturation of Digestive Tract in Rats. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 30–40. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef]
- Garruti, G.; Di Ciaula, A.; Wang, H.H.; Wang, D.Q.; Portincasa, P. Cross-Talk Between Bile Acids and Gastro-Intestinal and Thermogenic Hormones: Clues from Bariatric Surgery. Ann. Hepatol. 2017, 16, s68–s82. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Long, S.L.; Gahan, C.G.M.; Joyce, S.A. Interactions between gut bacteria and bile in health and disease. Mol. Asp. Med. 2017, 56, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Parseus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Stahlman, M.; Greiner, T.U.; Perkins, R.; Backhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Fukiya, S.; Islam, K.B.; Ooka, T.; Ogura, Y.; Hayashi, T.; Hagio, M.; Ishizuka, S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012, 3, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, F.; Liu, Y.; Gu, Z.; Zhang, L.; Lee, J.; He, L.; Vatsalya, V.; Zhang, H.G.; Deng, Z.; et al. Probiotic-derived nanoparticles inhibit ALD through intestinal miR194 suppression and subsequent FXR activation. Hepatology 2022. [Google Scholar] [CrossRef]
- Boursier, J.; Diehl, A.M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog. 2015, 11, e1004559. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.; Reily, M.D.; Lu, Z.; Lehman-McKeeman, L.D.; Cherrington, N.J. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharm. 2013, 268, 132–140. [Google Scholar] [CrossRef]
- Chavez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Liquori, G.E.; Mastrodonato, M.; Rossi, R.; Scillitani, G.; Gena, P.; Portincasa, P.; Calamita, G.; Ferri, D. Altered membrane glycoprotein targeting in cholestatic hepatocytes. Eur. J. Clin. Investig. 2010, 40, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Karpen, S.J.; Dawson, P.A.; Arrese, M.; Trauner, M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017, 65, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Bartolí, R.; Planas, R.; Hofmann, A.F.; Viñado, B.; Hagey, L.R.; Hernández, J.M.; Mañé, J.; Alvarez, M.A.; Ausina, V. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef]
- Clements, W.; Parks, R.; Erwin, P.; Halliday, M.; Barr, J.; Rowlands, B. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut 1996, 39, 587–593. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef]
- Sun, L.; Pang, Y.; Wang, X.; Wu, Q.; Liu, H.; Liu, B.; Liu, G.; Ye, M.; Kong, W.; Jiang, C. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm. Sin. B 2019, 9, 702–710. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.J.; Summanen, P.; Downes, J.; Roberts, M.C.; Wexler, H.; Finegold, S.M. Bilophila wadsworthia, gen. nov. and sp. nov., a unique gram-negative anaerobic rod recovered from appendicitis specimens and human faeces. J. Gen. Microbiol. 1989, 135, 3405–3411. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.W.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10(−/−) mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Ling, L.; Dinh, D.M.; DePaoli, A.M.; Lieu, H.D.; Harrison, S.A.; Sanyal, A.J. The Commensal Microbe Veillonella as a Marker for Response to an FGF19 Analog in NASH. Hepatology 2021, 73, 126–143. [Google Scholar] [CrossRef]
- Danneskiold-Samsøe, N.B.; Barros, H.D.d.F.Q.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Junior, M.R.M. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, R.; Zhou, X.; Liang, X.; Campbell, D.J.; Zhang, X.; Zhang, L.; Shi, R.; Wang, G.; Pandak, W.M.; et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 2014, 60, 908–918. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Guo, G.L.; Lambert, G.; Negishi, M.; Ward, J.M.; Brewer, H.B., Jr.; Kliewer, S.A.; Gonzalez, F.J.; Sinal, C.J. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003, 278, 45062–45071. [Google Scholar] [CrossRef]
- Kir, S.; Kliewer, S.A.; Mangelsdorf, D.J. Roles of FGF19 in liver metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 139–144. [Google Scholar] [CrossRef]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Loomba, R. Review article: The emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment. Pharm. 2012, 36, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.S.; Anderson, K.A.; Dewey, A.M.; Kavesh, M.H.; Green, R.M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid Res. 2011, 52, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Hata, T.; Yamazoe, Y.; Yoshinari, K. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 2014, 443, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Sabba, C.; Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 2016, 15, 51–69. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008, 237, 18–27. [Google Scholar] [CrossRef]
- Itoh, N. Hormone-like (endocrine) Fgfs: Their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res 2010, 342, 1–11. [Google Scholar] [CrossRef]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef]
- Nishimura, T.; Utsunomiya, Y.; Hoshikawa, M.; Ohuchi, H.; Itoh, N. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim. Biophys. Acta 1999, 1444, 148–151. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Xie, M.H.; Holcomb, I.; Deuel, B.; Dowd, P.; Huang, A.; Vagts, A.; Foster, J.; Liang, J.; Brush, J.; Gu, Q.; et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine 1999, 11, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.; Wang, D.Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Ahn, S.H.; Inagaki, T.; Choi, M.; Ito, S.; Guo, G.L.; Kliewer, S.A.; Gonzalez, F.J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 2007, 48, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Lundasen, T.; Galman, C.; Angelin, B.; Rudling, M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006, 260, 530–536. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Boney-Montoya, J.; Choi, M.; He, T.; Sunny, N.E.; Satapati, S.; Suino-Powell, K.; Xu, H.E.; Gerard, R.D.; Finck, B.N.; et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011, 13, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Kliewer, S.A.; Mangelsdorf, D.J. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012, 26, 312–324. [Google Scholar] [CrossRef]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Barcena-Varela, M.; Elizalde, M.; Jimenez, M.; Rodriguez-Ortigosa, C.M.; Corrales, F.J.; Fernandez-Barrena, M.G.; et al. Fibroblast Growth Factor 15/19 in Hepatocarcinogenesis. Dig. Dis. 2017, 35, 158–165. [Google Scholar] [CrossRef]
- Uriarte, I.; Latasa, M.U.; Carotti, S.; Fernandez-Barrena, M.G.; Garcia-Irigoyen, O.; Elizalde, M.; Urtasun, R.; Vespasiani-Gentilucci, U.; Morini, S.; de Mingo, A. Ileal FGF 15 contributes to fibrosis-associated hepatocellular carcinoma development. Int. J. Cancer 2015, 136, 2469–2475. [Google Scholar] [CrossRef]
- French, D.M.; Lin, B.C.; Wang, M.; Adams, C.; Shek, T.; Hotzel, K.; Bolon, B.; Ferrando, R.; Blackmore, C.; Schroeder, K.; et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS ONE 2012, 7, e36713. [Google Scholar] [CrossRef]
- Wu, X.; Lemon, B.; Li, X.; Gupte, J.; Weiszmann, J.; Stevens, J.; Hawkins, N.; Shen, W.; Lindberg, R.; Chen, J.L.; et al. C-terminal tail of FGF19 determines its specificity toward Klotho co-receptors. J. Biol. Chem. 2008, 283, 33304–33309. [Google Scholar] [CrossRef]
- Yang, C.; Jin, C.; Li, X.; Wang, F.; McKeehan, W.L.; Luo, Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS ONE 2012, 7, e33870. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Wang, M.; Blackmore, C.; Desnoyers, L.R. Liver-specific activities of FGF19 require Klotho beta. J. Biol. Chem. 2007, 282, 27277–27284. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.D.; Guo, G.L. Pharmacologic Modulation of Bile Acid-FXR-FGF15/FGF19 Pathway for the Treatment of Nonalcoholic Steatohepatitis. Handb. Exp. Pharm. 2019, 256, 325–357. [Google Scholar] [CrossRef]
- Naugler, W.E.; Tarlow, B.D.; Fedorov, L.M.; Taylor, M.; Pelz, C.; Li, B.; Darnell, J.; Grompe, M. Fibroblast Growth Factor Signaling Controls Liver Size in Mice with Humanized Livers. Gastroenterology 2015, 149, 728–740.e15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, C.; Chang, J.Y.; You, P.; Li, Y.; Jin, C.; Luo, Y.; Li, X.; McKeehan, W.L.; Wang, F. Hepatocyte FRS2α is essential for the endocrine fibroblast growth factor to limit the amplitude of bile acid production induced by prandial activity. Curr. Mol. Med. 2014, 14, 703–711. [Google Scholar] [CrossRef]
- Kurosu, H.; Choi, M.; Ogawa, Y.; Dickson, A.S.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Rosenblatt, K.P.; Kliewer, S.A.; Kuro, O.M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007, 282, 26687–26695. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Dumitrascu, D.L.; Portincasa, P. Gastrointestinal defects in gallstone and cholecystectomized patients. Eur. J. Clin. Investig. 2019, 49, e13066. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Lunardi Baccetto, R.; Wang, D.Q.; de Bari, O.; Krawczyk, M.; Portincasa, P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur. J. Clin. Investig. 2018, 48, e12958. [Google Scholar] [CrossRef]
- Diella, G.; Di Ciaula, A.; Lorusso, M.P.; Summo, C.; Caggiano, G.; Caponio, F.; Montagna, M.T.; Portincasa, P. Distinct Effects of two Almond Cultivars on Agreeability and Gastrointestinal Motility in Healthy Subjects: More than mere Nutraceuticals. J. Gastrointest. Liver Dis. JGLD 2018, 27, 31–39. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Peeters, T.L.; Stolk, M.F.; Nieuwenhuijs, V.B.; Portincasa, P.; Depoortere, I.; van Berge Henegouwen, G.P.; Akkermans, L.M. Motilin induces gall bladder emptying and antral contractions in the fasted state in humans. Gut 1998, 42, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Di Ciaula, A.; Wang, H.H.; Palasciano, G.; van Erpecum, K.J.; Moschetta, A.; Wang, D.Q. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008, 47, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Wang, L.; Chiang, J.Y.; Zhang, Y.; Klaassen, C.D.; Guo, G.L. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 2012, 56, 1034–1043. [Google Scholar] [CrossRef]
- Zollner, G.; Wagner, M.; Moustafa, T.; Fickert, P.; Silbert, D.; Gumhold, J.; Fuchsbichler, A.; Halilbasic, E.; Denk, H.; Marschall, H.U.; et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: Role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G923–G932. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.F.; Eloranta, J.J.; Vavricka, S.R.; Kullak-Ublick, G.A. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G476–G485. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Dawson, P.A.; Sinal, C.J.; Sehayek, E.; Gonzalez, F.J.; Breslow, J.; Ananthanarayanan, M.; Shneider, B.L. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 2003, 278, 19909–19916. [Google Scholar] [CrossRef]
- Yu, C.; Wang, F.; Jin, C.; Huang, X.; McKeehan, W.L. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J. Biol. Chem. 2005, 280, 17707–17714. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujimori, T.; Furuya, A.; Satoh, J.; Nabeshima, Y.; Nabeshima, Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J. Clin. Investig. 2005, 115, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, K.; Maeda, R.; Urakawa, I.; Yamazaki, Y.; Tanaka, T.; Ito, S.; Nabeshima, Y.; Tomita, T.; Odori, S.; Hosoda, K.; et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Kir, S.; Zhang, Y.; Gerard, R.D.; Kliewer, S.A.; Mangelsdorf, D.J. Nuclear receptors HNF4alpha and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 2012, 287, 41334–41341. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Choi, S.E.; Seok, S.M.; Yang, L.; Zuercher, W.J.; Xu, Y.; Willson, T.M.; Xu, H.E.; Kemper, J.K. Ligand-dependent regulation of the activity of the orphan nuclear receptor, small heterodimer partner (SHP), in the repression of bile acid biosynthetic CYP7A1 and CYP8B1 genes. Mol. Endocrinol. 2011, 25, 1159–1169. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5: A valuable metabolic target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Broeders, E.P.; Nascimento, E.B.; Havekes, B.; Brans, B.; Roumans, K.H.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Schoonjans, K. TGR5 and Immunometabolism: Insights from Physiology and Pharmacology. Trends Pharm. Sci. 2015, 36, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Schaap, F.G.; Trauner, M.; Jansen, P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef]
- Wang, D.Q.H.; Neuschwander-Tetri, B.A.; Portincasa, P. The Biliary System, Second Edition; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2017; Volume 8, p. i-178. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Owsley, E.; Matozel, M.; Hsu, P.; Novak, C.M.; Chiang, J.Y. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 2010, 52, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Sinal, C.J.; Tohkin, M.; Miyata, M.; Ward, J.M.; Lambert, G.; Gonzalez, F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000, 102, 731–744. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Damron, H.A.; Hillgartner, F.B. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J. Biol. Chem. 2009, 284, 10023–10033. [Google Scholar] [CrossRef]
- Kast, H.R.; Nguyen, C.M.; Sinal, C.J.; Jones, S.A.; Laffitte, B.A.; Reue, K.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: A molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 2001, 15, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Schoenfield, L.J.; Lachin, J.M. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: The National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann. Intern. Med. 1981, 95, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, Y.; Luo, Y.; Zhao, J.; Feng, C.; Xue, L.; Xu, J.; Wang, Q.; Yan, T.; Xiao, P.; et al. Intestinal farnesoid X receptor signaling controls hepatic fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159089. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.H.; Neuschwander-Tetri, B.A.; Portincasa, P. The Biliary System. Colloq. Ser. Integr. Syst. Physiol. Mol. Funct. 2012, 4, 1–148. [Google Scholar] [CrossRef]
- Wang, D.Q.; Portincasa, P.; Tso, P. Transintestinal cholesterol excretion: A secondary, nonbiliary pathway contributing to reverse cholesterol transport. Hepatology 2017, 66, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e6. [Google Scholar] [CrossRef]
- Deng, W.; Fan, W.; Tang, T.; Wan, H.; Zhao, S.; Tan, Y.; Oware, K.A.; Tan, J.; Li, J.; Qu, S. Farnesoid X Receptor Deficiency Induces Hepatic Lipid and Glucose Metabolism Disorder via Regulation of Pyruvate Dehydrogenase Kinase 4. Oxid. Med. Cell Longev. 2022, 2022, 3589525. [Google Scholar] [CrossRef] [PubMed]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011, 331, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lu, Y.; Li, X.Y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharm. Sin. 2015, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Daitoku, H.; Shimamoto, Y.; Matsuzaki, H.; Hirota, K.; Ishida, J.; Fukamizu, A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 2004, 279, 23158–23165. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Hart, S.N.; Kong, B.; Fang, J.; Zhong, X.B.; Guo, G.L. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 2010, 51, 1410–1419. [Google Scholar] [CrossRef]
- Zhan, L.; Liu, H.X.; Fang, Y.; Kong, B.; He, Y.; Zhong, X.B.; Fang, J.; Wan, Y.J.; Guo, G.L. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS ONE 2014, 9, e105930. [Google Scholar] [CrossRef]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Investig. 2006, 116, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.; Zhou, Y.; Kim, E.Y.; Lee, J.M.; Saha, P.K.; Anakk, S.; Moore, D.D. Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice. Hepatology 2017, 66, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Wagner, M.; Xiao, R.; Kim, K.H.; Feng, D.; Lazar, M.A.; Moore, D.D. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 2014, 516, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Cully, M. FXR and JAK step up to BAT. Nat. Rev. Drug Discov. 2015, 14, 91. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Stayrook, K.R.; Bramlett, K.S.; Savkur, R.S.; Ficorilli, J.; Cook, T.; Christe, M.E.; Michael, L.F.; Burris, T.P. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 2005, 146, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; John, L.M.; Adams, S.H.; Yu, X.X.; Tomlinson, E.; Renz, M.; Williams, P.M.; Soriano, R.; Corpuz, R.; Moffat, B.; et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004, 145, 2594–2603. [Google Scholar] [CrossRef]
- Somm, E.; Jornayvaz, F.R. Fibroblast Growth Factor 15/19: From Basic Functions to Therapeutic Perspectives. Endocr. Rev. 2018, 39, 960–989. [Google Scholar] [CrossRef]
- Morton, G.J.; Matsen, M.E.; Bracy, D.P.; Meek, T.H.; Nguyen, H.T.; Stefanovski, D.; Bergman, R.N.; Wasserman, D.H.; Schwartz, M.W. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Investig. 2013, 123, 4799–4808. [Google Scholar] [CrossRef]
- Taylor, R.; Magnusson, I.; Rothman, D.L.; Cline, G.W.; Caumo, A.; Cobelli, C.; Shulman, G.I. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J. Clin. Investig. 1996, 97, 126–132. [Google Scholar] [CrossRef]
- Benoit, B.; Meugnier, E.; Castelli, M.; Chanon, S.; Vieille-Marchiset, A.; Durand, C.; Bendridi, N.; Pesenti, S.; Monternier, P.A.; Durieux, A.C.; et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat. Med. 2017, 23, 990–996. [Google Scholar] [CrossRef]
- Massafra, V.; Milona, A.; Vos, H.R.; Ramos, R.J.J.; Gerrits, J.; Willemsen, E.C.L.; Ramos Pittol, J.M.; Ijssennagger, N.; Houweling, M.; Prinsen, H.; et al. Farnesoid X Receptor Activation Promotes Hepatic Amino Acid Catabolism and Ammonium Clearance in Mice. Gastroenterology 2017, 152, 1462–1476.e10. [Google Scholar] [CrossRef]
- Bag Soytas, R.; Suzan, V.; Arman, P.; Emiroglu Gedik, T.; Unal, D.; Cengiz, M.; Bolayirli, I.M.; Suna Erdincler, D.; Doventas, A.; Yavuzer, H. Association of FGF-19 and FGF-21 levels with primary sarcopenia. Geriatr. Gerontol. Int. 2021, 21, 959–962. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, J.; Li, Y.; Yang, F.; Yu, H.; Xue, M.; Zhang, F.; Jiang, X.; Ji, X.; Bao, Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann. Med. 2021, 53, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Jo, Y.H.; Li, X.; Schwartz, G.J.; Zhang, Y.; Dun, N.J.; Lyu, R.M.; Blouet, C.; Chang, J.K.; Chua, S., Jr. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 2014, 3, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Learned, R.M.; Rossi, S.J.; DePaoli, A.M.; Tian, H.; Ling, L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017, 1, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Chen, W.D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Rollins, B.J. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: Therapeutic opportunities and controversies. Microcirculation 2003, 10, 247–257. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-ĸB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 247–257. [Google Scholar]
- Renga, B.; Mencarelli, A.; Migliorati, M.; Cipriani, S.; D’Amore, C.; Distrutti, E.; Fiorucci, S. SHP-dependent and -independent induction of peroxisome proliferator-activated receptor-gamma by the bile acid sensor farnesoid X receptor counter-regulates the pro-inflammatory phenotype of liver myofibroblasts. Inflamm. Res. 2011, 60, 577–587. [Google Scholar] [CrossRef]
- Fiorucci, S.; Rizzo, G.; Antonelli, E.; Renga, B.; Mencarelli, A.; Riccardi, L.; Morelli, A.; Pruzanski, M.; Pellicciari, R. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor γ contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J. Pharmacol. Exp. Ther. 2005, 315, 58–68. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Ghosh, S.; Anderson, A.L.; Devereaux, M.W.; Balasubramaniyan, N.; D’Alessandro, A.; Orlicky, D.J.; Suchy, F.J.; Shearn, C.T.; Sokol, R.J. Pharmacologic activation of hepatic farnesoid X receptor prevents parenteral nutrition-associated cholestasis in mice. Hepatology 2022, 75, 252–265. [Google Scholar] [CrossRef]
- Schumacher, J.D.; Kong, B.; Wu, J.; Rizzolo, D.; Armstrong, L.E.; Chow, M.D.; Goedken, M.; Lee, Y.H.; Guo, G.L. Direct and Indirect Effects of Fibroblast Growth Factor (FGF) 15 and FGF19 on Liver Fibrosis Development. Hepatology 2020, 71, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Carulli, N.; Bertolotti, M.; Carubbi, F.; Concari, M.; Martella, P.; Carulli, L.; Loria, P. Review article: Effect of bile salt pool composition on hepatic and biliary functions. Aliment. Pharm. 2000, 14 (Suppl. 2), 14–18. [Google Scholar] [CrossRef] [PubMed]
- Merlen, G.; Ursic-Bedoya, J.; Jourdainne, V.; Kahale, N.; Glenisson, M.; Doignon, I.; Rainteau, D.; Tordjmann, T. Bile acids and their receptors during liver regeneration: “Dangerous protectors”. Mol. Asp. Med. 2017, 56, 25–33. [Google Scholar] [CrossRef]
- Donkers, J.M.; Roscam Abbing, R.L.P.; van de Graaf, S.F.J. Developments in bile salt based therapies: A critical overview. Biochem. Pharm. 2019, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Doignon, I.; Julien, B.; Serriere-Lanneau, V.; Garcin, I.; Alonso, G.; Nicou, A.; Monnet, F.; Gigou, M.; Humbert, L.; Rainteau, D.; et al. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J. Hepatol. 2011, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, R.; Patsenker, E.; de Gottardi, A.; Stickel, F.; Montani, M.; Stroka, D.; Candinas, D.; Beldi, G. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation 2014, 97, 892–900. [Google Scholar] [CrossRef]
- Miura, T.; Kimura, N.; Yamada, T.; Shimizu, T.; Nanashima, N.; Yamana, D.; Hakamada, K.; Tsuchida, S. Sustained repression and translocation of Ntcp and expression of Mrp4 for cholestasis after rat 90% partial hepatectomy. J. Hepatol. 2011, 55, 407–414. [Google Scholar] [CrossRef]
- Bhushan, B.; Borude, P.; Edwards, G.; Walesky, C.; Cleveland, J.; Li, F.; Ma, X.; Apte, U. Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am. J. Pathol. 2013, 183, 1518–1526. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, Y.; Wang, L.; Jin, W.; Liu, N.; Pan, H.; Liu, L.; Wagman, L.; Forman, B.M.; Huang, W. FXR regulates liver repair after CCl4-induced toxic injury. Mol. Endocrinol. 2010, 24, 886–897. [Google Scholar] [CrossRef]
- Huang, W.; Ma, K.; Zhang, J.; Qatanani, M.; Cuvillier, J.; Liu, J.; Dong, B.; Huang, X.; Moore, D.D. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 2006, 312, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Pean, N.; Doignon, I.; Garcin, I.; Besnard, A.; Julien, B.; Liu, B.; Branchereau, S.; Spraul, A.; Guettier, C.; Humbert, L.; et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology 2013, 58, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E. Bile acid flux is necessary for normal liver regeneration. PLoS ONE 2014, 9, e97426. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, L.T.; Rietkerk, M.; van Lienden, K.P.; van den Esschert, J.W.; Schaap, F.G.; van Gulik, T.M. Bile salts predict liver regeneration in rabbit model of portal vein embolization. J. Surg. Res. 2012, 178, 773–778. [Google Scholar] [CrossRef][Green Version]
- Hoekstra, L.T.; van Lienden, K.P.; Schaap, F.G.; Chamuleau, R.A.; Bennink, R.J.; van Gulik, T.M. Can plasma bile salt, triglycerides, and apoA-V levels predict liver regeneration? World J. Surg. 2012, 36, 2901–2908. [Google Scholar] [CrossRef]
- Fickert, P.; Fuchsbichler, A.; Marschall, H.U.; Wagner, M.; Zollner, G.; Krause, R.; Zatloukal, K.; Jaeschke, H.; Denk, H.; Trauner, M. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am. J. Pathol. 2006, 168, 410–422. [Google Scholar] [CrossRef]
- Gilgenkrantz, H.; Tordjmann, T. Bile acids and FGF receptors: Orchestrators of optimal liver regeneration. Gut 2015, 64, 1351–1352. [Google Scholar] [CrossRef]
- Padrissa-Altes, S.; Bachofner, M.; Bogorad, R.L.; Pohlmeier, L.; Rossolini, T.; Bohm, F.; Liebisch, G.; Hellerbrand, C.; Koteliansky, V.; Speicher, T.; et al. Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut 2015, 64, 1444–1453. [Google Scholar] [CrossRef]
- Park, Y.J.; Qatanani, M.; Chua, S.S.; LaRey, J.L.; Johnson, S.A.; Watanabe, M.; Moore, D.D.; Lee, Y.K. Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology 2008, 47, 1578–1586. [Google Scholar] [CrossRef]
- Modica, S.; Petruzzelli, M.; Bellafante, E.; Murzilli, S.; Salvatore, L.; Celli, N.; Di Tullio, G.; Palasciano, G.; Moustafa, T.; Halilbasic, E.; et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 2012, 142, 355–365.e4. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 2020, 61, 54–69. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.F.; Verkade, E.; Mulder, N.L.; de Vries, H.D.; Huijkman, N.; Koehorst, M.; Boer, T.; Wolters, J.C.; Bloks, V.W.; van de Sluis, B.; et al. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice. J. Lipid Res. 2020, 61, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Fukami, T.; Masuo, Y.; Brocker, C.N.; Xie, C.; Krausz, K.W.; Wolf, C.R.; Henderson, C.J.; Gonzalez, F.J. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 2016, 57, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.; Forrest, D.; Liu, L.; Wang, R.; Tai, J.; Deo, A.; Ling, V.; Bandiera, S. Hepatic bile acid metabolism and expression of cytochrome P450 and related enzymes are altered in Bsep (−/−) mice. Mol. Cell Biochem. 2014, 389, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. Progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol. 2014, 4, 25–36. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Paumgartner, G.; Wahlstrom, A.; Schwabl, P.; Reiberger, T.; Leditznig, N.; Stojakovic, T.; Rohr-Udilova, N.; Chiba, P.; Marschall, H.U.; et al. Metabolic preconditioning protects BSEP/ABCB11(−/−) mice against cholestatic liver injury. J. Hepatol. 2017, 66, 95–101. [Google Scholar] [CrossRef]
- Lee, C.S.; Kimura, A.; Wu, J.F.; Ni, Y.H.; Hsu, H.Y.; Chang, M.H.; Nittono, H.; Chen, H.L. Prognostic roles of tetrahydroxy bile acids in infantile intrahepatic cholestasis. J. Lipid Res. 2017, 58, 607–614. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Inflammation and Cell Death During Cholestasis: The Evolving Role of Bile Acids. Gene Expr. 2019, 19, 215–228. [Google Scholar] [CrossRef]
- Yerushalmi, B.; Dahl, R.; Devereaux, M.W.; Gumpricht, E.; Sokol, R.J. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology 2001, 33, 616–626. [Google Scholar] [CrossRef]
- Grattagliano, I.; Montezinho, L.P.; Oliveira, P.J.; Fruhbeck, G.; Gomez-Ambrosi, J.; Montecucco, F.; Carbone, F.; Wieckowski, M.R.; Wang, D.Q.; Portincasa, P. Targeting mitochondria to oppose the progression of nonalcoholic fatty liver disease. Biochem. Pharm. 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Reinehr, R.; Graf, D.; Haussinger, D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 2003, 125, 839–853. [Google Scholar] [CrossRef]
- Miyoshi, H.; Rust, C.; Roberts, P.J.; Burgart, L.J.; Gores, G.J. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 1999, 117, 669–677. [Google Scholar] [CrossRef]
- Allen, K.; Jaeschke, H.; Copple, B.L. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011, 178, 175–186. [Google Scholar] [CrossRef]
- Csanaky, I.L.; Aleksunes, L.M.; Tanaka, Y.; Klaassen, C.D. Role of hepatic transporters in prevention of bile acid toxicity after partial hepatectomy in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G419–G433. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.; Wagner, M.; Dietrich, C.G.; Trauner, M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim. Biophys. Acta 2007, 1773, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhang, M.; Huang, M.; Rizzolo, D.; Armstrong, L.E.; Schumacher, J.D.; Chow, M.D.; Lee, Y.H.; Guo, G.L. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis. 2019, 51, 570–576. [Google Scholar] [CrossRef]
- Kong, B.; Huang, J.; Zhu, Y.; Li, G.; Williams, J.; Shen, S.; Aleksunes, L.M.; Richardson, J.R.; Apte, U.; Rudnick, D.A.; et al. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G893–G902. [Google Scholar] [CrossRef]
- Uriarte, I.; Fernandez-Barrena, M.G.; Monte, M.J.; Latasa, M.U.; Chang, H.C.; Carotti, S.; Vespasiani-Gentilucci, U.; Morini, S.; Vicente, E.; Concepcion, A.R.; et al. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut 2013, 62, 899–910. [Google Scholar] [CrossRef]
- Kong, B.; Sun, R.; Huang, M.; Chow, M.D.; Zhong, X.B.; Xie, W.; Lee, Y.H.; Guo, G.L. Fibroblast Growth Factor 15-Dependent and Bile Acid-Independent Promotion of Liver Regeneration in Mice. Hepatology 2018, 68, 1961–1976. [Google Scholar] [CrossRef]
- Cai, S.Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef]
- Schumacher, J.D.; Kong, B.; Pan, Y.; Zhan, L.; Sun, R.; Aa, J.; Rizzolo, D.; Richardson, J.R.; Chen, A.; Goedken, M.; et al. The effect of fibroblast growth factor 15 deficiency on the development of high fat diet induced non-alcoholic steatohepatitis. Toxicol. Appl. Pharm. 2017, 330, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, S.Y.; Boyer, J.L. Mechanisms of bile acid mediated inflammation in the liver. Mol. Asp. Med. 2017, 56, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Meadows, V.; Kennedy, L.; Ekser, B.; Kyritsi, K.; Kundu, D.; Zhou, T.; Chen, L.; Pham, L.; Wu, N.; Demieville, J.; et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology 2021, 74, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.L.; Coulter, S.; Liddle, C.; Wong, A.; Eastham-Anderson, J.; French, D.M.; Peterson, A.S.; Sonoda, J. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS ONE 2011, 6, e17868. [Google Scholar] [CrossRef]
- Luo, J.; Ko, B.; Elliott, M.; Zhou, M.; Lindhout, D.A.; Phung, V.; To, C.; Learned, R.M.; Tian, H.; DePaoli, A.M.; et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci. Transl. Med. 2014, 6, 247ra100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, X.; Phung, V.; Lindhout, D.A.; Mondal, K.; Hsu, J.Y.; Yang, H.; Humphrey, M.; Ding, X.; Arora, T.; et al. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014, 74, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kurosu, H.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Kuro-o, M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 2007, 104, 7432–7437. [Google Scholar] [CrossRef]
- Chen, M.M.; Hale, C.; Stanislaus, S.; Xu, J.; Veniant, M.M. FGF21 acts as a negative regulator of bile acid synthesis. J. Endocrinol. 2018, 237, 139–152. [Google Scholar] [CrossRef]
- Zhang, J.; Gupte, J.; Gong, Y.; Weiszmann, J.; Zhang, Y.; Lee, K.J.; Richards, W.G.; Li, Y. Chronic Over-expression of Fibroblast Growth Factor 21 Increases Bile Acid Biosynthesis by Opposing FGF15/19 Action. EBioMedicine 2017, 15, 173–183. [Google Scholar] [CrossRef]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef]
- Kim, A.M.; Somayaji, V.R.; Dong, J.Q.; Rolph, T.P.; Weng, Y.; Chabot, J.R.; Gropp, K.E.; Talukdar, S.; Calle, R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 2017, 19, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef]
- Luo, Y.; Decato, B.E.; Charles, E.D.; Shevell, D.E.; McNaney, C.; Shipkova, P.; Apfel, A.; Tirucherai, G.S.; Sanyal, A.J. Pegbelfermin selectively reduces secondary bile acid concentrations in patients with non-alcoholic steatohepatitis. JHEP Rep. 2022, 4, 100392. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Pellicciari, R.; Costantino, G.; Camaioni, E.; Sadeghpour, B.M.; Entrena, A.; Willson, T.M.; Fiorucci, S.; Clerici, C.; Gioiello, A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004, 47, 4559–4569. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Pockros, P.J.; Kremer, A.E.; Pares, A.; Forman, L.M.; Drenth, J.P.H.; Ryder, S.D.; Terracciano, L.; Jin, Y.; Liberman, A.; et al. Long-Term Obeticholic Acid Therapy Improves Histological Endpoints in Patients With Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1170–1178.e6. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Dyson, J.K.; Alexander, G.J.M.; Chapman, M.H.; Collier, J.; Hübscher, S.; Patanwala, I.; Pereira, S.P.; Thain, C.; Thorburn, D.; et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018, 67, 1568–1594. [Google Scholar] [CrossRef]
- Chapman, R.W.; Lynch, K.D. Obeticholic acid-a new therapy in PBC and NASH. Br. Med. Bull. 2020, 133, 95–104. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Zheng, L.; Kim, Y.; Fang, B.; Liu, B.; Valdez, R.A.; Dietrich, W.F.; Rucker, P.V.; Chianelli, D.; Schmeits, J.; et al. Tropifexor-Mediated Abrogation of Steatohepatitis and Fibrosis Is Associated With the Antioxidative Gene Expression Profile in Rodents. Hepatol. Commun. 2019, 3, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Chen, J.; Desai, S.; Vaidya, S.; Neelakantham, S.; Zhang, J.; Gan, L.; Danis, K.; Laffitte, B.; Klickstein, L.B. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Novel Non-Bile Acid FXR Agonist Tropifexor (LJN452) in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2020, 9, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Nord, S.L.; Burton, D.; Oduyebo, I.; Zhang, Y.; Chen, J.; Im, K.; Bhad, P.; Badman, M.K.; Sanders, D.S.; et al. Randomised clinical trial: Significant biochemical and colonic transit effects of the farnesoid X receptor agonist tropifexor in patients with primary bile acid diarrhoea. Aliment. Pharm. 2020, 52, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Seyedkazemi, S.; Francque, S.; Sanyal, A.; Rinella, M.; Charlton, M.; Loomba, R.; Ratziu, V.; Kochuparampil, J.; Fischer, L.; et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp. Clin. Trials 2020, 88, 105889. [Google Scholar] [CrossRef]
- Camilleri, M.; Nord, S.L.; Burton, D.; Oduyebo, I.; Zhang, Y.M.; Chen, J.; Bhad, P.; Badman, M.K.; Sanders, D.; Walters, J.R. A Double-Blind, Randomized, Placebo-Controlled, Crossover, Multiple-Dose Study of Tropifexor, a Non Bile Acid Fxr Agonist, in Patients with Primary Bile Acid Diarrhea. Gastroenterology 2019, 156, S204–S205. [Google Scholar] [CrossRef]
- Schramm, C.; Hirschfield, G.; Mason, A.L.; Wedemeyer, H.; Klickstein, L.; Neelakantham, S.; Koo, P.; Sanni, J.; Badman, M.; Jones, D. Early assessment of safety and efficacy of tropifexor, a potent non bile-acid FXR agonist, in patients with primary biliary cholangitis: An interim analysis of an ongoing phase 2 study. J. Hepatol. 2018, 68, S103. [Google Scholar] [CrossRef]
- Sanyal, A.; Lopez, P.; Lawitz, E.; Kim, W.; Huang, J.-F.; Andreone, P.; Goh, G.B.B.; Chen, Y.-C.; Ratziu, V.; Kim, Y.J. Tropifexor, a farnesoid X receptor agonist for the treatment of nonalcoholic steatohepatitis–Interim results based on baseline body mass index from first two parts of Phase 2b study FLIGHT-FXR. J. Hepatol. 2019, 70, E796–E797. [Google Scholar] [CrossRef]
- Schramm, C.; Wedemeyer, H.; Mason, A.; Hirschfield, G.M.; Levy, C.; Kowdley, K.V.; Milkiewicz, P.; Janczewska, E.; Malova, E.S.; Sanni, J.; et al. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomised trial in patients with primary biliary cholangitis. JHEP Rep. 2022, 4, 100544. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef]
- Trauner, M.; Bowlus, C.L.; Gulamhusein, A.; Hameed, B.; Caldwell, S.H.; Shiffman, M.L.; Landis, C.; Muir, A.J.; Billin, A.; Xu, J.; et al. Safety and sustained efficacy of the farnesoid X receptor (FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC. Clin. Gastroenterol. Hepatol. 2022, S1542-3565(22)00720-0. [Google Scholar] [CrossRef]
- Erken, R.; Andre, P.; Roy, E.; Kootstra, N.; Barzic, N.; Girma, H.; Laveille, C.; Radreau-Pierini, P.; Darteil, R.; Vonderscher, J.; et al. Farnesoid X receptor agonist for the treatment of chronic hepatitis B: A safety study. J. Viral Hepat. 2021, 28, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Chianelli, D.; Rucker, P.V.; Roland, J.; Tully, D.C.; Nelson, J.; Liu, X.; Bursulaya, B.; Hernandez, E.D.; Wu, J.; Prashad, M.; et al. Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2020, 63, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.K.; Singh, N.; Kumari, R.; Mishra, J.S.; Tripathi, S.; Banerjee, P.; Shah, P.; Kukshal, V.; Tyagi, A.M.; Gaikwad, A.N.; et al. Bile acid receptor agonist GW4064 regulates PPARgamma coactivator-1alpha expression through estrogen receptor-related receptor alpha. Mol. Endocrinol. 2011, 25, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Lee, K.J.; Shim-Lopez, J.; Lee, J.; Wagner, B.; Smith, N.D.; Chen, H.C.; Lawitz, E.J. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Marzano, M.; Fosso, B.; Colliva, C.; Notario, E.; Passeri, D.; Intranuovo, M.; Gioiello, A.; Adorini, L.; Pesole, G.; Pellicciari, R.; et al. Farnesoid X receptor activation by the novel agonist TC-100 (3alpha, 7alpha, 11beta-Trihydroxy-6alpha-ethyl-5beta-cholan-24-oic Acid) preserves the intestinal barrier integrity and promotes intestinal microbial reshaping in a mouse model of obstructed bile acid flow. Biomed. Pharm. 2022, 153, 113380. [Google Scholar] [CrossRef]
- Nara, S.J.; Jogi, S.; Cheruku, S.; Kandhasamy, S.; Jaipuri, F.; Kathi, P.K.; Reddy, S.; Sarodaya, S.; Cook, E.M.; Wang, T.; et al. Discovery of BMS-986339, a Pharmacologically Differentiated Farnesoid X Receptor Agonist for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2022, 65, 8948–8960. [Google Scholar] [CrossRef]
- Cao, S.; Yang, X.; Zhang, Z.; Wu, J.; Chi, B.; Chen, H.; Yu, J.; Feng, S.; Xu, Y.; Li, J.; et al. Discovery of a tricyclic farnesoid X receptor agonist HEC96719, a clinical candidate for treatment of non-alcoholic steatohepatitis. Eur. J. Med. Chem. 2022, 230, 114089. [Google Scholar] [CrossRef]
- Gege, C.; Hambruch, E.; Hambruch, N.; Kinzel, O.; Kremoser, C. Nonsteroidal FXR Ligands: Current Status and Clinical Applications. Handb. Exp. Pharm. 2019, 256, 167–205. [Google Scholar] [CrossRef]
- Liu, X.; Xue, R.; Ji, L.; Zhang, X.; Wu, J.; Gu, J.; Zhou, M.; Chen, S. Activation of farnesoid X receptor (FXR) protects against fructose-induced liver steatosis via inflammatory inhibition and ADRP reduction. Biochem. Biophys. Res. Commun. 2014, 450, 117–123. [Google Scholar] [CrossRef]
- Al-Khaifi, A.; Rudling, M.; Angelin, B. An FXR Agonist Reduces Bile Acid Synthesis Independently of Increases in FGF19 in Healthy Volunteers. Gastroenterology 2018, 155, 1012–1016. [Google Scholar] [CrossRef]
- Traussnigg, S.; Halilbasic, E.; Hofer, H.; Munda, P.; Stojakovic, T.; Fauler, G.; Kashofer, K.; Krssak, M.; Wolzt, M.; Trauner, M. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien. Klin. Wochenschr. 2021, 133, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Crittenden, D.B.; Eng, C.; Zhang, Q.; Guo, P.; Chung, D.; Fenaux, M.; Klucher, K.; Jones, C.; Jin, F.; et al. Safety, Pharmacokinetics, Pharmacodynamics, and Formulation of Liver-Distributed Farnesoid X-Receptor Agonist TERN-101 in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2021, 10, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Kawashima, Y.; Sakai, M.; Matsubayashi, M.; Motoki, K.; Miyajima, Y.; Watanabe, Y.; Chikamatsu, N.; Taniguchi, T.; Tokuyama, R. Discovery, optimization, and evaluation of non-bile acid FXR/TGR5 dual agonists. Sci. Rep. 2021, 11, 9196. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Liu, X.Y.; Zhan, W. Farnesoid X receptor agonist INT-767 attenuates liver steatosis and inflammation in rat model of nonalcoholic steatohepatitis. Drug Des. Devel. 2018, 12, 2213–2221. [Google Scholar] [CrossRef]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; Vavassori, P.; Brancaleone, V.; Fiorucci, S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim. Biophys. Acta 2010, 1802, 363–372. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Nader, F.; Loomba, R.; Anstee, Q.M.; Ratziu, V.; Harrison, S.; Sanyal, A.J.; Schattenberg, J.M.; Barritt, A.S.; et al. Obeticholic Acid Impact on Quality of Life in Patients With Nonalcoholic Steatohepatitis: REGENERATE 18-Month Interim Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2050–2058.e12. [Google Scholar] [CrossRef] [PubMed]
- Tully, D.C.; Rucker, P.V.; Chianelli, D.; Williams, J.; Vidal, A.; Alper, P.B.; Mutnick, D.; Bursulaya, B.; Schmeits, J.; Wu, X. Discovery of tropifexor (LJN452), a Highly Potent Non-Bile Acid FXR agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH); ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- ClinicalTrials.gov—Studies on Tropifexor. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=tropifexor&cntry=&state=&city=&dist= (accessed on 18 November 2022).
- ClinicalTrials.gov—Studies on FXF agonists. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=FXR+agonist&cntry=&state=&city=&dist=&Search=Search (accessed on 18 November 2022).
- Oduyebo, I.; Camilleri, M.; Nelson, A.D.; Khemani, D.; Nord, S.L.; Busciglio, I.; Burton, D.; Rhoten, D.; Ryks, M.; Carlson, P.; et al. Effects of NGM282, an FGF19 variant, on colonic transit and bowel function in functional constipation: A randomized phase 2 trial. Am. J. Gastroenterol. 2018, 113, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Jia, Y.; Ma, A.; He, J.; Shao, C.; Li, X.; Wang, S.; Yang, B.; Zhou, H. Dapagliflozin protects against nonalcoholic steatohepatitis in db/db mice. Front. Pharm. 2022, 13, 934136. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Aich, P.; Singh, S.P.; Serviddio, G.; Vendemiale, G. Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease. World J. Gastroenterol. 2022, 28, 3243–3257. [Google Scholar] [CrossRef]
- Wu, X.; Ge, H.; Gupte, J.; Weiszmann, J.; Shimamoto, G.; Stevens, J.; Hawkins, N.; Lemon, B.; Shen, W.; Xu, J.; et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J. Biol. Chem. 2007, 282, 29069–29072. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).