Abstract
Background: Selenium is a trace element that has been reported to be effective in regulating glucose and lipid metabolism. However, there is conflicting evidence from different clinical trials of selenium supplementation in treating cardiometabolic diseases (CMDs). Objective: This meta-analysis aimed to identify the effects of selenium supplementation on insulin resistance, glucose homeostasis, and lipid profiles in patients with CMDs. Methods: Randomized controlled trials (RCTs) of selenium supplementation for treating CMDs were screened in five electronic databases. Insulin levels, homeostatic model assessment of insulin resistance (HOMA-IR), fasting plasma glucose (FPG), and glycosylated hemoglobin A1C (HbA1c) were defined as the primary outcome markers, and lipid profiles were considered the secondary outcome markers. Results: Ten studies involving 526 participants were included in the meta-analysis. The results suggested that selenium supplementation significantly reduced serum insulin levels (standardized men difference [SMD]: −0.53; 95% confidence interval [CI] [−0.84, −0.21], p = 0.001, I2 = 68%) and HOMA-IR (SMD: −0.50, 95% CI [−0.86, −0.14], p = 0.006, I2 = 75%) and increased high-density lipoprotein cholesterol (HDL-C) levels (SMD: 0.97; 95% CI [0.26, 1.68], p = 0.007, I2 = 92%), but had no significant effect on FPG, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C). Conclusion: Current evidence supports the beneficial effects of selenium supplementation on reducing insulin levels, HOMA-IR, and increasing HDL-C levels. Selenium supplementation may be an effective strategy for reducing insulin resistance in patients with CMDs. However, more high-quality clinical studies are needed to improve the certainty of our estimates.
1. Introduction
Cardiometabolic diseases (CMDs) begin with clinically high-risk states ranging from insulin resistance (IR) to prediabetes states (e.g., obesity) and metabolic syndrome (MS), which then progress to type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [1]. Most individuals diagnosed with CMDs also experience additional cardiometabolic risks, including obesity, dyslipidemia, hyperinsulinemia, and thrombosis [2]. Unhealthy diets and accelerated population aging have resulted in an annual increase in the prevalence and mortality of CMDs, which places a significant economic burden on the healthcare systems of various countries [3,4]. Studies have shown that these metabolic disorders may be influenced by nutrition. Individual nutrient and dietary supplements composed of sufficient nutrients may be associated with reduced cardiometabolic risks, suggesting that nutritional intervention measures are effective in managing these diseases [5,6,7,8].
Selenium is a micronutrient that is vital for human health, and its content in soil varies across different regions. In many countries, including China, selenium-containing soil is poor, and people are prone to selenium deficiency [9,10]. Selenium can exist in nature as inorganic forms (e.g., selenate and selenite) and organic forms (e.g., selenocysteine [Sec]) [11]. Selenium is highly absorbed and distributed throughout the body; in particular, organic selenium is more stable and bioavailable than inorganic selenium [11]. Sec is the main form of selenium in cells, and Sec-containing protein, namely selenoprotein (e.g., selenoprotein P, glutathione peroxidases [GPxs], and thioredoxin reductase [TrxRs]), is mainly responsible for the biological role of selenium in the human body [11,12,13,14]. Selenium is incorporated into selenoproteins, which have broad pleiotropic functions, such as antioxidant and anti-inflammatory properties [15]. Oxidative stress, which is an imbalance between antioxidant defense and prooxidant substances (e.g., reactive oxygen species [ROS] and reactive nitrogen species [RNS]), causes oxidative damage through various mechanisms, including lipid peroxidative damage, DNA damage, and protein oxidation [16,17]. Lately, increasing evidence has shown that the progression of insulin resistance, the pathogenesis of T2DM, and its microvascular ailments and macrovascular complications are significantly regulated by oxidative stress [18,19]. Higher oxidative stress is directly related to the emergence of CMDs [20,21]. Selenium is a well-known antioxidant; in particular, the selenoproteins GPxs and TrxRs are involved in antioxidant defenses and protection against oxidative damage [22]. Supplementing selenium could significantly reduce ROS, increase superoxide dismutase and GPxs activity, and reduce inflammatory cytokine content [23]. A meta-analysis of 13 trials found that selenium supplementation alleviated oxidative stress by raising the total antioxidant capacity and GPxs levels and lowering serum malonaldehyde [17]. It has been proposed that selenium is a hormetic chemical, a substance with a biphasic dose-response that is poisonous at high levels but beneficial at low concentrations [22]. Supra-nutritional levels of selenium produce ROS, which then disturb the redox states of cells [24], increase oxidative stress, and damage tissues and organs [25]. Therefore, maintaining an optimal selenium status is crucial to maintaining redox equilibrium.
Numerous studies have highlighted the significance of selenium and selenoproteins in the prevention and treatment of chronic metabolic diseases such as MS, T2DM, and CVD [26,27,28]. Huang et al. [29] reported that low selenium levels were related to an elevated risk of metabolic disorders, poor prognosis, and mortality. Interestingly, Kamali et al. [30] observed that selenium supplementation significantly improved glucose metabolism by decreasing fasting plasma glucose (FPG), insulin, and homeostatic model assessment of insulin resistance (HOMA-IR), and also increased high-density lipoprotein-cholesterol (HDL-C) levels, but did not affect other lipid profiles. However, selenium status has been reported to be positively associated with markers of insulin resistance and lipid profiles by Cardoso et al. [31] and Ju et al. [32]. On the other hand, a previous meta-analysis reported that selenium supplementation significantly alleviated oxidative stress and inflammation, but did not improve the blood lipid status [33]. To the best of our knowledge, the exact role of selenium in glycolipid metabolism in patients with CMDs remains undetermined. Therefore, to address these issues, we analyzed the impacts of selenium supplementation on glucose and lipid metabolism in CMDs, with the aim of verifying whether selenium supplementation could be a complementary treatment strategy for CMDs.
2. Methods
This meta-analysis strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34]. The study protocol has been registered and published in PROSPERO with ID: CRD42022353393.
2.1. Search Strategy
Searches of the literature for this meta-analysis were conducted using PubMed, Cochrane Library, Embase, Scopus, and Web of Science databases up to 31 July 2022. The key search terms for searching the databases included the following: selenium, selenite, selenate, trace element, cardiometabolic disease, diabetes mellitus, T2DM, coronary heart disease, heart failure, hypertension, hyperlipidemia, metabolic syndrome, stroke, obesity, randomized controlled trial, RCT, random*. In some cases, we may have added or changed the retrieved keywords depending on the characteristics of the databases (Supplementary Table S1). Moreover, we manually checked the reference lists of the eligible articles to identify extra pertinent research. Two reviewers (J.O. and Y.C.) conducted the literature search independently, and any discrepancies were resolved by consensus.
2.2. Study Selection
Two authors (J.O. and Y.C.) individually filtered all eligible studies using strict inclusion and exclusion criteria. Any differences in opinion were settled through consensus or discussion with Drs. Bai and Wang. The reasons for the exclusion of studies in each phase were recorded. Eligible studies were required to meet the following inclusion criteria according to PICOS: (1) Types of population (P): patients with diseases related to CMDs, such as diabetes mellitus, coronary heart disease, heart failure, hypertension, stroke, metabolic syndrome, and obesity. (2) Types of interventions (I): the experimental group received selenium supplementation but the control group did not. Selenium supplementation in all forms, including inorganic, organic, synthetic, and selenium-enriched yeast, was considered. The treatment dose and period were not limited. (3) Types of comparison (C): the control group received placebo or conventional treatment. (4) Types of outcomes (O): primary outcomes: insulin levels, HOMA-IR, FPG, and glycosylated hemoglobin A1C (HbA1c); secondary outcomes: lipid profiles, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and HDL-C. (5) Types of study design (S): randomized controlled trial (RCT) only. The exclusion criteria were as follows: (1) Repeat published studies; (2) conference abstracts; and (3) in vitro and animal studies.
2.3. Data Collection Process
Two reviewers (J.O. and Y.C.) independently collected the following data from the included studies using standardized forms: (1) the characteristics of selected articles, such as author(s), journal of publication, publication year, study design, study location, registration, or not, the number of participants, interventions, treatment period; (2) characteristics of participants, such as disease type, mean age, gender; and (3) clinical outcomes.
2.4. Risk of Bias Assessment
We assessed the risk of bias of the included studies according to the Cochrane Collaboration Risk of Bias tool. The assessed domains included the following: methods of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias [35]. Each study was classified as low, unclear, or high risk based on these domains.
2.5. Data Synthesis and Statistical Analysis
The effect of selenium supplementation on relevant outcomes was assessed as the changes (mean ± standard deviation [SD]) before and after treatment in the experimental and the control groups. If the mean values of the changes before and after treatment were unreported, they were calculated by subtracting the mean at the baseline from the mean at the end of the follow-up. When the SDs of the changes before and after treatment were not reported, they were computed according to the number of patients, standard errors, 95% confidence interval (CI), interquartile ranges, or p-values. If the missing SDs were still unavailable, they were calculated using the correlation formula, and the correlation coefficient was cautiously assumed to be 0.5 [36,37]. For studies with multiple intervention groups, we combined relevant groups into a single treatment group. All related calculation formulas were referred to the Cochrane Handbook for Systematic Reviews of Intervention [38].
Data were evaluated using Review Manager version 5.3 and STATA version 17.0 for a more comprehensive assessment of outcomes. The heterogeneity between studies was assessed using Cochrane’s Q test and was quantified by the I2 test. Heterogeneity was rated as low, moderate, or high when the value of I2 was <50%, 50–75%, or >75%, respectively [39]. When the heterogeneity was low (I2 < 50%), data were pooled by applying the fixed-effects model; otherwise, the random-effects model was applied [40]. Effect sizes are presented as the standardized mean difference (SMD) with 95% CI. If sufficient studies (≥10) were included, funnel plots and Egger’s test were applied to determine whether there was publication bias. A p-value of <0.05 was considered statistically significant.
2.6. Analysis of Subgroups or Subsets
In cases where significant heterogeneity was noted among studies, sensitivity analysis or subgroup analyses were performed to identify its possible sources. Sensitivity analysis was performed by removing each study sequentially to evaluate the influence of each study on the overall effect size. Subgroup analysis was conducted according to the type of disease of the participants.
3. Results
3.1. Literature Selection
We retrieved 4688 studies from five electronic databases. Two studies were manually retrieved. Then, 2530 studies were retained after excluding 2160 duplicates, and a further 2503 studies were eliminated after reading the title and abstract, leaving 27 studies that met the screening criteria for full-text evaluation. Finally, 10 RCTs [30,41,42,43,44,45,46,47,48,49] were included in this meta-analysis. The screening process is depicted in Figure 1.
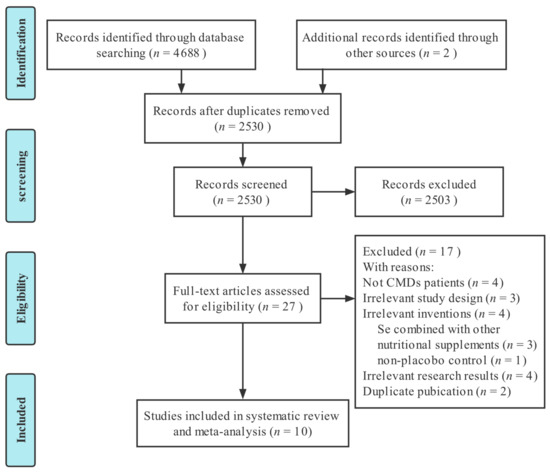
Figure 1.
PRISMA flow chart for selection and screening of the studies.
3.2. Study Characteristics
All 10 studies included in this meta-analysis were randomized, double-blind, placebo-controlled trials, with 526 participants, including 272 in the selenium group (experimental group) and 254 in the control group. The treatment period ranged from 4 to 24 weeks. All 10 studies were conducted in Iran. Except for Faghihi 2014 [48], the remaining nine studies have completed clinical trial registration. Faghihi 2014 [48] reported participants’ selenium concentration as deficient state at baseline, and the remaining studies did not report participants’ selenium status. In the included studies, the forms of selenium supplementation were mainly selenium yeast and sodium selenite, but three studies did not mention the form of selenium supplementation. Five studies [37,41,43,45,48] recruited patients with diabetes mellitus or complications of diabetes mellitus (e.g., diabetic nephropathy), three studies [30,42,44] recruited patients with cardiovascular disease, one study [46] recruited patients with diabetes mellitus combined with coronary heart disease, and one study [49] recruited obese patients (Table 1).

Table 1.
Characteristics of the included studies.
3.3. Risk of Bias Assessments
All except two studies [46,49] were rated as having a low risk of selection bias for adopting appropriate random sequence generation and allocation concealment methods. Farrokhian 2016 [46] and Alizadeh 2012 [49] were assessed as having an unclear risk of selection bias because they reported random sequence generation methods, but did not report allocation concealment methods. All of the 10 studies were rated as carrying a low risk of performance bias and concealment bias due to complete reporting of blinding implementation. Eight studies [30,41,42,44,45,46,47,49] were rated as having a low risk of attrition bias, but Najib 2020 [43] and Faghihi 2014 [48] were rated as high risk due to unbalanced and unexplained loss at follow-up. Farrokhian 2016 [46] and Alizadeh 2012 [49] were rated as high risk of reporting bias because the primary outcomes were changed after the protocol registration. Due to the lack of a registered protocol [48] or the inability to report several secondary outcomes [41,42,43,45], five studies were rated as unknown risks of report bias. Farrokhian 2016 [45] was rated a high risk of other bias because of the inconsistency in the types of hypoglycemic drugs taken between the selenium and control groups, which may have affected the effect evaluation (Figure 2 and Figure 3).
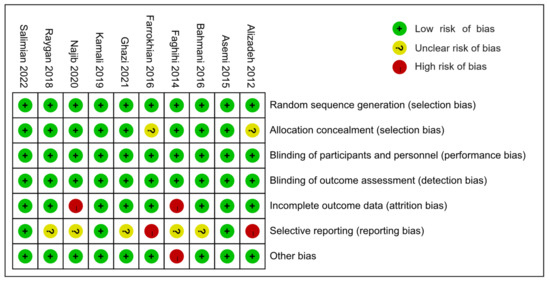
Figure 2.
Summary of the risks of bias.
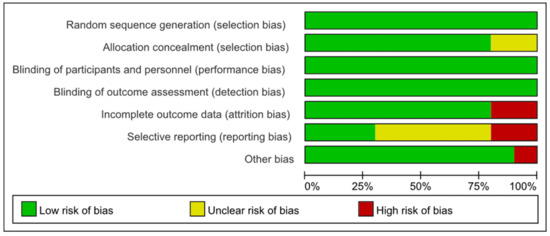
Figure 3.
Risks of bias graph expressed as percentages.
3.4. Meta-Analysis
3.4.1. Primary Outcomes
All 10 studies with 526 participants evaluated the effects of serum insulin levels and HOMA-IR. The heterogeneity of serum insulin levels and HOMA-IR were moderate (I2 = 68%, I2 = 75%). Pooled results obtained by employing a random-effects model demonstrated that selenium supplementation remarkably lowered serum insulin levels (SMD: −0.53, 95% CI [−0.84, −0.21], p = 0.001) and decreased HOMA-IR (SMD: −0.50, 95% CI [−0.86, −0.14], p = 0.006) (Figure 4 and Figure 5). To resolve heterogeneity, sensitivity analysis was conducted by excluding the studies one by one. The pooled results were broadly consistent with the above analysis (Supplementary Figures S1 and S2), and the heterogeneity was largely affected by Faghihi 2014 [48], which was excluded. Nine studies [30,41,42,43,44,45,46,47,49] remained after the exclusion, with no heterogeneity in the pooled results (all I2 = 0%), indicating that Faghihi 2014 [48] was a major factor in the source of heterogeneity of insulin levels and HOMA-IR. This may be due to a baseline difference in the hypoglycemic drugs taken between the selenium and control groups in Faghihi’s study. An analysis was then conducted with the fixed-effects model, and the result confirmed the previous observation that supplementing with selenium was associated with lower serum insulin levels (SMD: −0.67, 95% CI [−0.86, −0.48], p < 0.0001) and HOMA-IR (SMD: −0.67, 95% CI [−0.86, −0.48], p < 0.0001) (Figure 6 and Figure 7).
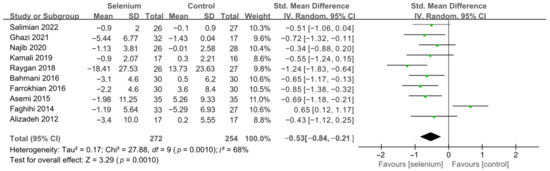
Figure 4.
Forest plot of insulin levels [30,41,42,43,44,45,46,47,48,49].
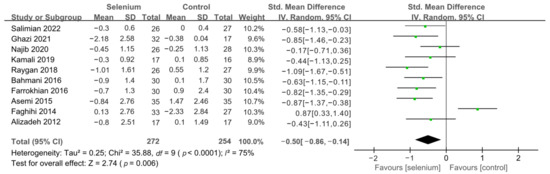
Figure 5.
Forest plot of HOMA-IR [30,41,42,43,44,45,46,47,48,49].
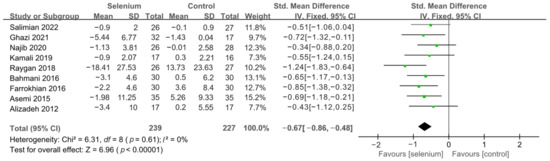
Figure 6.
Forest plot of insulin levels after excluding Faghihi 2014 [30,41,42,43,44,45,46,47,49].
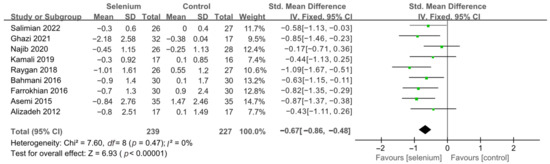
Figure 7.
Forest plot of HOMA-IR after excluding Faghihi 2014 [30,41,42,43,44,45,46,47,49].
The effect of selenium supplementation on FPG was assessed in 492 participants through nine studies [30,41,42,43,44,45,46,47,48]. The heterogeneity between studies was high (I2 = 91%). Pooled analysis from the random-effects model indicated that the selenium group and the control group had similar effects on FPG (SMD: 0.06, 95% CI [−0.56, 0.68], p = 0.86) (Supplementary Figure S3). To resolve heterogeneity, sensitivity analysis was conducted by excluding the studies one by one. The results showed that although the pooled results were stable, the heterogeneity was not resolved (Supplementary Figure S4). Then, subgroup analysis was conducted based on the underlying diseases of the participants. As shown in Figure 8, the FPG levels in patients with cardiovascular disease were significantly lower in the selenium group than in the control group (SMD: −0.42, 95% CI: [−0.77, −0.07], p = 0.02), with no heterogeneity (I2 = 0%). However, there was no statistical difference in terms of FPG between the selenium and control groups in the other two subgroups (Figure 8).
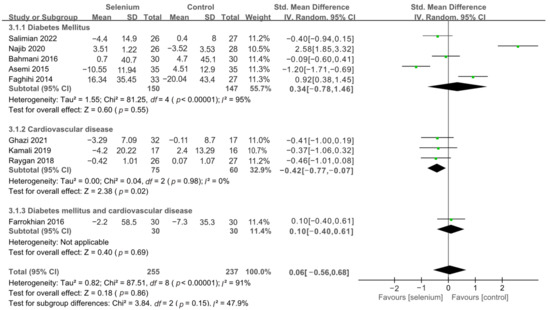
Figure 8.
Subgroup analysis of FPG [30,41,42,43,44,45,46,47,48].
Only two studies [43,48], including 114 participants, assessed the effect of selenium supplementation on HbA1c. The heterogeneity between studies was high (I2 = 85%). Thus, we did not perform a meta-analysis of HbA1c. Both studies reported reductions in HbA1c after treatment in both the selenium and control groups, in which Najib 2020 [43] reported a more significant reduction in HbA1c in the selenium group compared to the control group.
3.4.2. Secondary Outcomes
Nine studies [30,41,42,44,45,46,47,48,49] with 492 patients evaluated the effects of TC, TG, and LDL-C, and five studies [30,41,44,45,46] with 259 patients evaluated the effects of VLDL-C. The heterogeneity of TC, TG, and VLDL-C was insignificant (all I2 = 0). Unfortunately, the pooled results from the fixed-effects model demonstrated that selenium supplements did not significantly lower TC, TG, and VLDL-C in patients with CMDs (SMD: −0.07, 95% CI [−0.25, 0.12], p = 0.48, SMD: −0.12, 95% CI [−0.30, 0.06], p = 0.20, and SMD: −0.08, 95% CI [−0.33, 0.16], p = 0.51, respectively) (Figure 9, Figure 10 and Figure 11). The heterogeneity of LDL-C was high (I2 = 79%). Pooled results from the random-effects model demonstrated no significant difference in LDL-C between the two groups (SMD: −0.35, 95% CI [−0.76, 0.06], p = 0.10) (Figure 12).
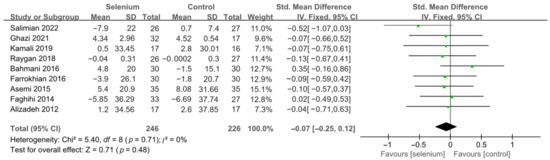
Figure 9.
Forest plot of TC [30,41,42,44,45,46,47,48,49].
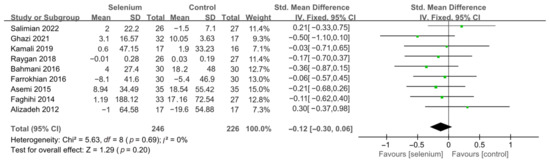
Figure 10.
Forest plot of TG [30,41,42,44,45,46,47,48,49].
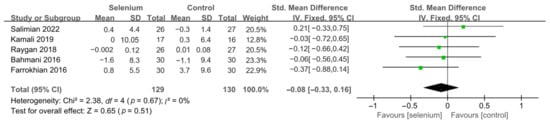
Figure 11.
Forest plot of VLDL-C [30,41,44,45,46].
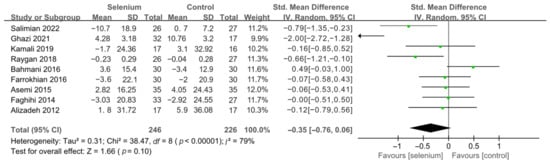
Figure 12.
Forest plot of LDL-C [30,41,42,44,45,46,47,48,49].
A total of nine studies [30,41,42,44,45,46,47,48,49], including 492 patients, evaluated the effects of HDL-C. The pooled results from the random-effects model indicated that selenium supplementation remarkably increased HDL-C levels (SMD: 0.97, 95% CI [0.26, 1.68], p = 0.007), with high heterogeneity across studies (I2 = 92%) (Supplementary Figure S5). To resolve heterogeneity, a sensitivity analysis was conducted by excluding each study separately. The results showed that the pooled results were broadly consistent with the above analysis (Supplementary Figure S6), and the heterogeneity was largely affected by Ghazi 2021 [42]. Eight studies [30,41,44,45,46,47,48,49] remained after excluding Ghazi 2021 [42], and pooled results showed that the heterogeneity between studies was decreased (I2 = 58%) (Supplementary Figure S7). The participants of Ghazi 2021 [42] were patients with atherosclerosis, and dyslipidemia is closely related to atherosclerosis. Therefore, considering that the source of heterogeneity may be related to the participants’ underlying disease, the included studies were divided into subgroups based on the participants’ disease type. As shown in Figure 13, the HDL-C levels were significantly increased in the diabetes mellitus subgroup and cardiovascular disease subgroup (SMD: 0.50, 95% CI [0.10, 0.91], p = 0.02 and SMD: 4.22, 95% CI [1.06, 7.37], p = 0.009), with significant heterogeneity among studies (I2 = 59% and I2 = 97%). However, there was no statistical difference between the selenium and control groups in the other two subgroups (Figure 13).
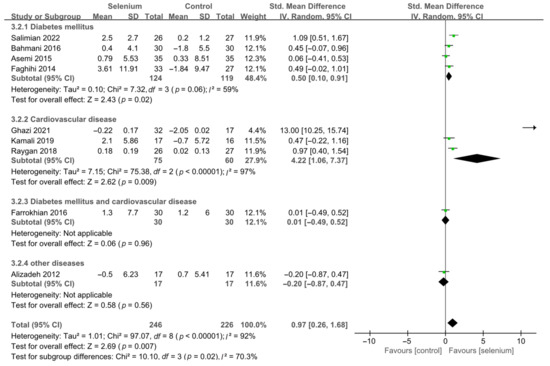
Figure 13.
Subgroup analysis of HDL-C [30,41,42,44,45,46,47,48,49].
3.5. Publication Bias
Funnel plots of insulin levels and HOMA-IR were drawn with Review Manager version 5.3, and Egger’s test was conducted to quantify the publication bias with Stata version 17.0. The two funnel plots were substantially symmetrical (Figure 14). The results of Egger’s test for insulin levels and HOMA-IR were p = 0.678 and p = 0.908, respectively, indicating that there was no publication bias.
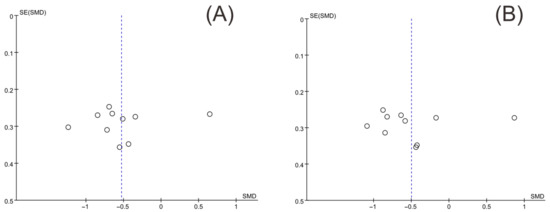
Figure 14.
Funnel plot of insulin levels and HOMA-IR. (A) Insulin levels, (B) HOMA-IR.
4. Discussion
Over the last decade, increasing attention has been paid to the selenium status in patients with various cardiometabolic diseases and the association between selenium status and mortality risk of various cardiometabolic diseases. Several meta-analyses have indicated that individuals with cardiometabolic diseases tend to have lower selenium levels than healthy individuals [50,51,52], and that selenium levels are negatively associated with mortality among patients with MS, T2DM, and CVD [53,54,55]. Alterations in the metabolism of glucose and lipids characterize metabolic disorders [56]. Insulin has regulatory effects on glucose, which are mainly classified into two aspects: promoting glucose absorption into liver cells, muscle cells, and adipose tissue, and inhibiting glycogenolysis and gluconeogenesis in the liver [57]. Insulin resistance is a common pathophysiological status in which individuals have decreased insulin sensitivity and impaired glucose regulation by insulin, resulting in glucose intolerance [58]. It is well-established that insulin resistance underpins many chronic metabolic diseases [59], and cardiometabolic risks such as obesity and dyslipidemia can exacerbate insulin resistance and impel the progression of CMDs [1]. Studies have shown that the micronutrient selenium can regulate the human body’s insulin sensitivity, and selenium in the form of selenate is known to act as an insulin mimetic with a role in maintaining blood sugar homeostasis [60,61]. Expression of selenoprotein P plays a crucial role in pancreatic β-cell function by preventing ferroptosis and thus maintaining glutathione peroxidase 4 (Gpx4) and cell viability, and also by inhibiting stress-induced degradation of nascent granules, a novel regulatory pathway for insulin [62], thus maintaining cellular proinsulin levels [63]. Furthermore, a meta-analysis performed by Tabrizi et al. [64] supported the positive effect of selenium supplements on lipid profiles. Of note, that selenium supplementation makes the most sense when there is deficit of selenium [14,65].
In this meta-analysis, we examined the impact of selenium supplementation on the markers of insulin resistance, glucose, as well as blood lipid profiles in patients with CMDs. The level of glucose in the blood is one of the most significant homeostatic indicators and is strictly regulated [66]. The pathways and regulation of glucose metabolism include glycolysis/glycogenolysis, gluconeogenesis, pentose phosphate pathway (PPP), insulin signaling pathway, and many others [67], some of which can be regulated by insulin [57]. HOMA-IR is often used in investigating and quantifying insulin resistance because of the simplicity of the underlying mathematical model (HOMA-IR = fasting glucose [mg] × fasting insulin [mu/L]/22.5) [68]. Furthermore, HbA1c is an essential marker of long-term glycemic control that reflects a cumulative glycemic level over the past two to three months [69]. Therefore, insulin levels, HOMA-IR, FPG and HbA1c were used to evaluate the selenium supplementation on glycemic control. In this study, comprehensive pooled results from 10 RCTs involving 526 patients supported the favorable effects of selenium supplementation in decreasing serum insulin levels and HOMA-IR. Moreover, selenium supplementation may increase HDL-C levels, but the effectiveness of selenium supplementation on FPG, TC, TG, LDL-C, and VLDL-C levels was unclear. The current results suggest that selenium supplementation may be an effective treatment for reducing insulin resistance.
The findings reveal that selenium supplementation could reduce insulin levels and HOMA-IR in patients with CMDs, but the effect on FPG was ambiguous. This result is similar to that of the meta-analysis conducted by Strozyk et al. in 2017, which included four RCTs [70]. Their study focused on assessing the efficacy of selenium supplementation in T2DM, and the results supported that selenium supplementation significantly improved insulin resistance. However, our study also focused on other cardiometabolic diseases, such as cardiovascular disease, and collected RCTs that have been updated recently. Therefore, 10 RCTs were included in this meta-analysis. The pooled results derived from the included studies are in line with those of previous animal studies [71,72,73], in that supplemental selenium therapy had a significantly protective anti-diabetic effect. Selenium nanoparticles (selenium-NPs) are a new supplemental form of selenium that is readily absorbed and has low toxicity. According to Abdulmalek et al. [74], treating diabetic rats with selenium-NPs (0.1 and 0.4 mg/kg) and/or metformin (100 mg/kg) separately or together, led to a remarkable decrease in FBG and insulin levels, suggesting that selenium and its derivatives play a significant role in the maintenance of glucose homeostasis. There is a lack of positive evidence regarding the effect of selenium supplementation on HbA1c. A cross-sectional study reported that obese participants with lower selenium intakes exhibited higher concentrations of HbA1c [75]. In this regard, the effect of selenium supplementation on long-term glycemic control deserves further attention.
The pooled results of this study also demonstrated that selenium supplementation increased HDL-C levels, but had little effect on other blood lipids. In addition, in the subgroup of disease types, we found that selenium supplementation increased HDL-C levels more significantly in participants with cardiovascular disease, followed by those with diabetes mellitus. HDL-C is essential for reverse cholesterol transport and has anti-inflammatory, anti-atherogenic, and anti-thrombotic effects [76]. The interaction of these properties contributes to the cardioprotective properties of HDL-C. Thus, appropriate selenium supplementation may contribute to the improvement of CMDs, particularly cardiovascular disease.
Some statistical heterogeneity was discovered in the pooled analysis of insulin levels, HOMA-IR, FPG, and HDL-C. The results were similar before and after sensitivity analysis, suggesting that the results were stable and reliable. In terms of insulin levels and HOMA-IR outcomes, we considered that Faghihi’s study [48] administered inconsistent types or combinations of antidiabetic drugs at baseline, which might be an essential source of heterogeneity in the pooled analysis. In Faghihi’s study [48], approximately 85.2% of participants in the control group received combined antidiabetic medication, compared to only 66.6% of participants in the selenium group. Additionally, subgroup analysis suggested that the inconsistency of participants’ underlying diseases is a major source of heterogeneity in terms of FPG and HDL-C. In the future, more evaluable studies should be included in the analysis to better systematically evaluate the effectiveness of selenium supplementation in different types of CMDs.
This meta-analysis has several strengths. First, two reviewers independently used a comprehensive search strategy to identify all trials evaluating the effect of selenium supplementation in patients with CMDs and used standardized templates to extract data from included trials to guarantee data accuracy and reduce the impact of study design faults. Second, most included RCTs were high-quality with appropriate randomization, allocation concealment, and double-blinding methods. Third, no publication bias was found in this meta-analysis. Furthermore, we performed a thorough sensitivity analysis to examine the stability of our results. However, there are several limitations that should be considered. First, as the control group was placebo instead of different doses of selenium, the optimal dose of selenium supplementation cannot be accurately determined in this study. Second, the number of participants in the included RCTs was relatively small, with none having more than 100. Third, as only two studies evaluated HbA1c and there was high heterogeneity in the pooled analysis results, we could only perform a systematic review, which may affect the reliability and comprehensiveness of the evaluation of the efficacy of selenium supplementation on the HbA1c control. Fourth, although trials of the effects of selenium supplementation on CMDs have been conducted in countries other than Iran, trials in which other nutritional supplements were supplemented in addition to selenium in the intervention [77], and trials in which markers related to glucolipid metabolism were not reported [78] were excluded from this meta-analysis, and the final relevant included studies were all conducted in Iran, which may limit the generalizability. According to this, the recommendations of potential effects of selenium supplementation conclusions should be drawn with caution. Finally, we suggest that future clinical trials should pay more attention to the different doses of selenium supplements and the baseline level of serum selenium of CMDs patients, in order to further illuminate the therapeutic effects of selenium supplementation on CMDs.
5. Conclusions
This meta-analysis demonstrated that selenium supplementation may reduce the levels of serum insulin and HOMA-IR, and increase serum HDL-C levels, suggesting that selenium supplementation may be beneficial for reducing insulin resistance in patients with CMDs. This finding may provide support to prospective studies looking into selenium supplementation to manage cardiometabolic risk factors. However, in the case of selenium excess, the efficacy of selenium supplementation on glucolipid metabolism needs further evaluation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14224933/s1, Table S1. Search strategy for each electronic database; Figure S1. Sensitivity analysis of insulin levels; Figure S2. Sensitivity analysis of HOMA-IR; Figure S3. Forest plot of FPG; Figure S4. Sensitivity analysis of FPG; Figure S5. Forest plot of HDL-C; Figure S6. Sensitivity analysis of HDL-C; Figure S7. Forest plot of HDL-C after excluding Ghazi 2021 [42].
Author Contributions
Conceptualization and design, A.W., R.B. and Z.G.; software, J.O., Y.C. and Y.S.; validation, A.W. and R.B.; writing—original draft preparation, J.O., Y.C.; writing—review and editing, A.W. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82174216 AND No. 82004194), the Fundamental Research Funds for the Central public welfare research institutes (No. ZZ13-YQ-005, No. ZZ13-YQ-005-C1, No. ZZ13-YQ-014-C1 and No. ZZ13-YQ-014) and Scientific Fund of National Clinical Research Center for Chinese Medicine Cardiology (No. CMC2022009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, F.; Moellering, D.R.; Garvey, W.T. The progression of cardiometabolic disease: Validation of a new cardiometabolic disease staging system applicable to obesity. Obesity 2014, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, A.; Kontopantelis, E.; Adeniji, C.; van Marwijk, H.; McMillian, B.; Bower, P.; Panagioti, M. Interventions Using Wearable Physical Activity Trackers Among Adults With Cardiometabolic Conditions: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2116382. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.Y.; Wang, L.S.; Jiang, H.R.; Jia, X.F.; Wang, Z.H.; Wang, H.J.; Zhang, B.; Ding, G.G. Epidemiological characteristics and trends of cardiometabolic risk factors in residents aged 18–64 years in 15 provinces of China. Zhonghua Liu Xing Bing Xue Za Zhi 2022, 43, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Madlala, S.S.; Hill, J.; Kunneke, E.; Kengne, A.P.; Peer, N.; Faber, M. Dietary Diversity and its Association with Nutritional Status, Cardiometabolic Risk Factors and Food Choices of Adults at Risk for Type 2 Diabetes Mellitus in Cape Town, South Africa. Nutrients 2022, 14, 3191. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Kazemi, A.; Bartolomaeus, T.U.P.; Martami, F.; Noormohammadi, M.; Salari, A.; Löber, U.; Balou, H.A.; Forslund, S.K.; Mahdavi-Roshan, M. The effect of probiotic and synbiotic supplementation on lipid parameters among patients with cardiometabolic risk factors: A systematic review and meta-analysis of clinical trials. Cardiovasc. Res. 2022; in press. [Google Scholar] [CrossRef]
- Jamilian, M.; Modarres, S.Z.; Siavashani, M.A.; Karimi, M.; Mafi, A.; Ostadmohammadi, V.; Asemi, Z. The Influences of Chromium Supplementation on Glycemic Control, Markers of Cardio-Metabolic Risk, and Oxidative Stress in Infertile Polycystic ovary Syndrome Women Candidate for In vitro Fertilization: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2018, 185, 48–55. [Google Scholar] [CrossRef]
- Gil, Á.; Ortega, R.M. Introduction and Executive Summary of the Supplement, Role of Milk and Dairy Products in Health and Prevention of Noncommunicable Chronic Diseases: A Series of Systematic Reviews. Adv. Nutr. 2019, 10, S67–S73. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, Y.K.; Lee, J.; Kim, E. Could nutrient supplements provide additional glycemic control in diabetes management? A systematic review and meta-analysis of randomized controlled trials of as an add-on nutritional supplementation therapy. Arch. Pharmacal Res. 2022, 45, 185–204. [Google Scholar] [CrossRef]
- Bitterli, C.; Bañuelos, G.S.; Schulin, R. Use of transfer factors to characterize uptake of selenium by plants. J. Geochem. Explor. 2010, 107, 206–216. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium Geochemistry and Health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wang, C.; Zhao, Z.; Fan, Y. Systematic Review and Meta-Analysis of Oxidative Stress and Antioxidant Markers in Oral Lichen Planus. Oxidative Med. Cell. Longev. 2021, 2021, 9914652. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of selenium supplementation on antioxidant markers: A systematic review and meta-analysis of randomized controlled trials. Hormones 2019, 18, 451–462. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Gunawardena, H.P.; Silva, R.; Sivakanesan, R.; Ranasinghe, P.; Katulanda, P. Poor Glycaemic Control Is Associated with Increased Lipid Peroxidation and Glutathione Peroxidase Activity in Type 2 Diabetes Patients. Curr. Med. Res. Opin. 2019, 2019, 9471697. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Liu, S.; Zhang, Q.; Wang, P.; Cao, Y.; Wu, L. Supplementation with selenium attenuates autism-like behaviors and improves oxidative stress, inflammation and related gene expression in an autism disease model. J. Nutr. Biochem. 2022, 107, 109034. [Google Scholar] [CrossRef]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Xu, G.-B.; Xia, T.-A. Prevalence rate of metabolic syndrome and dyslipidemia in a large professional population in Beijing. Atherosclerosis 2006, 184, 188–192. [Google Scholar] [CrossRef]
- Kohler, L.N.; Florea, A.; Kelley, C.P.; Chow, S.; Hsu, P.; Batai, K.; Saboda, K.; Lance, P.; Jacobs, E.T. Higher Plasma Selenium Concentrations Are Associated with Increased Odds of Prevalent Type 2 Diabetes. J. Nutr. 2018, 148, 1333–1340. [Google Scholar] [CrossRef]
- Yin, T.; Zhu, X.; Xu, D.; Lin, H.; Lu, X.; Tang, Y.; Shi, M.; Yao, W.; Zhou, Y.; Zhang, H.; et al. The Association Between Dietary Antioxidant Micronutrients and Cardiovascular Disease in Adults in the United States: A Cross-Sectional Study. Front. Nutr. 2021, 8, 799095. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Kamali, A.; Amirani, E.; Asemi, Z. Effects of Selenium Supplementation on Metabolic Status in Patients Undergoing for Coronary Artery Bypass Grafting (CABG) Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2019, 191, 331–337. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Braat, S.; Graham, R.M. Selenium Status Is Associated With Insulin Resistance Markers in Adults: Findings From the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front. Nutr. 2021, 8, 696024. [Google Scholar] [CrossRef]
- Ju, W.; Ji, M.; Li, X.; Li, Z.; Wu, G.; Fu, X.; Yang, X.; Gao, X. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018, 37, 1512–1517. [Google Scholar] [CrossRef]
- Ju, W.; Li, X.; Li, Z.; Wu, G.R.; Fu, X.F.; Yang, X.M.; Zhang, X.Q.; Gao, X.B. The effect of selenium supplementation on coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2017, 44, 8–16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yuan, D.; Sharma, H.; Krishnan, A.; Vangaveti, V.N.; Malabu, U.H. Effect of glucagon-like peptide 1 receptor agonists on albuminuria in adult patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2022, 24, 1869–1881. [Google Scholar] [CrossRef]
- Li, T.; Yu, T.; Hawkins, B.S.; Dickersin, K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PLoS ONE 2015, 10, e0133023. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Akbari, M.; Moosazadeh, M.; Ghahramani, S.; Tabrizi, R.; Kolahdooz, F.; Asemi, Z.; Lankarani, K.B. High prevalence of hypertension among Iranian children and adolescents: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 1155–1163. [Google Scholar] [CrossRef]
- Salimian, M.; Soleimani, A.; Bahmani, F.; Tabatabaei, S.M.H.; Asemi, Z.; Talari, H.R. The effects of selenium administration on carotid intima-media thickness and metabolic status in diabetic hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2022, 47, 58–62. [Google Scholar] [CrossRef]
- Ghazi, M.K.K.; Ghaffari, S.; Naemi, M.; Salehi, R.; Sadeghi, M.T.; Barati, M.; Shabestari, A.N.; Kafil, B.; Alamdari, N.M.; Soleimanzadeh, H.; et al. Effects of sodium selenite and selenium-enriched yeast on cardiometabolic indices of patients with atherosclerosis: A double-blind randomized clinical trial study. J. Cardiovasc. Thorac. Res. 2021, 13, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Najib, F.S.; Poordast, T.; Nia, M.R.; Dabbaghmanesh, M.H. Effects of selenium supplementation on glucose homeostasis in women with gestational diabetes mellitus: A randomized, controlled trial. Int. J. Reprod. Biomed. 2020, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Behnejad, M.; Ostadmohammadi, V.; Bahmani, F.; Mansournia, M.A.; Karamali, F.; Asemi, Z. Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2018, 120, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Kia, M.; Soleimani, A.; Asemi, Z.; Esmaillzadeh, A. Effect of Selenium Supplementation on Glycemic Control and Lipid Profiles in Patients with Diabetic Nephropathy. Biol. Trace Elem. Res. 2016, 172, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Farrokhian, A.; Bahmani, F.; Taghizadeh, M.; Mirhashemi, S.M.; Aarabi, M.; Raygan, F.; Aghadavod, E.; Asemi, Z. Selenium Supplementation Affects Insulin Resistance and Serum hs-CRP in Patients with Type 2 Diabetes and Coronary Heart Disease. Horm. Metab. Res. 2016, 48, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef]
- Faghihi, T.; Radfar, M.; Barmal, M.; Amini, P.; Qorbani, M.; Abdollahi, M.; Larijani, B. A Randomized, Placebo-Controlled Trial of Selenium Supplementation in Patients With Type 2 Diabetes: Effects on Glucose Homeostasis, Oxidative Stress, and Lipid Profile. Am. J. Ther. 2014, 21, 491–495. [Google Scholar] [CrossRef]
- Alizadeh, M.; Safaeiyan, A.; Ostadrahimi, A.; Estakhri, R.; Daneghian, S.; Ghaffari, A.; Gargari, B.P. Effect of L-Arginine and Selenium Added to a Hypocaloric Diet Enriched with Legumes on Cardiovascular Disease Risk Factors in Women with Central Obesity: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Nutr. Metab. 2012, 60, 157–168. [Google Scholar] [CrossRef]
- Yang, L.; Qi, M.; Du, X.; Xia, Z.; Fu, G.; Chen, X.; Liu, Q.; Sun, N.; Shi, C.; Zhang, R. Selenium concentration is associated with occurrence and diagnosis of three cardiovascular diseases: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2022, 70, 126908. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Y.; Ji, Y.; Yu, H.; Li, Y.; Piao, C.; Xie, L. The association between serum selenium level and gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2022, 38, e3522. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; de Araújo, D.S.C.; da Cunha Soares, T.; Cruz, K.J.C.; Henriques, G.S.; do Nascimento Marreiro, D. Nutritional status of selenium in overweight and obesity: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 862–884. [Google Scholar] [CrossRef]
- Kuria, A.; Tian, H.; Li, M.; Wang, Y.; Aaseth, J.O.; Zang, J.; Cao, Y. Selenium status in the body and cardiovascular disease: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 3616–3625. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.; Liu, Z.; Guo, H.; Liang, J.; Zhang, Y. Associations of the Dietary Iron, Copper, and Selenium Level With Metabolic Syndrome: A Meta-Analysis of Observational Studies. Front. Nutr. 2021, 8, 810494. [Google Scholar] [CrossRef]
- Qiu, Z.; Geng, T.; Wan, Z.; Lu, Q.; Guo, J.; Liu, L.; Pan, A.; Liu, G. Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes. Am. J. Clin. Nutr. 2022, 115, 53–60. [Google Scholar] [CrossRef]
- Díaz-Ruiz, M.; Martínez-Triguero, M.L.; López-Ruiz, A.; Fernández-de la Cruz, F.; Bañuls, C.; Hernández-Mijares, A. Metabolic disorders and inflammation are associated with familial combined hyperlipemia. Clin. Chim. Acta 2019, 490, 194–199. [Google Scholar] [CrossRef]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc transporters and insulin resistance: Therapeutic implications for type 2 diabetes and metabolic disease. J. Biomed. Sci. 2017, 24, 87. [Google Scholar] [CrossRef]
- Ogawa-Wong, A.N.; Berry, M.J.; Seale, L.A. Selenium and Metabolic Disorders: An Emphasis on Type 2 Diabetes Risk. Nutrients 2016, 8, 80. [Google Scholar] [CrossRef]
- Ezaki, O. The insulin-like effects of selenate in rat adipocytes. J. Biol. Chem. 1990, 265, 1124–1128. [Google Scholar] [CrossRef]
- Pasquier, A.; Vivot, K.; Erbs, E.; Spiegelhalter, C.; Zhang, Z.; Aubert, V.; Liu, Z.; Senkara, M.; Maillard, E.; Pinget, M.; et al. Lysosomal degradation of newly formed insulin granules contributes to β cell failure in diabetes. Nat. Commun. 2019, 10, 3312. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, N.; Nakao, S.; Mita, Y.; Arisawa, K.; Hoshi, T.; Toyama, T.; Ishii, K.-A.; Takamura, T.; Noguchi, N.; Saito, Y. Role of selenoprotein P expression in the function of pancreatic β cells: Prevention of ferroptosis-like cell death and stress-induced nascent granule degradation. Free Radic. Biol. Med. 2022, 183, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Akbari, M.; Moosazadeh, M.; Lankarani, K.B.; Heydari, S.T.; Kolahdooz, F.; Mohammadi, A.A.; Shabani, A.; Badehnoosh, B.; Jamilian, M.; et al. The Effects of Selenium Supplementation on Glucose Metabolism and Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2017, 49, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.H. Four grams of glucose. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, M.S.; Doberne, L.; Kraemer, F.; Tobey, T.; Reaven, G. Assessment of Insulin Resistance with the Insulin Suppression Test and the Euglycemic Clamp. Diabetes 1981, 30, 387–392. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Stróżyk, A.; Osica, Z.; Przybylak, J.D.; Kołodziej, M.; Zalewski, B.M.; Mrozikiewicz-Rakowska, B.; Szajewska, H. Effectiveness and safety of selenium supplementation for type 2 diabetes mellitus in adults: A systematic review of randomised controlled trials. J. Hum. Nutr. Diet. 2019, 32, 635–645. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Zeng, S.; Ke, Y.; Liu, Y.; Shen, Y.; Zhang, L.; Li, C.; Liu, A.; Shen, L.; Hu, X.; Wu, H.; et al. Synthesis and antidiabetic properties of chitosan-stabilized selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 115–121. [Google Scholar] [CrossRef]
- Oztürk, Z.; Gurpinar, T.; Vural, K.; Boyacıoglu, S.; Korkmaz, M.; Var, A. Effects of selenium on endothelial dysfunction and metabolic profile in low dose streptozotocin induced diabetic rats fed a high fat diet. Biotech. Histochem. 2015, 90, 506–515. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Balbaa, M. Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: Diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS ONE 2019, 14, e0220779. [Google Scholar] [CrossRef]
- Kazemi, A.; Shim, S.R.; Jamali, N.; Hassanzadeh-Rostami, Z.; Soltani, S.; Sasani, N.; Mohsenpour, M.A.; Firoozi, D.; Basirat, R.; Hosseini, R.; et al. Comparison of nutritional supplements for glycemic control in type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Res. Clin. Pract. 2022, 191, 110037. [Google Scholar] [CrossRef]
- Bhale, A.S.; Venkataraman, K. Leveraging knowledge of HDLs major protein ApoA1: Structure, function, mutations, and potential therapeutics. Biomed. Pharmacother. 2022, 154, 113634. [Google Scholar] [CrossRef]
- Sena-Evangelista, K.C.M.; Pedrosa, L.F.C.; Paiva, M.S.M.O.; Dias, P.C.S.; Ferreira, D.Q.C.; Cozzolino, S.M.F.; Faulin, T.E.S.; Abdalla, D.S.P. The Hypolipidemic and Pleiotropic Effects of Rosuvastatin Are Not Enhanced by Its Association with Zinc and Selenium Supplementation in Coronary Artery Disease Patients: A Double Blind Randomized Controlled Study. PLoS ONE 2015, 10, e0119830. [Google Scholar] [CrossRef]
- Gharipour, M.; Sadeghi, M.; Haghjooy-Javanmard, S.; Hamledari, H.; Khosravi, E.; Dianatkhah, M.; Vaseghi, G. Effects of selenium intake on the expression of prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2) and matrix metallopeptidase-9 genes in the coronary artery disease: Selenegene study, a double-blind randomized controlled trial. ARYA Atheroscler. J. 2021, 17, 2093. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).