Exposure to (Poly)phenol Metabolites after a Fruit and Vegetable Supplement Intake: A Double-Blind, Cross-Over, Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of a Nutritional Supplement Containing 5 Servings of Fruit and Vegetable (Poly)phenols
2.1.1. Total Phenolic Content
2.1.2. Antioxidant Capacity
2.1.3. HPLC Analysis of Phenolic Compounds in the Supplement
2.2. Pharmacokinetics and Urinary Excretion
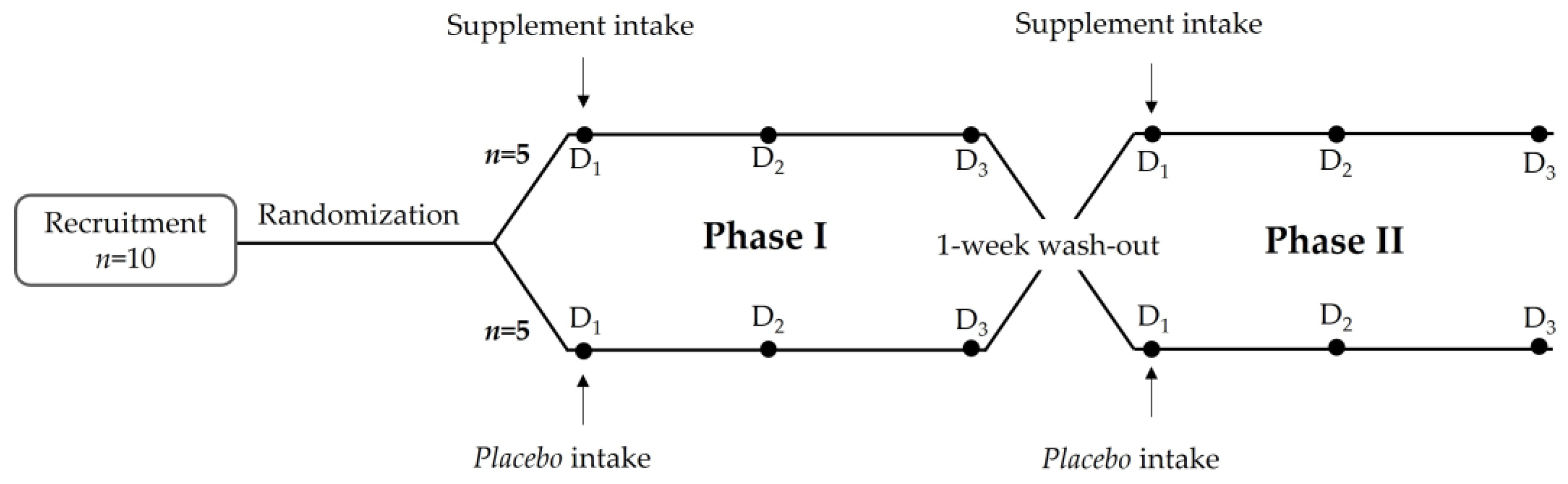
2.2.1. Subjects
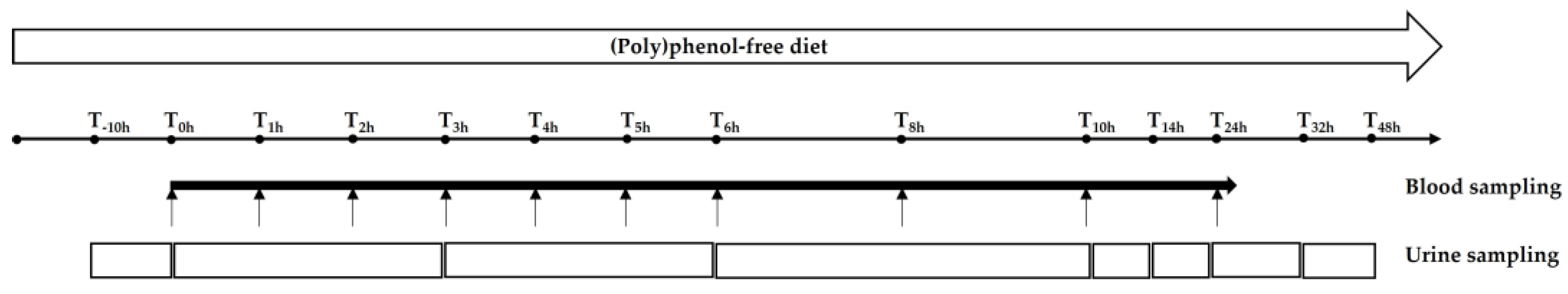
2.2.2. Study Design
2.2.3. Plasma Pharmacokinetics
2.2.4. Urinary Excretion
2.2.5. Plasma and Urine UHPLC-ESI-MS/MS Analysis
2.2.6. Data Analysis
3. Results
3.1. Total Phenolic Content and Antioxidant Capacity
3.2. HPLC Analysis If Phenolic Compounds in the Supplement
3.3. Characteristics of the Subjects
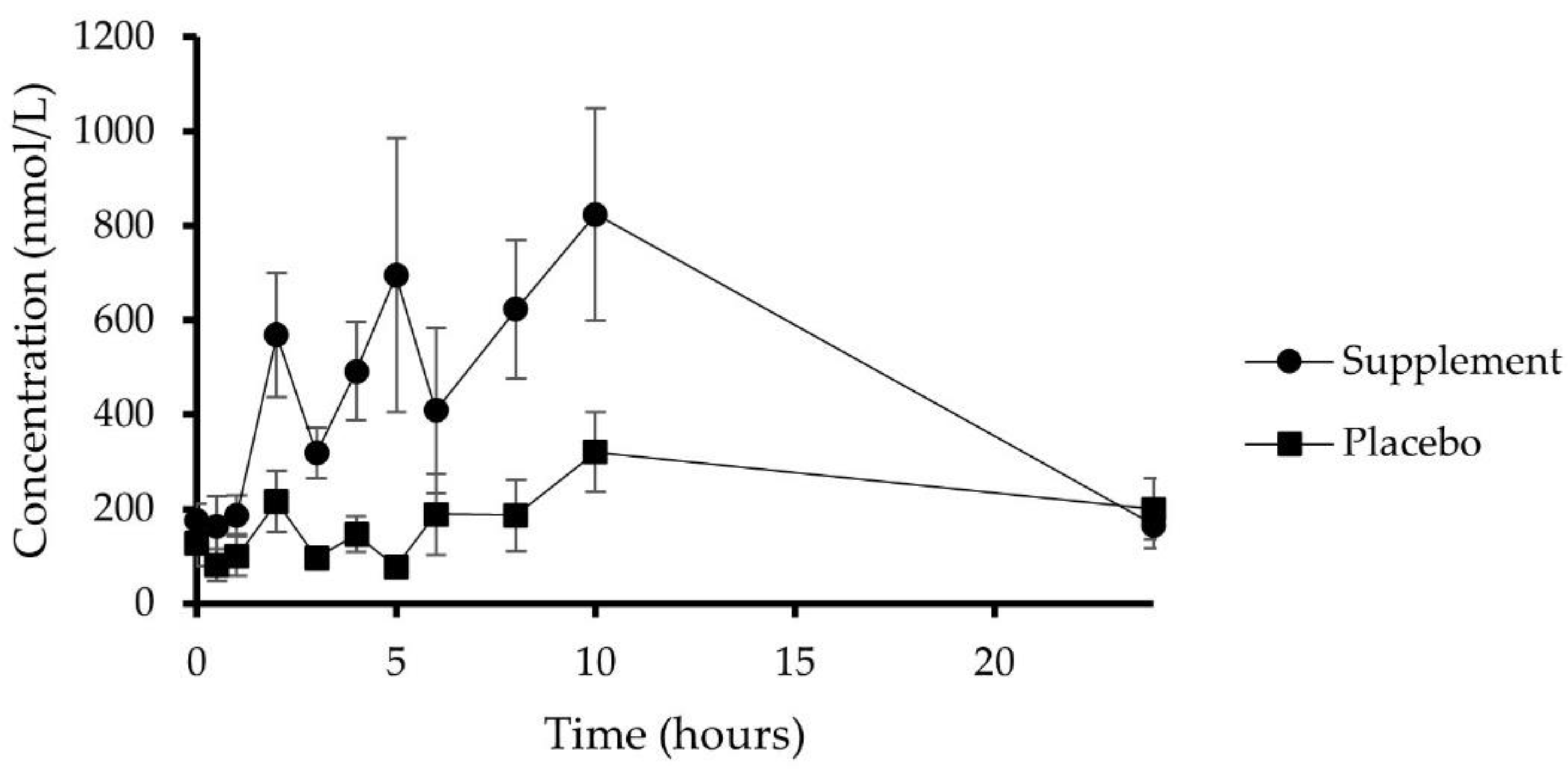
3.4. Plasma Pharmacokinetics
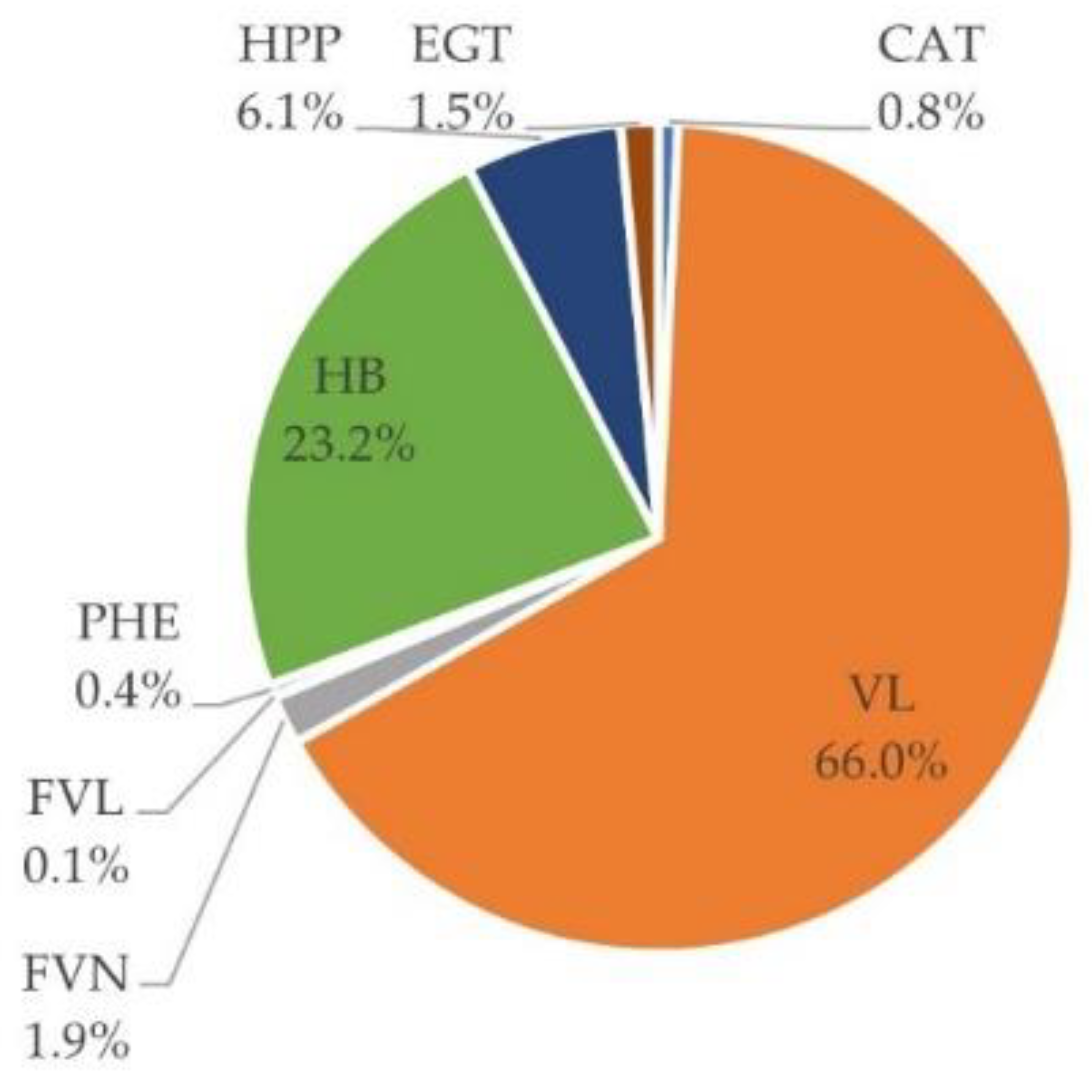
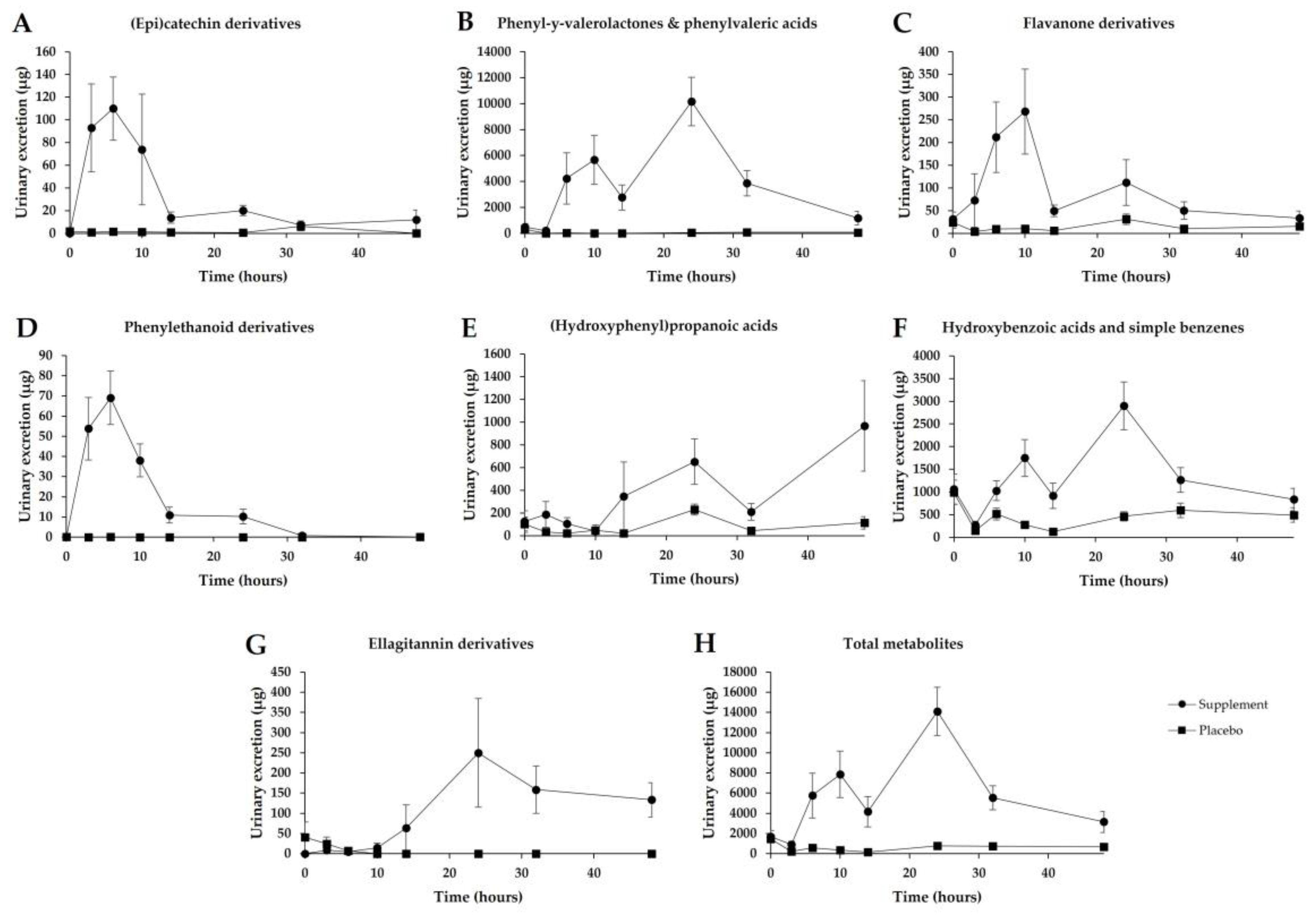
3.5. Urinary Excretion
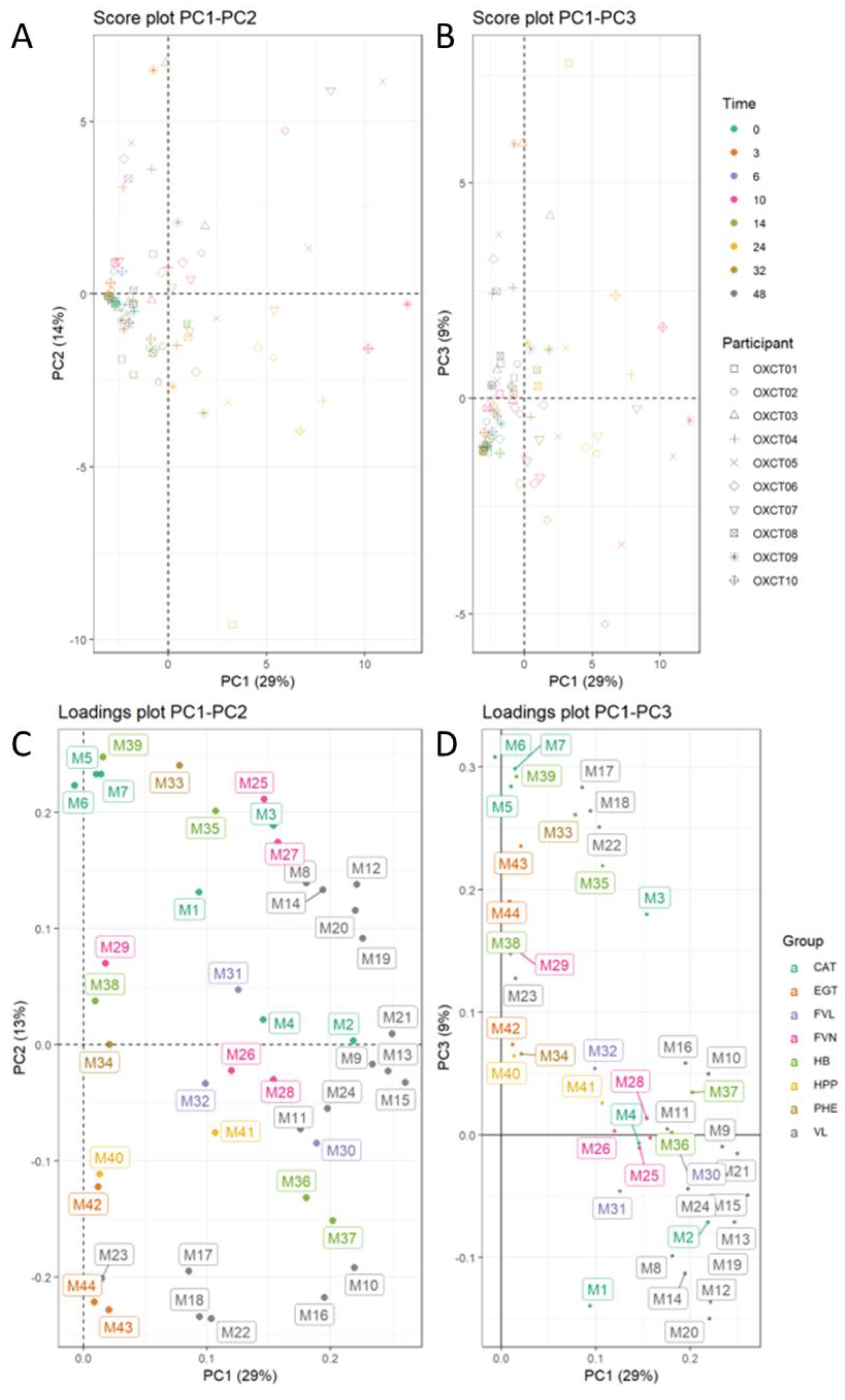
3.6. Inter-Individual Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial Factors in Eastern Europe (HAPIEE) Study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary polyphenol intake, blood pressure, and hypertension: A systematic review and meta-analysis of observational studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary Reference Intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Ziaullah; Rupasinghe, H.P.V. Application of NMR spectroscopy in plant polyphenols associated with human health. Appl. NMR Spectrosc. 2015, 2, 3–92. [Google Scholar]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Fruit and Vegetables for Health—Report of a Joint FAO/WHO Workshop; FAO/WHO: Rome, Italy, 2004. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: http://www.who.int/elena/titles/bbc/fruit_vegetables_ncds/en/ (accessed on 23 November 2018).
- Leenders, M.; Sluijs, I.; Ros, M.M.; Boshuizen, H.C.; Siersema, P.D.; Ferrari, P.; Weikert, C.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.C.; et al. Fruit and vegetable consumption and mortality. Am. J. Epidemiol. 2013, 178, 590–602. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.A.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and vegetable intake and mortality: Results from 2 prospective cohort studies of US men and women and a meta-analysis of 26 cohort studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Hartley, L.; Igbinedion, E.; Holmes, J.; Flowers, N.; Thorogood, M.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2013, 6, CD009874. [Google Scholar]
- Rekhy, R.; McConchie, R. Promoting consumption of fruit and vegetables for better health. Have campaigns delivered on the goals? Appetite 2014, 79, 113–123. [Google Scholar] [CrossRef]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409. [Google Scholar] [CrossRef]
- Kantar World Panel. Les Fruits et Légumes Les Plus Consommés en 2018. Available online: https://www.lesfruitsetlegumesfrais.com/filiere-et-metiers/les-chiffres-cles/les-produits-les-plus-consommes (accessed on 23 November 2018).
- Brat, P.; Georgé, S.; Bellamy, A.; Chaffaut, L.D.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef]
- Cases, J.; Romain, C.; Marín-Pagán, C.; Chung, L.H.; Rubio-Pérez, J.M.; Laurent, C.; Gaillet, S.; Prost-Camus, E.; Prost, M.; Alcarar, P.E. Supplementation with a polyphenol-rich extract, Perfload, improves physical performance during high-intensity exercise: A randomized, double blind, crossover trial. Nutrients 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus Officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica Granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Cossu, M.; Mena, P.; Sayegh, M.; Ray, S.; Del Rio, D. (Poly)phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition 2015, 3, 11–19. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Vijayananthan, A.; Nawawi, O. The importance of Good Clinical Practice Guidelines and its role in clinical trials. Biomed. Imaging Interv. J. 2008, 4, e5. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Brindani, N.; Mena, P.; Calani, L.; Benzie, I.; Choi, S.-W.; Brighenti, F.; Zanardi, F.; Curti, C.; Del Rio, D. Synthetic and analytical strategies for the quantification of phenyl-γ-valerolactone conjugated metabolites in human urine. Mol. Nutr. Food Res. 2017, 61, 1700077. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. MixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.E.; Lamprecht, M.; et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Di Pede, G.; Mena, P.; Bresciani, L.; Almutairi, T.M.; Del Rio, D.; Clifford, M.N.; Crozier, A. Human colonic catabolism of dietary flavan-3-ol bioactives. Mol. Asp. Med. 2022, in press. [CrossRef] [PubMed]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the major phenolic compounds in pomegranate juices by HPLC-DAD-ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Kashiwada, Y.; Ikeshiro, Y.; Hatano, Y.; Shida, Y.; Horie, M.; Matsugo, S.; Konishi, T. Complete assignment of bilberry (Vaccinium Myrtillus L.) anthocyanins separated by capillary zone electrophoresis. Chem. Pharm. Bull. 2004, 52, 226–229. [Google Scholar] [CrossRef]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of grape and wine anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef]
- McCall, D.O.; McGartland, C.P.; McKinley, M.C.; Patterson, C.C.; Sharpe, P.; McCance, D.R.; Young, I.S.; Woodside, J.V. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation 2009, 119, 2153–2160. [Google Scholar] [CrossRef]
- Baldrick, F.R.; Woodside, J.V.; Elborn, J.S.; Young, I.S.; McKinley, M.C. Biomarkers of fruit and vegetable intake in human intervention studies: A systematic review. Crit. Rev. Food Sci. Nutr. 2011, 51, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Muli, S.; Huybrechts, I.; Nöthlings, U.; Ahrens, W.; Scalbert, A.; Floegel, A. Assessment of fruit and vegetables intake with biomarkers in children and adolescents and their level of validation: A systematic review. Metabolites 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Cas, A.D.; Bonadonna, R.; Brighenti, F.; Del Rio, D. Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds. Am. J. Clin. Nutr. 2021, 114, 2107–2118. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53, S44–S53. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.M.; Stehle, P.; Simon, M.-C. Microbial metabolites of flavan-3-ols and their biological activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Riedl, K.M.; Cooperstone, J.L.; Högel, J.; Bosy-Westphal, A.; Schwartz, S.J.; Carle, R.; Schweiggert, R.M. Urinary excretion of citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol. Nutr. Food Res. 2016, 60, 2602. [Google Scholar] [CrossRef]
- Castello, F.; Fernández-Pachón, M.S.; Cerrillo, I.; Escudero-López, B.; Ortega, Á.; Rosi, A.; Bresciani, L.; Del Rio, D.; Mena, P. Absorption, metabolism, and excretion of orange juice (poly)phenols in humans: The effect of a controlled alcoholic fermentation. Arch. Biochem. Biophys. 2020, 695, 108627. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human Absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea Europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Calatayud, M.; Romo-vaquero, M.; Espín, J.C.; Selva, M.V.; Collado, M.C. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef]
- Morand, C.; De Roos, B.; Garcia-Conesa, M.T.; Gibney, E.R.; Landberg, R.; Manach, C.; Milenkovic, D.; Rodriguez-Mateos, A.; Van de Wiele, T.; Tomas-Barberan, F. Why interindividual variation in response to consumption of plant food bioactives matters for future personalised nutrition. Proc. Nutr. Soc. 2020, 79, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Yuan, J.-M.; Yang, C.S.; Van Den Berg, D.J.; Lee, M.-J.; Gao, Y.-T.; Yu, M.C. Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int. J. Mol. Epidemiol. Genet. 2010, 1, 114. [Google Scholar] [PubMed]
- Ung, d.; Nagar, S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab. Dispos. 2007, 35, 740–746. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Lee, P.G.; Lee, U.J.; Song, H.; Choi, K.Y.; Kim, B.G. Recent advances in the microbial hydroxylation and reduction of soy isoflavones. FEMS Microbiol. Lett. 2018, 365, fny195. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Mena, P.; Favari, C.; Acharjee, A.; Chernbumroong, S.; Bresciani, L.; Curti, C.; Brighenti, F.; Heiss, C.; Rodriguez-Mateos, A.; Del Rio, D. Metabotypes of flavan-3-ol colonic metabolites after cranberry intake: Elucidation and statistical approaches. Eur. J. Nutr. 2022, 61, 1299–1317. [Google Scholar] [CrossRef]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Curti, C.; Brindani, N.; Battistini, L.; Sartori, A.; Pelosi, G.; Mena, P.; Brighenti, F.; Zanardi, F.; Del Rio, D. Catalytic, Enantioselective Vinylogous Mukaiyama Aldol Reaction of Furan-Based Dienoxy Silanes: A Chemodivergent Approach to γ-Valerolactone Flavan-3-ol Metabolites and δ-Lactone Analogues. Adv. Synth. Catal. 2015, 357, 4082–4092. [Google Scholar] [CrossRef]
| Five Fruits and Vegetables—400 g | Supplement—450 mg | |
|---|---|---|
| Total (poly)phenol content (mg GAE) | 228 ± 23 | 230 ± 23 |
| Total antioxidant capacity (mg GAE) | 396 ± 20 | 383 ± 19 |
| Family | Mean (mg/450 mg) |
|---|---|
| Flavan-3-ols | 50.7 ± 3.2 |
| Flavanones | 39.1 ± 2.9 |
| Ellagitannins | 26.7 ± 2.0 |
| Secoiridois and Phenylethanoids | 22.2 ± 0.9 |
| Anthocyanins | 6.1 ± 1.0 |
| TOTAL (poly)phenols | 144.8 ± 9.9 |
| Compounds | Mean (µg/450 mg) |
|---|---|
| Flavan-3-ols | |
| (+)-Catechin | 4100.4 ± 169.5 |
| (−)-Epicatechin | 4737.9 ± 69.0 |
| (+)-Gallocatechin | 399.9 ± 5.9 |
| (−)-Epigallocatechin | 1989.7 ± 67.6 |
| (Epi)catechin gallate | 28,879.6 ± 639.5 |
| (Epi)gallocatechin-methylgallate | 294.6 ± 3.6 |
| (−)-Epigallocatechin gallate | 27,109.5 ± 587.8 |
| (Epi)gallocatechin gallate | 1516.6 ± 76.1 |
| Procyanidin dimer A-type | 4441.0 ± 363.0 |
| Procyanidin dimer B-type | 571.7 ± 33.7 |
| Procyanidin dimer B-type | 914.9 ± 47.7 |
| Procyanidin dimer B-type | 432.5 ± 25.9 |
| Procyanidin dimer B-type | 276.0 ± 5.1 |
| Prodelphinidin dimer B-type (1 unit of (epi)GC + 1(epi)C) | 70.7 ± 2.2 |
| Prodelphinidin dimer B-type (1 unit of (epi)GC + 1(epi)C) | 115.7 ± 3.7 |
| Prodelphinidin dimer B-type gallate (1 unit of (epi)GC + 1(epi)C) | 1339.0 ± 96.7 |
| Prodelphinidin tetramer B-type gallate (2 unit of (epi)GC + 2(epi)C) | 303.2 ± 14.9 |
| Prodelphinidin tetramer B-type gallate (2 unit of (epi)GC + 2(epi)C) | 257.0 ± 48.6 |
| Prodelphinidin dimer B-type gallate (2 units (epi)GC) | 83.6 ± 3.6 |
| Procyanidin trimer B-type | n.q. |
| Procyanidin trimer B-type | n.q. |
| Procyanidin trimer B-type | n.q. |
| (Epi)catechin-gallate dimer | n.q. |
| Sum of Flavan-3-ols (mg/450 mg) | 77.83 ± 0.91 |
| Ellagitannins | |
| Ellagic acid | 293.9 ± 8.5 |
| Galloyl-hexoside | 1.7 ± 0.3 |
| Galloyl-hexoside | 10.9 ± 0.4 |
| Galloyl-hexoside | 27.2 ± 1.2 |
| Galloyl-hexoside | 21.6 ± 1.5 |
| Galloyl-hexoside | 22.3 ± 0.8 |
| Ellagic acid hexoside | 116.6 ± 9.1 |
| Ellagic acid dimethyl ether glucurionide | 5.3 ± 1.0 |
| Galloyl-HHDP-hexoside | 69.9 ± 2.2 |
| Galloyl-HHDP-hexoside | 4.5 ± 0.6 |
| Galloyl-HHDP-hexoside | 49.5 ± 3.0 |
| Galloyl-HHDP-hexoside | 55.4 ± 4.6 |
| Galloyl-HHDP-hexoside | 88.2 ± 1.6 |
| Galloyl-HHDP-hexoside | 360.7 ± 27.8 |
| Galloyl-HHDP-hexoside | 252.0 ± 9.3 |
| Gallagyl-hexoside (Punicalin α/A) | 93.1 ± 2.3 |
| Gallagyl-hexoside (Punicalin β/B) | 1266.9 ± 156.5 |
| Bis-HHDP-hexoside (Pedunculagin I isomer) | 41.0 ± 0.5 |
| Bis-HHDP-hexoside (Pedunculagin I isomer) | 249.7 ± 10.2 |
| Bis-HHDP-hexoside (Pedunculagin I isomer) | 238.7 ± 11.7 |
| Galloyl-bis-HHDP-hexoside (Casuarinin) | 145.0 ± 9.3 |
| Punicalagin isomer | 1026.7 ± 59.8 |
| HHDP-gallagyl-hexoside (Punicalagin α) | 10,935.5 ± 1237.4 |
| HHDP-gallagyl-hexoside (Punicalagin β) | 24,246.1 ± 1017.9 |
| Di(HHDP-galloylglucose)-pentoside | 2029.8 ± 182.0 |
| Di(HHDP-galloylglucose)-pentoside | 181.8 ± 12.0 |
| Di(HHDP-galloylglucose)-pentoside | 662.2 ± 42.6 |
| Sum of Ellagitannins (mg/450 mg) | 42.50 ± 2.22 |
| Phenylethanoids | |
| Tyrosol (tentative identification) | 159.0 ± 8.7 |
| Hydroxytyrosol | 21,065.2 ± 865.2 |
| Hydroxytyrosol-O-hexoside | 10,467.2 ± 367.0 |
| Oleoside | 517.1 ± 12.0 |
| Verbascoside (Caffeoyl-phenylethanoid glycoside) | 488.9 ± 15.0 |
| Verbascoside (Caffeoyl-phenylethanoid glycoside) | 81.8 ± 4.1 |
| Sum of Phenylethanoids (mg/450 mg) | 32.78 ± 1.25 |
| Flavanones | |
| Naringenin | 56.6 ± 2.2 |
| Eriodyctiol | 9.0 ± 0.5 |
| Tetrahydroxyflavanone | 6.6 ± 0.2 |
| Naringenin-O-glucoside | 7.4 ± 0.8 |
| Tetrahydroxyflavanone-O-rhamnoside (tentative identification) | 22.2 ± 0.3 |
| Hesperetin-O-hexoside | 681.5 ± 19.9 |
| Naringenin-7-O-rutinoside (Narirutin) | 427.8 ± 4.2 |
| Naringenin-7-O-neohesperidoside (Naringin) | 17,923.8 ± 297.3 |
| Naringenin-O-neohesperidoside | 69.9 ± 1.3 |
| Isosakuranetin-O-rutinoside (Didymin) | 739.6 ± 6.9 |
| Eriocitrin or Neoeriocitrin | n.q. |
| Naringenin-C-dihexoside | 37.9 ± 1.2 |
| Hesperetin-7-O-rutinoside (Hesperidin) | 7734.6 ± 635.6 |
| Hesperetin-7-O-neohesperidoside (Neohesperidin) | 90.6 ± 7.3 |
| Sum of Flavanones (mg/450 mg) | 27.81 ± 0.95 |
| Seco-iridoids | |
| Oleuropein aglycone | n.q. |
| Oleuropein aglycone | n.q. |
| Oleuropein aglycone | n.q. |
| Oleuropein aglycone | n.q. |
| Oleuropein aglycone | n.q. |
| Oleuropein | 15,980.1 ± 1067.3 |
| Oleuropein | 3930.0 ± 200.7 |
| Sum of Seco-iridoids (mg/450 mg) | 19.91 ± 1.27 |
| Flavones | |
| Apigenin | n.q. |
| Trihydroxyflavone | 221.7 ± 2.7 |
| Apigenin-O-hexoside | 100.4 ± 2.8 |
| Luteolin-O-hexoside | 597.1 ± 18.5 |
| Luteolin-O-hexoside | 3465.2 ± 142.5 |
| Luteolin-O-hexoside | 1420.6 ± 62.3 |
| Luteolin-O-hexoside | 509.2 ± 4.3 |
| Dihydroluteolin-O-hexoside | 264.7 ± 3.3 |
| Apigenin-O-rutinoside | 239.2 ± 2.5 |
| Luteolin-O-rutinoside | 297.7 ± 4.5 |
| Luteolin-O-rutinoside | 207.9 ± 8.2 |
| Chrysoeriol-O-rutinoside or Diosmetin-O-rutinoside | 35.3 ± 0.4 |
| Luteolin-O-dihexoside (tentative identification) | 50.5 ± 7.4 |
| Luteolin-O-dihexoside | 32.0 ± 1.1 |
| Sum of Flavones (mg/450 mg) | 7.44 ± 0.10 |
| Flavonols | |
| Quercetin | 3448.8 ± 256.9 |
| Rhamnetin | 1.7 ± 0.1 |
| Isorhamnetin | 8.0 ± 0.4 |
| Myricetin | 7.5 ± 0.4 |
| Quercetin-O-pentoside | 67.8 ± 2.5 |
| Myricetin-O-rhamnoside | 115.4 ± 11.3 |
| Quercetin-O-hexoside | 428.0 ± 10.2 |
| Quercetin-O-glucuronide | 706.1 ± 16.5 |
| Myricetin-O-hexoside | 330.5 ± 5.9 |
| Kaempferol-O-rutinoside | 86.4 ± 4.2 |
| Tetrahydroxy-dimethoxyflavone-O- hexoside (Syringetin-O-hexoside) | 70.6 ± 1.6 |
| Isorhamnetin-O- dirhamnoside | 225.6 ± 6.5 |
| Quercetin-O-rutinoside (Rutin) | 187.4 ± 8.6 |
| Isorhamnetin-O-rutinoside | 106.6 ± 1.6 |
| Sum of Flavonols (mg/450 mg) | 5.79 ± 0.25 |
| Hydroxybenzoic acids | |
| 3-Hydroxybenzoic acid | 365.5 ± 25.9 |
| 4-Hydroxybenzoic acid | 191.7 ± 4.5 |
| Hydroxybenzoic acid | 349.5 ± 12.1 |
| 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | 152.9 ± 7.3 |
| Dihydroxybenzoic acid | 98.9 ± 7.7 |
| Dihydroxyphenylacetic acid | 110.1 ± 34.6 |
| Dihydroxyphenylacetic acid | 186.3 ± 26.8 |
| Dihydroxyphenylacetic acid | 183.3 ± 9.0 |
| Gallic acid | 1804.3 ± 38.2 |
| Hydroxyphenyllactic acid | n.q. |
| Ethyl-gallate | 415.5 ± 11.2 |
| Sum of Hydroxybenzoic acids (mg/450 mg) | 3.81 ± 0.01 |
| Anthocyanins | |
| Cyanidin-3-O-hexoside | 392.9 ± 5.1 |
| Malvidin-3-O-arabinoside | 39.8 ± 0.3 |
| Peonidin-3-O-hexoside | 156.6 ± 7.1 |
| Delphinidin 3-O-hexoside | 370.6 ± 24.6 |
| Petunidin-3-O-hexoside | 251.6 ± 5.4 |
| Malvidin-3-O-hexoside | 285.4 ± 11.4 |
| Cyanidin-3-O-rutinoside | 87.3 ± 0.9 |
| Delphinidin 3-O-rutinoside | 106.5 ± 3.4 |
| Sum of Anthocyanins (mg/450 mg) | 1.69 ± 0.05 |
| Hydroxycinnamic acids | |
| Caffeic acid | 206.6 ± 2.0 |
| 5-Caffeoylquinic acid | 382.7 ± 8.6 |
| Ferulic acid-O-hexoside | 26.4 ± 1.5 |
| Sum of Hydroxycinnamic acids (mg/450 mg) | 0.62 ± 0.01 |
| Stilbenoids | |
| Piceid (Resveratrol-O-glucoside) | 514.2 ± 13.6 |
| Sum of Stilbenoids (mg/450 mg) | 0.51 ± 0.01 |
| Gallotannins | |
| Digalloylglucose | 82.9 ± 1.8 |
| Digalloylglucose | 6.5 ± 0.1 |
| Digalloylglucose | 27.5 ± 0.9 |
| Digalloylglucose | 49.3 ± 4.9 |
| Digalloylglucose | 33.1 ± 0.3 |
| Trigalloylglucose | 112.7 ± 1.4 |
| Sum of Gallotannins (mg/450 mg) | 0.31 |
| Dihydrochalcones | |
| Phloretin | 32.0 ± 0.3 |
| Sum of Dihydrochalcones (mg/450 mg) | 0.03 ± 0.01 |
| Coumarin | |
| Scopoletin-O-hexoside | n.q. |
| Sum of Coumarin (mg/450 mg) | n.q. |
| TOTAL (POLY)PHENOL | 221.1 ± 9.5 (mg/450 mg) 594.9 ± 14.5 (µmol/450 mg) |
| Id. | Phenolic Metabolites | AUC0-24 (CV) (nmol/L·h) | Tpres (h) | Tmax (h) | Cmax (nmol/L) |
|---|---|---|---|---|---|
| (Epi)catechin derivatives | |||||
| 5 | (Epi)catechin-sulfate_isomer 1 | 18.41 ± 4.09 (70) | 6.65 ± 2.03 | 8.15 ± 2.69 | 4.72 ± 0.96 |
| 4 | (Epi)catechin-glucuronide_isomer 2 | 43.70 ± 6.66 (48) | 10.30 ± 2.29 | 3.60 ± 0.91 | 10.88 ± 2.44 |
| 3 | Methoxy(epi)gallocatechin glucuronide | 9.57 ± 2.70 (89) | 4.86 ± 1.64 | 3.14 ± 1.20 | 4.72 ± 0.62 |
| Sum of (Epi)catechin derivatives | 71.68 ± 9.08 (40) | 13.35 ± 2.53 | 3.10 ± 0.87 | 16.37 ± 2.13 | |
| Flavanone derivatives | |||||
| 26 | Hesperetin-diglucuronide | 5.09 ± 1.32 (82) | 6.17 ± 2.04 | 10.78 ± 2.55 | 1.49 ± 0.37 |
| 27 | Naringenin-glucuronide | 339.74 ± 75.66 (70) | 14.70 ± 2.57 | 5.30 ± 0.98 | 95.42 ± 29.73 |
| 28 | Hesperetin-7-glucuronide | 152.05 ± 27.84 (58) | 17.83 ± 1.54 | 7.11 ± 0.90 | 19.95 ± 2.31 |
| 29 | Hesperetin-sulfate | 173.05 ± 55.23 (101) | 15.35 ± 1.92 | 6.50 ± 0.86 | 31.62 ± 11.38 |
| Sum of flavanone derivatives | 669.93 ± 98.61 (47) | 21.65 ± 0.42 | 5.30 ± 1.17 | 124.75 ± 39.01 | |
| Other flavonoid derivatives | |||||
| 48 | Luteolin-sulfate | 3.41 ± 0.67 (62) | 7.60 ± 2.19 | 1.55 ± 0.24 | 0.44 ± 0.14 |
| Phenylethanoid derivatives | |||||
| 33 | 2-(Phenyl)ethanol-3′-glucuronide (Hydroxytyrosol-glucuronide) | 50.92 ± 5.85 (36) | 8.45 ± 2.19 | 1.45 ± 0.32 | 24.94 ± 5.63 |
| Phenyl-y-valerolactones and phenyl valeric acids | |||||
| 20 | 5-(Methoxyhydroxyphenyl)-γ-valerolactone-sulfate | 31.12 ± 8.29 (84) | 16.25 ± 1.44 | 8.25 ± 0.80 | 7.75 ± 2.52 |
| 9 | 5-(Methoxy-hydroxyphenyl)-γ-valerolactone- glucuronide | 7.63 ± 2.52 (105) | 9.00 ± 3.06 | 10.00 ± 3.15 | 1.54 ± 0.35 |
| 16 | 5-(5′-Hydroxyphenyl)-γ-valerolactone-3′-sulfate | 30.46 ± 11.85 (123) | 10.57 ± 2.72 | 8.88 ± 2.41 | 9.97 ± 4.31 |
| 21 | 5-(3′-Hydroxyphenyl)-γ-valerolactone-4′-sulfate | 141.70 ± 50.37 (112) | 12.15 ± 1.84 | 7.00 ± 0.75 | 40.05 ± 16.85 |
| 10 | 5-(5′-Hydroxyphenyl)-γ-valerolactone-3′-glucuronide | 125.55 ± 47.68 (120) | 14.44 ± 2.42 | 12.78 ± 2.85 | 14.96 ± 5.61 |
| 13 | 5-(Phenyl)-γ-valerolactone-sulfate-glucuronide | 10.24 ± 3.34 (103) | 10.17 ± 2.42 | 8.67 ± 2.17 | 1.55 ± 0.29 |
| 22 | 5-(Methoxyphenyl)-γ-valerolactone-sulfate | 18.59 ± 8.47 (144) | 7.13 ± 2.33 | 6.50 ± 1.28 | 7.34 ± 4.47 |
| 23 | 5-Phenyl-γ-valerolactone-3′-sulfate | 10.11 ± 3.89 (122) | 9.33 ± 2.45 | 8.22 ± 2.13 | 2.15 ± 0.48 |
| 17 | 5-(Phenyl)-γ-valerolactone-3′-glucuronide | 22.00 ± 6.41 (92) | 7.13 ± 2.27 | 5.88 ± 0.95 | 6.34 ± 1.66 |
| 15 | 4-Hydroxy-5-(hydroxyphenyl)valeric acid-sulfate | 61.35 ± 23.81 (123) | 13.06 ± 2.97 | 6.19 ± 1.06 | 13.10 ± 3.94 |
| 18 | 4-Hydroxy-5-(phenyl)valeric acid-sulfate | 47.47 ± 18.41 (123) | 10.30 ± 2.28 | 5.60 ± 0.88 | 11.73 ± 2.95 |
| 14 | 5-(Methoxyphenyl)valeric acid-glucuronide | 39.35 ± 14.74 (118) | 12.06 ± 2.47 | 7.22 ± 0.91 | 8.80 ± 3.60 |
| Sum of Phenyl-y-valerolactones and phenyl valeric acids | 545.58 ± 155.32 (90) | 21.60 ± 0.72 | 8.50 ± 2.30 | 84.55 ± 36.40 | |
| (Hydroxyphenyl)propanoic acids | |||||
| 41 | 3-(4′-hydroxyphenyl)propanoic acid-3′-sulfate (Dihydrocaffeic acid-sulfate) | 27.41 ± 11.21 (129) | 9.06 ± 2.56 | 4.56 ± 1.13 | 18.24 ± 11.27 |
| 46 | 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate (Dihydroferulic acid-sulfate) | 558.28 ± 531.21 (301) | 7.14 ± 3.61 | 6.43 ± 2.97 | 168.73 ± 156.13 |
| 40 | 3-(3′-Hydroxyphenyl)propanoic acid (3-(3-Hydroxyphenyl)propionic acid) | 1554.29 ± 644.33 (131) | 7.50 ± 2.38 | 7.13 ± 0.77 | 443.09 ± 99.28 |
| Sum of (hydroxyphenyl)propanoic acids | 2139.98 ± 953.73 (141) | 12.60 ± 2.76 | 5.50 ± 1.01 | 425.53 ± 122.90 | |
| Hydroxybenzoic acids and simple benzenes | |||||
| 45 | 4-Hydroxybenzoic acid-3-sulfate (Protocatechuic acid-3-sulfate) | 196.06 ± 32.72 (53) | 13.45 ± 2.80 | 2.10 ± 0.46 | 43.60 ± 7.34 |
| 47 | 4′-Hydroxyhippuric acid | 5719.44 ± 1327.87 (73) | 21.55 ± 0.74 | 6.50 ± 1.28 | 816.10 ± 269.47 |
| 36 | Dihydroxybenzene-sulfate (Pyrogallol-sulfate) | 1677.03 ± 322.08 (61) | 23.30 ± 0.27 | 7.10 ± 0.86 | 185.03 ± 34.67 |
| 37 | Methoxyhydroxybenzene-sulfate (Pyrogallol-methoxy-sulfate) | 885.81 ± 266.69 (95) | 21.35 ± 1.41 | 7.00 ± 0.93 | 108.13 ± 24.62 |
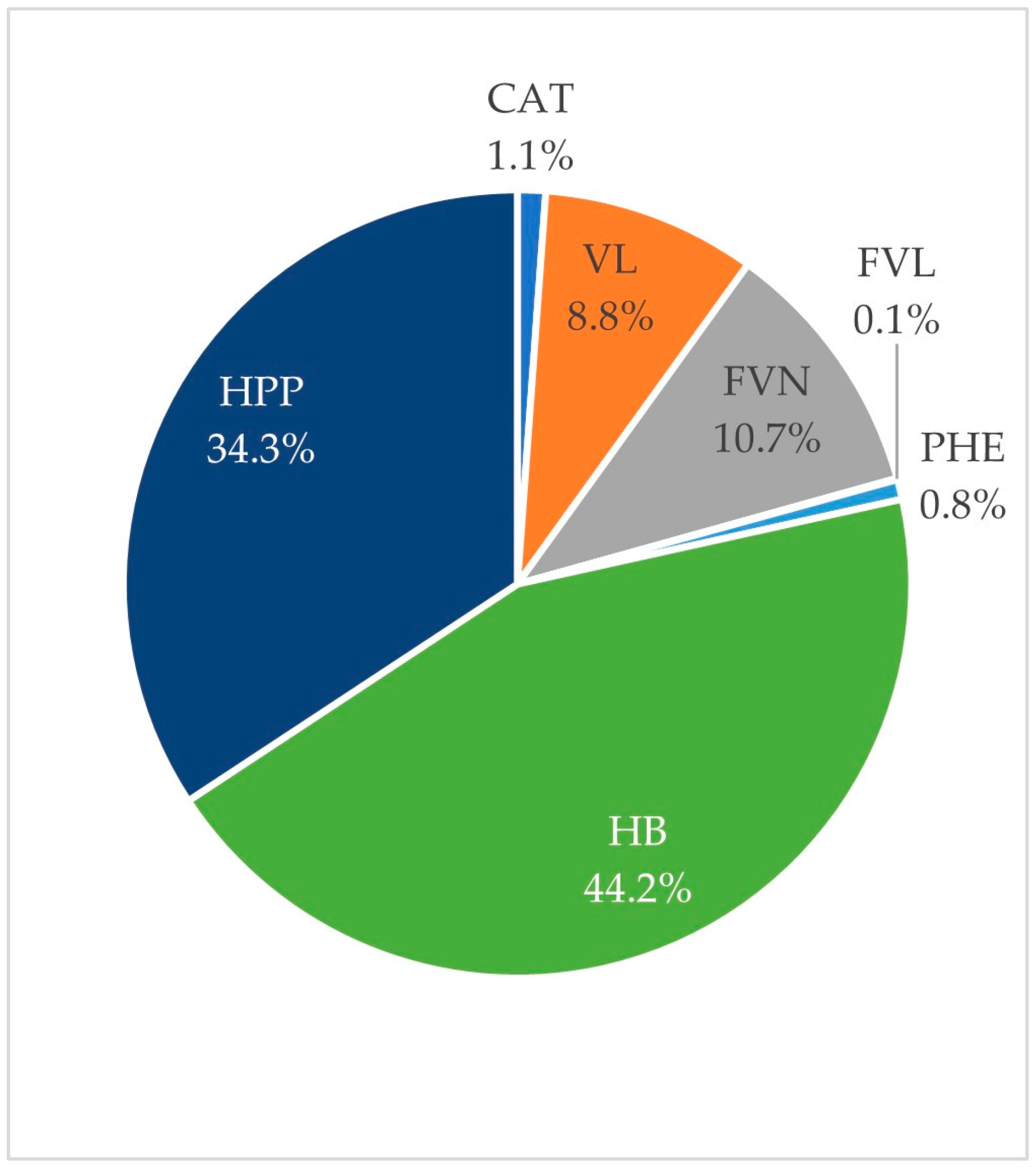
| Sum of hydroxybenzoic acids and simple benzenes | 8478.34 ± 1448.99 (54) | 23.60 ± 0.22 | 7.70 ± 0.86 | 958.18 ± 269.69 | |
| SUM of all metabolites | 11,959.85 ± 2200.65 (58) | 23.80 ± 0.24 | 6.90 ± 0.96 | 1271.11 ± 281.33 |
| Id. | Phenolic Metabolites | 0 (h) | 0–3 (h) | 3–6 (h) | 6–10 (h) | 10–14 (h) | 14–24 (h) | 24–32 (h) | 32–48 (h) | Total (0–48 h) | CV (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Epi)catechin derivatives | |||||||||||
| 5 | (Epi)catechin-sulfate_isomer 1 | nd | 14.26 ± 7.60 | 10.63 ± 3.02 | 0.49 ± 0.49 | nd | nd | nd | 2.28 ± 2.28 | 27.65 ± 8.42 | 96 |
| 6 | (Epi)catechin-sulfate_isomer 2 | nd | 36.14 ± 15.62 | 29.95 ± 11.99 | 0.00 ± 0.00 | 1.66 ± 1.20 | 4.12 ± 2.29 | 2.06 ± 2.06 | 9.52 ± 6.72 | 83.45 ± 19.91 | 75 |
| 2 | (Epi)catechin-glucuronide_isomer 1 | nd | 0.17 ± 0.17 | 2.60 ± 1.15 | 9.09 ± 3.87 | 3.10 ± 1.10 | 5.67 ± 1.80 | 1.72 ± 0.92 | nd | 22.35 ± 5.67 | 80 |
| 4 | (Epi)catechin-glucuronide_isomer 2 | nd | 0.59 ± 0.59 | 14.00 ± 7.73 | 53.64 ± 44.88 | 3.19 ± 1.65 | 3.65 ± 2.21 | 1.22 ± 1.22 | nd | 76.29 ± 45.22 | 187 |
| 7 | Methoxy(epi)catechin-sulfate | nd | 39.90 ± 19.16 | 48.81 ± 12.64 | 7.02 ± 4.78 | 4.33 ± 2.25 | 5.00 ± 3.44 | 2.12 ± 2.12 | nd | 107.19 ± 26.64 | 79 |
| 1 | Methoxy-(epi)catechin-glucuronide | nd | nd | 0.59 ± 0.52 | 1.29 ± 0.94 | 0.74 ± 0.36 | 0.24 ± 0.22 | nd | nd | 2.86 ± 1.48 | 164 |
| 3 | Methoxy-(epi)gallocatechin-glucuronide | nd | 1.91 ± 0.99 | 3.38 ± 0.81 | 2.36 ± 1.11 | 0.78 ± 0.33 | 1.33 ± 0.61 | 0.18 ± 0.18 | nd | 9.94 ± 2.24 | 71 |
| Sum of (Epi)catechin derivatives | nd | 92.97 ± 38.65 | 109.96 ± 27.97 | 73.89 ± 48.67 | 13.80 ± 5.01 | 20.00 ± 4.58 | 7.30 ± 3.54 | 11.80 ± 8.79 | 329.72 ± 86.05 | 83 | |
| Flavanone derivatives | |||||||||||
| 29 | Hesperetin-sulfate | nd | 2.03 ± 2.03 | 1.19 ± 0.81 | 0.23 ± 0.23 | nd | 1.22 ± 0.90 | 0.33 ± 0.33 | 2.21 ± 2.21 | 7.21 ± 5.41 | 237 |
| 28 | Hesperetin-7-glucuronide | 14.48 ± 5.94 | 4.02 ± 2.90 | 20.07 ± 4.27 | 28.56 ± 5.46 | 9.57 ± 2.19 | 30.72 ± 8.04 | 19.07 ± 5.57 | 22.33 ± 5.83 | 148.81 ± 14.88 | 32 |
| 26 | Hesperetin-diglucuronide | nd | 0.05 ± 0.05 | 0.16 ± 0.08 | 0.12 ± 0.07 | 0.10 ± 0.05 | 0.45 ± 0.26 | 0.16 ± 0.07 | 0.04 ± 0.04 | 1.09 ± 0.36 | 104 |
| 27 | Naringenin-glucuronide | 16.31 ± 16.31 | 65.92 ± 53.49 | 187.75 ± 72.43 | 237.98 ± 90.88 | 39.21 ± 11.90 | 78.88 ± 45.12 | 30.40 ± 16.54 | 9.13 ± 9.13 | 665.58 ± 148.66 | 71 |
| 25 | Naringenin-diglucuronide | 0.17 ± 0.17 | 0.56 ± 0.38 | 2.47 ± 1.02 | 1.66 ± 1.01 | 0.46 ± 0.21 | 0.54 ± 0.28 | 0.09 ± 0.09 | nd | 5.95 ± 1.56 | 83 |
| Sum of flavanone derivatives | 30.96 ± 15.78 | 72.59 ± 58.76 | 211.64 ± 77.44 | 268.55 ± 93.64 | 49.33 ± 12.85 | 111.81 ± 50.11 | 50.04 ± 19.22 | 33.70 ± 15.01 | 828.64 ± 159.16 | 61 | |
| Other flavonoid derivatives | |||||||||||
| 30 | Luteolin-glucuronide | nd | 1.84 ± 1.25 | 2.67 ± 0.96 | 3.39 ± 2.32 | 1.80 ± 0.84 | 5.97 ± 2.77 | 3.07 ± 2.06 | 6.12 ± 2.55 | 24.88 ± 7.86 | 100 |
| 31 | Myricetin-glucuronide | nd | 0.00 ± 0.00 | 0.72 ± 0.49 | 2.78 ± 1.05 | 1.81 ± 0.68 | 3.68 ± 1.04 | 1.30 ± 0.56 | nd | 10.29 ± 2.19 | 67 |
| 32 | Quercetin-diglucuronide | nd | 0.04 ± 0.04 | 0.39 ± 0.19 | 0.50 ± 0.25 | 0.21 ± 0.19 | 0.42 ± 0.25 | 0.30 ± 0.23 | nd | 1.86 ± 1.01 | 171 |
| Sum of other flavonoid derivatives | nd | 1.88 ± 1.29 | 3.78 ± 1.42 | 6.68 ± 2.59 | 3.83 ± 1.14 | 10.07 ± 2.88 | 4.67 ± 2.16 | 6.12 ± 2.55 | 37.03 ± 7.60 | 65 | |
| Phenylethanoid derivatives | |||||||||||
| 34 | Oleuropein-sulfate | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.03 ± 0.01 | nd | nd | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.02 | 0.10 ± 0.03 | 106 |
| 33 | 2-(Phenyl)ethanol-3′-glucuronide (Hydroxytyrosol-glucuronide) | nd | 53.75 ± 15.51 | 69.04 ± 13.20 | 38.02 ± 8.17 | 10.90 ± 3.90 | 10.23 ± 3.71 | 0.75 ± 0.75 | nd | 182.69 ± 19.99 | 35 |
| Sum of phenylethanoid derivatives | 0.02 ± 0.02 | 53.76 ± 15.51 | 69.06 ± 13.20 | 38.02 ± 8.17 | 10.90 ± 3.90 | 10.24 ± 3.72 | 0.77 ± 0.74 | 0.02 ± 0.02 | 182.79 ± 19.98 | 35 | |
| Phenyl-y-valerolactones and phenylvaleric acids | |||||||||||
| 8 | 5-(Dihydroxyphenyl)-γ-valerolactone-glucuronide | nd | nd | 23.24 ± 17.36 | 26.86 ± 11.59 | 6.17 ± 3.84 | 5.33 ± 2.23 | nd | nd | 61.61 ± 29.59 | 152 |
| 20 | 5-(Methoxy-hydroxyphenyl)-γ-valerolactone--sulfate | nd | 1.15 ± 0.85 | 123.73 ± 64.97 | 287.71 ± 81.19 | 98.28 ± 40.32 | 116.82 ± 44.46 | 21.40 ± 9.99 | 2.02 ± 2.02 | 651.12 ± 170.13 | 83 |
| 9 | 5-(Methoxyhydroxyphenyl)-γ-valerolactone- glucuronide | nd | nd | 9.83 ± 6.55 | 18.07 ± 6.62 | 9.28 ± 2.54 | 16.44 ± 4.84 | 4.47 ± 2.31 | nd | 58.09 ± 11.33 | 62 |
| 16 | 5-(5′-Hydroxyphenyl)-γ-valerolactone-3′-sulfate | 18.25 ± 10.84 | 4.64 ± 3.47 | 439.69 ± 219.42 | 1256.55 ± 516.93 | 944.18 ± 474.02 | 3986.28 ± 711.64 | 1721.60 ± 393.97 | 586.15 ± 240.93 | 8957.33 ± 1361.49 | 48 |
| 21 | 5-(3′-Hydroxyphenyl)-γ-valerolactone-4′-sulfate | 344.60 ± 186.50 | 166.29 ± 126.77 | 2548.76 ± 1207.06 | 2277.16 ± 795.52 | 919.49 ± 314.48 | 3618.13 ± 985.44 | 1346.37 ± 481.02 | 389.78 ± 215.48 | 11,610.60 ± 1923.81 | 52 |
| 10 | 5-(5′-Hydroxyphenyl)-γ-valerolactone-3′-glucuronide | 5.34 ± 5.34 | 0.00 ± 0.00 | 101.70 ± 51.36 | 316.86 ± 132.04 | 199.53 ± 88.98 | 624.93 ± 108.36 | 281.58 ± 60.75 | 65.29 ± 25.62 | 1595.23 ± 260.71 | 52 |
| 13 | 5-(Phenyl)-γ-valerolactone-sulfate-glucuronide | 0.34 ± 0.34 | 0.13 ± 0.13 | 9.26 ± 4.10 | 23.29 ± 7.81 | 10.14 ± 3.00 | 20.29 ± 4.37 | 7.77 ± 2.43 | 1.43 ± 0.97 | 72.65 ± 15.10 | 66 |
| 22 | 5-(Methoxy-phenyl)-γ-valerolactone-sulfate | 5.66 ± 3.78 | 2.16 ± 1.13 | 7.50 ± 2.92 | 18.92 ± 9.27 | 12.82 ± 4.86 | 22.47 ± 5.87 | 9.17 ± 3.61 | 4.24 ± 2.18 | 82.94 ± 15.05 | 57 |
| 23 | 5-(Phenyl)-γ-valerolactone-3′-sulfate | 41.07 ± 30.35 | 4.29 ± 2.19 | 52.98 ± 22.54 | 175.24 ± 98.80 | 76.01 ± 39.48 | 345.14 ± 198.47 | 100.92 ± 46.88 | 34.61 ± 16.03 | 830.26 ± 275.43 | 105 |
| 17 | 5-(Phenyl)-γ-valerolactone-3′-glucuronide | 15.01 ± 15.01 | 15.42 ± 15.42 | 196.48 ± 138.99 | 169.91 ± 105.95 | 65.96 ± 36.27 | 468.44 ± 330.34 | 81.71 ± 28.26 | 16.56 ± 16.56 | 1029.49 ± 394.77 | 121 |
| 15 | 4-Hydroxy-5-(Hydroxyphenyl)valeric acid-sulfate | 31.48 ± 18.11 | 9.56 ± 5.01 | 268.65 ± 125.49 | 432.54 ± 133.32 | 216.01 ± 58.49 | 571.58 ± 137.24 | 216.26 ± 60.42 | 30.47 ± 14.26 | 1776.56 ± 310.38 | 55 |
| 19 | 4-Hydroxy-5-(methoxy-phenyl)valeric acid--sulfate | nd | 0.30 ± 0.21 | 3.34 ± 1.93 | 6.42 ± 1.94 | 1.84 ± 0.84 | 4.02 ± 1.53 | 0.29 ± 0.15 | nd | 16.20 ± 4.31 | 84 |
| 11 | 4-Hydroxy-5-(methoxyphenyl)valeric acid-glucuronide (4-Hydroxy-5-phenylvaleric acid-methoxy-glucuronide) | nd | nd | 4.79 ± 2.48 | 6.48 ± 4.24 | 8.52 ±3.93 | 18.97 ± 6.71 | 1.78 ±1.78 | nd | 40.54 ± 10.11 | 79 |
| 18 | 4-Hydroxy-5-(phenyl)valeric acid-sulfate | 0.81 ± 0.61 | 0.23 ± 0.11 | 2.28 ± 1.17 | 4.40 ± 2.19 | 2.01 ± 0.97 | 10.89 ± 6.29 | 3.86 ± 1.36 | 1.41 ± 0.57 | 25.89 ± 8.19 | 100 |
| 24 | 5-(Hydroxyphenyl)valeric acid-sulfate | 5.68 ± 5.68 | 0.58 ± 0.42 | 5.06 ± 5.06 | 7.41 ± 4.74 | 4.35 ± 2.69 | 34.54 ± 13.80 | 31.20 ± 5.27 | 29.85 ± 15.45 | 118.67 ± 27.65 | 74 |
| 12 | 5-(Methoxyphenylvaleric) acid-sulfate | nd | 1.90 ± 1.90 | 374.69 ± 221.67 | 543.24 ± 149.94 | 169.00 ± 78.02 | 277.94 ± 104.02 | 42.67 ± 21.21 | 5.27 ± 3.42 | 1414.71 ± 421.96 | 94 |
| 14 | 5-(Methoxy-phenylvaleric) acid--glucuronide | 8.97 ± 6.01 | nd | 59.80 ± 41.17 | 90.37 ± 34.08 | 22.28 ± 11.86 | 14.60 ± 7.48 | nd | 3.07 ± 3.07 | 199.09 ± 78.07 | 124 |
| Sum of phenyl-y-valerolactones and phenylvaleric acids | 477.22 ± 266.28 | 206.65 ± 148.90 | 4231.78 ± 1978.16 | 5661.43 ± 1887.41 | 2765.88 ± 963.32 | 10,156.83 ± 1864.13 | 3871.06 ± 972.99 | 1170.14 ± 510.79 | 28,540.99 ± 3805.96 | 42 | |
| (Hydroxyphenyl)propanoic acids | |||||||||||
| 41 | 3-(4′-hydroxyphenyl)propanoic acid-3′-sulfate (Dihydrocaffeic acid-sulfate) | 27.49 ± 27.49 | 101.08 ± 52.47 | 103.59 ± 54.34 | 41.08 ± 16.51 | 56.67 ± 24.78 | 389.70 ± 95.00 | 208.69 ± 73.47 | 289.45 ± 118.16 | 1217.76 ± 256.95 | 67 |
| 40 | 3-(3′-Hydroxyphenyl)propanoic acid (3-(3-Hydroxyphenyl)propionic acid) | 96.20 ± 96.20 | 85.67 ± 85.67 | nd | nd | 287.94 ± 287.94 | 262.06 ± 138.26 | nd | 676.70 ± 383.40 | 1408.57 ± 544.97 | 122 |
| Sum of (Hydroxyphenyl)propanoic acids | 123.69 ± 97.07 | 186.75 ± 112.79 | 103.59 ± 54.34 | 41.08 ± 16.51 | 344.61 ± 305.36 | 651.76 ± 199.89 | 208.69 ± 73.47 | 966.15 ± 399.30 | 2626.33 ± 618.94 | 75 | |
| Hydroxybenzoic acids and simple benzenes | |||||||||||
| 39 | 3,5-Dimethoxy-4-hydroxybenzoic acid (Syringic acid) | 0.71 ± 0.46 | 8.71 ± 8.71 | 3.89 ± 3.81 | 3.57 ± 2.82 | 1.42 ± 1.38 | 3.63 ± 2.95 | 3.33 ± 2.94 | 8.73 ± 8.35 | 33.92 ± 30.72 | 286 |
| 38 | Methoxy-hydroxybenzoic acid-sulfate (Gallic acid-methoxy-sulfate) | 1.18 ± 1.18 | 18.56 ± 8.39 | 26.07 ± 5.72 | 2.89 ± 1.25 | 1.18 ± 1.18 | 2.43 ± 1.72 | 2.23 ± 1.49 | 2.34 ± 2.34 | 56.87 ± 13.94 | 78 |
| 35 | 4-Hydroxybenzoic acid-3-glucuronide (Protocatechuic acid-3-glucuronide) | 1.13 ± 1.13 | 14.20 ± 4.88 | 24.68 ± 5.82 | 12.53 ± 4.10 | 3.10 ± 1.12 | 4.51 ± 1.34 | nd | nd | 60.16 ± 9.86 | 52 |
| 36 | Dihydroxybenzene-sulfate (Pyrogallol-sulfate) | 747.18 ± 254.96 | 125.75 ± 42.49 | 549.56 ± 129.84 | 948.39 ± 201.27 | 504.78 ± 150.35 | 1676.23 ± 346.95 | 721.74 ± 169.19 | 541.99 ± 169.88 | 5815.62 ± 765.07 | 42 |
| 37 | Methoxyhydroxybenzene-sulfate (Pyrogallol-methoxy-sulfate) | 311.66 ± 96.06 | 104.78 ± 28.89 | 425.64 ± 95.78 | 783.20 ± 204.13 | 409.48 ± 127.88 | 1211.89 ± 191.45 | 538.08 ± 118.63 | 284.32 ± 101.86 | 4069.06 ± 502.55 | 39 |
| Sum of hydroxybenzoic acids and simple benzenes | 1061.79 ± 329.42 | 272.00 ± 85.28 | 1029.84 ± 219.14 | 1750.58 ± 408.15 | 919.95 ± 279.48 | 2898.70 ± 524.69 | 1265.38 ± 274.37 | 837.38 ± 246.45 | 10,035.62 ± 1234.49 | 39 | |
| Ellagitannin derivatives | |||||||||||
| 42 | 8-Hydroxy-urolithin-3-glucuronide (Urolithin A-glucuronide) | nd | 8.35 ± 7.92 | 4.88 ± 4.63 | 2.42 ± 2.30 | 18.31 ± 14.57 | 68.99 ± 33.01 | 69.22 ± 21.30 | 96.89 ± 37.45 | 269.07 ± 84.25 | 99 |
| 43 | 9-Hydroxy-urolithin-3-glucuronide (Isourolithin A-glucuronide) | nd | nd | nd | 6.91 ± 6.55 | 5.49 ± 5.21 | 36.41 ± 34.54 | 14.38 ± 9.83 | 5.33 ± 5.05 | 68.53 ± 55.19 | 255 |
| 44 | Urolithin-3-glucuronide (Urolithin B-glucuronide) | nd | nd | nd | 5.31 ± 5.04 | 39.56 ± 37.53 | 144.45 ± 90.90 | 74.85 ± 48.79 | 30.99 ± 29.40 | 295.17 ± 185.26 | 198 |
| Sum of ellagitannin derivatives | nd | 8.35 ± 7.92 | 4.88 ± 4.63 | 14.64 ± 11.53 | 63.37 ± 57.13 | 249.86 ± 135.01 | 158.45 ± 58.48 | 133.21 ± 42.40 | 632.76 ± 251.20 | 126 | |
| SUM of all metabolites | 1693.68 ± 579.94 | 894.12 ± 325.73 | 5764.04 ± 2219.80 | 7853.42 ± 2304.78 | 4165.34 ± 1501.54 | 14,084.29 ± 2394.88 | 5550.52 ± 1194.80 | 3145.19 ± 1052.77 | 43,150.60 ± 5117.69 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romain, C.; Bresciani, L.; Muralidharan, J.; Mena, P.; Chung, L.H.; Alcaraz, P.E.; Del Rio, D.; Cases, J. Exposure to (Poly)phenol Metabolites after a Fruit and Vegetable Supplement Intake: A Double-Blind, Cross-Over, Randomized Trial. Nutrients 2022, 14, 4913. https://doi.org/10.3390/nu14224913
Romain C, Bresciani L, Muralidharan J, Mena P, Chung LH, Alcaraz PE, Del Rio D, Cases J. Exposure to (Poly)phenol Metabolites after a Fruit and Vegetable Supplement Intake: A Double-Blind, Cross-Over, Randomized Trial. Nutrients. 2022; 14(22):4913. https://doi.org/10.3390/nu14224913
Chicago/Turabian StyleRomain, Cindy, Letizia Bresciani, Jananee Muralidharan, Pedro Mena, Linda H. Chung, Pedro E. Alcaraz, Daniele Del Rio, and Julien Cases. 2022. "Exposure to (Poly)phenol Metabolites after a Fruit and Vegetable Supplement Intake: A Double-Blind, Cross-Over, Randomized Trial" Nutrients 14, no. 22: 4913. https://doi.org/10.3390/nu14224913
APA StyleRomain, C., Bresciani, L., Muralidharan, J., Mena, P., Chung, L. H., Alcaraz, P. E., Del Rio, D., & Cases, J. (2022). Exposure to (Poly)phenol Metabolites after a Fruit and Vegetable Supplement Intake: A Double-Blind, Cross-Over, Randomized Trial. Nutrients, 14(22), 4913. https://doi.org/10.3390/nu14224913










