Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of Fungiform Papillae in a Genetically Homogeneous Sardinian Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. PROP Taster Status Classification
2.4. Sweet Taste Sensitivity Assessments
2.4.1. Sucrose Detection Threshold Measurements
2.4.2. Suprathreshold Sucrose Measurements
2.5. Density Assessments of the Fungiform Taste Papillae
2.6. Molecular Analysis
2.7. Statistical Analyses
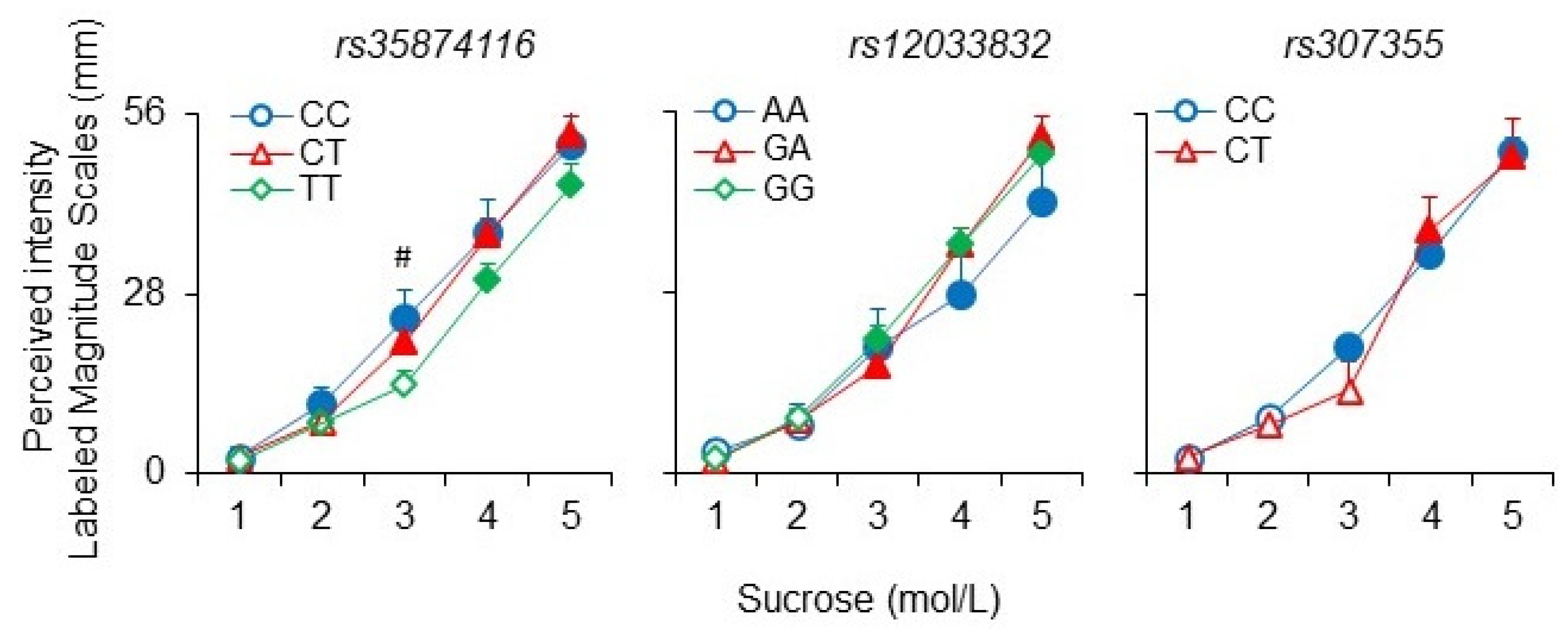
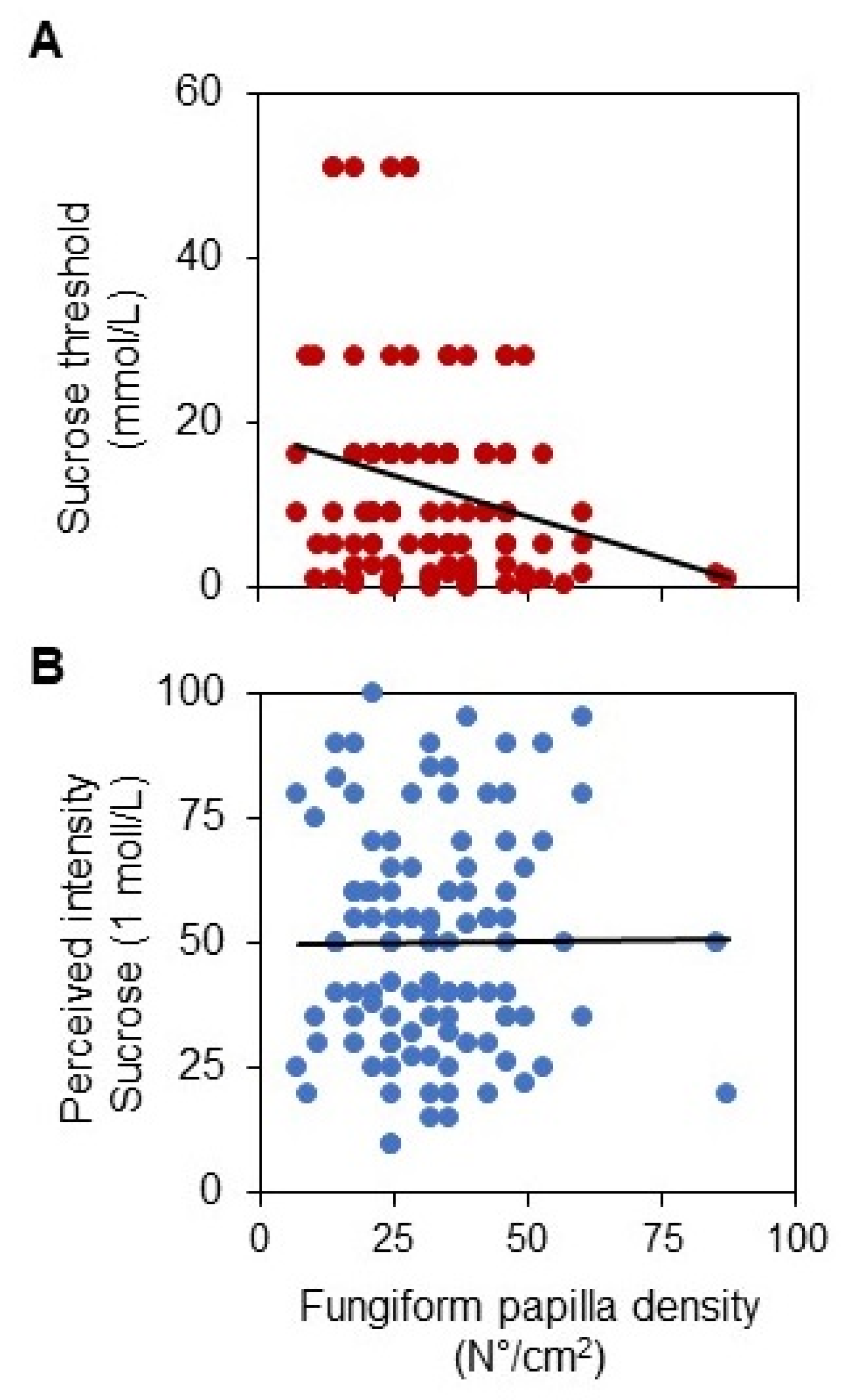
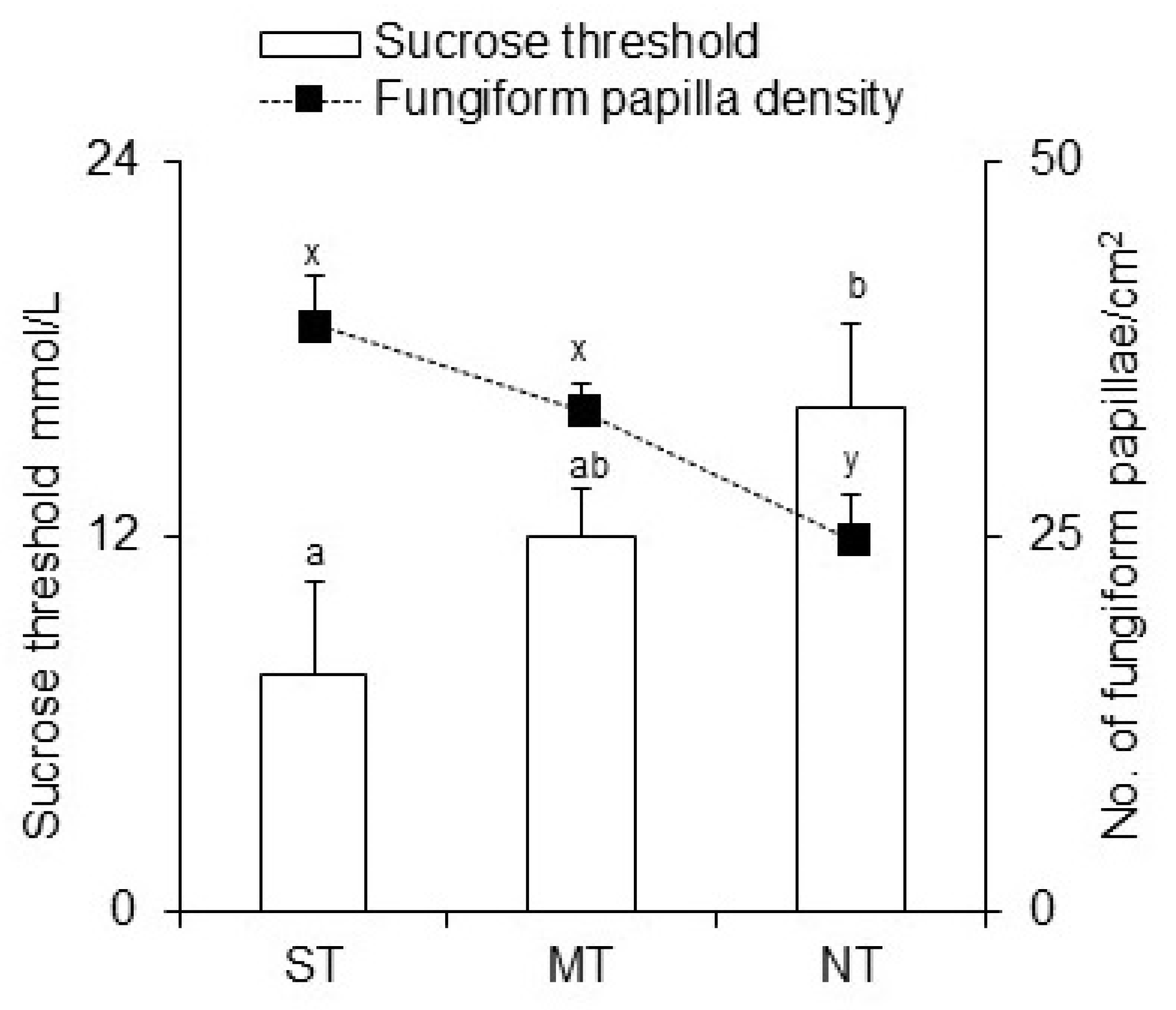
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- El-Sohemy, A.; Stewart, L.; Khataan, L.; Fontaine-Bisson, B.; Kwong, P.; Ozsungur, S.; Cornelis, M.C. Nutrigenomics of taste—Impact on food preferences and food production. Forum. Nutr. 2007, 60, 176–182. [Google Scholar] [PubMed]
- Garcia-Bailo, B.; Toguri, C.; Eny, K.M.; El-Sohemy, A. Genetic variation in taste and its influence on food selection. Omics 2009, 13, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Biarnes, X.; Marchiori, A.; Giorgetti, A.; Lanzara, C.; Gasparini, P.; Carloni, P.; Born, S.; Brockhoff, A.; Behrens, M.; Meyerhof, W. Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLoS ONE 2010, 5, e12394. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Psychophysical dissection of genotype effects on human bitter perception. Chem. Senses 2011, 36, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Padiglia, A.; Zonza, A.; Atzori, E.; Chillotti, C.; Calò, C.; Tepper, B.J.; Barbarossa, I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010, 92, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Methven, L.; Allen, V.J.; Withers, C.A.; Gosney, M.A. Ageing and taste. Proc. Nutr. Soc. 2012, 71, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Whissell-Buechy, D.; Wills, C. Male and female correlations for taster (P.T.C.) phenotypes and rate of adolescent development. Ann. Hum. Biol. 1989, 16, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Goldstein, G.L.; Daun, H.; Tepper, B.J. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol. Behav. 2007, 90, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Prutkin, J.; Fisher, E.M.; Etter, L.; Fast, K.; Gardner, E.; Lucchina, L.A.; Snyder, D.J.; Tie, K.; Weiffenbach, J.; Bartoshuk, L.M. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol. Behav. 2000, 69, 161–173. [Google Scholar] [CrossRef]
- Miller, I.J.; Reedy, F.E. Variations in human taste bud density and taste intensity perception. Physiol. Behav. 1990, 47, 1213–1219. [Google Scholar] [CrossRef]
- Segovia, C.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. A quantitative study of fungiform papillae and taste pore density in adults and children. Dev. Brain Res. 2002, 138, 135–146. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhang, H.Y.; Wang, X.F.; Zhan, Y.H.; Deng, S.P.; Qin, Y.M. The relationship between fungiform papillae density and detection threshold for sucrose in the young males. Chem Senses 2009, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jilani, H.; Ahrens, W.; Buchecker, K.; Russo, P.; Hebestreit, A. Association between the number of fungiform papillae on the tip of the tongue and sensory taste perception in children. Food Nutr. Res. 2017, 61, 1348865. [Google Scholar] [CrossRef] [PubMed]
- Essick, G.; Chopra, A.; Guest, S.; McGlone, F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol. Behav. 2003, 80, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Atzori, E.; Cabras, S.; Zonza, A.; Calò, C.; Muroni, P.; Nieddu, M.; Padiglia, A.; Sogos, V.; Tepper, B.J.; et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS ONE 2013, 8, e74151. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Melis, M.; Pani, D.; Cosseddu, P.; Usai, I.; Crnjar, R.; Bonfiglio, A.; Tomassini Barbarossa, I. First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Sci. Rep. 2017, 7, 40353. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Melis, M.; Koelliker, Y.; Gasparini, P.; Ahijevych, K.L.; Tomassini Barbarossa, I. Factors Influencing the Phenotypic Characterization of the Oral Marker, PROP. Nutrients 2017, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R.; Tepper, B.J.; Rietzschel, J.; Prescott, J. Human hedonic responses to sweetness: Role of taste genetics and anatomy. Physiol. Behav. 2007, 91, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Yousaf, N.Y.; Mattes, M.Z.; Cabras, T.; Messana, I.; Crnjar, R.; Tomassini Barbarossa, I.; Tepper, B.J. Sensory perception of and salivary protein response to astringency as a function of the 6-n-propylthioural (PROP) bitter-taste phenotype. Physiol. Behav. 2017, 173, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Tomassini Barbarossa, I. Taste Perception of Sweet, Sour, Salty, Bitter, and Umami and Changes Due to l-Arginine Supplementation, as a Function of Genetic Ability to Taste 6-n-Propylthiouracil. Nutrients 2017, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Sollai, G.; Muroni, P.; Crnjar, R.; Barbarossa, I.T. Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients 2015, 7, 2068–2084. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Duffy, V.B. Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses 2007, 32, 225–236. [Google Scholar] [CrossRef]
- Duffy, V.B.; Bartoshuk, L.M. Food acceptance and genetic variation in taste. J. Am. Diet Assoc. 2000, 100, 647–655. [Google Scholar] [CrossRef]
- Keller, K.L.; Steinmann, L.; Nurse, R.J.; Tepper, B.J. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite 2002, 38, 3–12. [Google Scholar] [CrossRef]
- Tepper, B.J.; Nurse, R.J. PROP taster status is related to fat perception and preference. Ann. N. Y. Acad. Sci. 1998, 855, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, I.T.; Carta, G.; Murru, E.; Melis, M.; Zonza, A.; Vacca, C.; Muroni, P.; Di Marzo, V.; Banni, S. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition 2013, 29, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Melis, M.; Pintus, S.; Pintus, P.; Piras, C.A.; Muredda, L.; Demurtas, D.; Di Marzo, V.; Banni, S.; Barbarossa, I.T. Participants with Normal Weight or with Obesity Show Different Relationships of 6-n-Propylthiouracil (PROP) Taster Status with BMI and Plasma Endocannabinoids. Sci. Rep. 2017, 7, 1361. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2014, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Cohen, N.A. Bitter taste receptors in innate immunity: T2R38 and chronic rhinosinusitis. J. Rhinol.-Otol. 2017, 5, 12–18. [Google Scholar]
- Melis, M.; Errigo, A.; Crnjar, R.; Pes, G.M.; Tomassini Barbarossa, I. TAS2R38 bitter taste receptor and attainment of exceptional longevity. Sci. Rep. 2019, 9, 18047. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Grzeschuchna, L.; Sollai, G.; Hummel, T.; Tomassini Barbarossa, I. Taste disorders are partly genetically determined: Role of the TAS2R38 gene, a pilot study. Laryngoscope 2019, 129, E307–E312. [Google Scholar] [CrossRef]
- Cossu, G.; Melis, M.; Sarchioto, M.; Melis, M.; Melis, M.; Morelli, M.; Tomassini Barbarossa, I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov. Disord. 2018, 33, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Banni, S.; Melis, M.; Crnjar, R.; Tomassini Barbarossa, I. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients 2014, 6, 3363–3381. [Google Scholar] [CrossRef]
- Melis, M.; Mastinu, M.; Sollai, G.; Paduano, D.; Chicco, F.; Magrì, S.; Usai, P.; Crnjar, R.; Tepper, B.J.; Barbarossa, I.T. Taste Changes in Patients with Inflammatory Bowel Disease: Associations with PROP Phenotypes and polymorphisms in the salivary protein, Gustin and CD36 Receptor Genes. Nutrients 2020, 12, 409. [Google Scholar] [CrossRef]
- Bartoshuk, L.M. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science 1979, 205, 934–935. [Google Scholar] [CrossRef] [PubMed]
- Gent, J.; Bartoshuk, L. Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem. Senses 1983, 7, 265–272. [Google Scholar] [CrossRef]
- Feeney, E.; O’Brien, S.; Scannel, A.; Markey, A.; Gibney, E.R. Perceived sucrose intensity is related to 6-n-propylthiouracil sensitivity and to markers of sugar intake. Proc. Nutr. Soc. 2010, 69, E23. [Google Scholar] [CrossRef]
- Bartoshuk, L.; Fast, K.; Karrer, T.; Marino, S.; Price, R.; Reed, D. PROP supertasters and the perception of sweetness and bitterness. Chem. Senses 1992, 17, 594. [Google Scholar]
- Bartoshuk, L.M.; Rifkin, B.; Marks, L.E.; Hooper, J.E. Bitterness of KCl and benzoate: Related to genetic status for sensitivity to PTC/PROP. Chem. Senses 1988, 13, 517–528. [Google Scholar] [CrossRef]
- Prescott, J.; Swain-Campbell, N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem. Senses 2000, 25, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Reed, D.R.; Snyder, D.J.; Bartoshuk, L.M. Bitter Receptor Gene (TAS2R38), 6-n-Propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcohol. Clin. Exp. Res. 2004, 28, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Kirkmeyer, S.V.; Tepper, B.J. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem. Senses 2003, 28, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Bajec, M.R.; Pickering, G.J. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol. Behav. 2008, 95, 581–590. [Google Scholar] [CrossRef]
- Shahbake, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005, 1052, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.D.; Bartoshuk, L.M.; Dichello, S.Z.; Panzini, L.; Weiffenbach, J.M.; Duffy, V.B. Association between 6-n-propylthiouracil (PROP) bitterness and colonic neoplasms. Dig. Dis. Sci. 2005, 50, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Carrai, M.; Steinke, V.; Vodicka, P.; Pardini, B.; Rahner, N.; Holinski-Feder, E.; Morak, M.; Schackert, H.K.; Gorgens, H.; Stemmler, S.; et al. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: A case-control study in two independent populations of Caucasian origin. PLoS ONE 2011, 6, e20464. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M.; Ng, X.; Boyd, L.; Skinner, V.; Wai, R.; Tang, S.; Naylor, C.; Yates, Z.; Choi, J.H.; Roach, P.; et al. TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps. Food Funct. 2011, 2, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.; Kim, U.K.; Bamshad, M.J.; Larsen, J.; Jorde, L.B.; Drayna, D. Natural Selection and Molecular Evolution in PTC, a Bitter-Taste Receptor Gene. Am. J. Hum. Genet. 2004, 74, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Boxer, E.E.; Garneau, N.L. Rare haplotypes of the gene TAS2R38 confer bitter taste sensitivity in humans. SpringerPlus 2015, 4, 505. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henderson, S.A.; Shore, A.B. Genetic sensitivity to 6-n-propylthiouracil (PROP) and hedonic responses to bitter and sweet tastes. Chem. Senses 1997, 22, 27–37. [Google Scholar] [CrossRef]
- Drewnowski, A.; Henderson, S.A.; Shore, A.B.; Barratt-Fornell, A. Nontasters, tasters, and supertasters of 6-n-propylthiouracil (PROP) and hedonic response to sweet. Physiol. Behav. 1997, 62, 649–655. [Google Scholar] [CrossRef]
- Ly, A.; Drewnowski, A. PROP (6-n-Propylthiouracil) tasting and sensory responses to caffeine, sucrose, neohesperidin dihydrochalcone and chocolate. Chem. Senses 2001, 26, 41–47. [Google Scholar] [CrossRef]
- Keskitalo, K.; Silventoinen, K.; Tuorila, H.; Perola, M.; Pietiläinen, K.H.; Rissanen, A.; Kaprio, J. Genetic and environmental contributions to food use patterns of young adult twins. Physiol. Behav. 2008, 93, 235–242. [Google Scholar] [CrossRef]
- Stevens, J.C.; Cain, W.S. Changes in taste and flavor in aging. Crit. Rev. Food Sci. Nutr. 1993, 33, 27–37. [Google Scholar] [CrossRef]
- Fukunaga, A.; Uematsu, H.; Sugimoto, K. Influences of aging on taste perception and oral somatic sensation. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 109–113. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kodama, H.; Satoh, T.; Adachi, K.; Watanabe, S.; Yokote, Y.; Sakagami, H. Diurnal changes in salivary amino acid concentrations. Vivo 2010, 24, 837–842. [Google Scholar]
- Rodin, J. Insulin levels, hunger, and food intake: An example of feedback loops in body weight regulation. Health Psychol. 1985, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.G.; Eny, K.M.; Cockburn, M.; Chiu, W.; Nielsen, D.E.; Duizer, L.; El-Sohemy, A. Variation in the TAS1R2 Gene, Sweet Taste Perception and Intake of Sugars. J. Nutr. Nutr. 2015, 8, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fushan, A.A.; Simons, C.T.; Slack, J.P.; Manichaikul, A.; Drayna, D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr. Biol. 2009, 19, 1288–1293. [Google Scholar] [CrossRef]
- Chamoun, E.; Liu, A.S.; Duizer, L.M.; Feng, Z.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W.L. Single nucleotide polymorphisms in sweet, fat, umami, salt, bitter and sour taste receptor genes are associated with gustatory function and taste preferences in young adults. Nutr. Res. 2021, 85, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Riduan Bahauddin, A.; Shaari, N.; Mohd Shariff, Z.; Karim, R. Association Between TAS1R2 Gene Polymorphism (rs12033832) and Sweet Taste Perception Amongst Malay Obese and Nonobese Subjects Mal. J. Med. Health Sci. 2020, 16, 4–12. [Google Scholar]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Max, M.; Shanker, Y.G.; Huang, L.; Rong, M.; Liu, Z.; Campagne, F.; Weinstein, H.; Damak, S.; Margolskee, R.F. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001, 28, 58–63. [Google Scholar] [CrossRef]
- Kitagawa, M.; Kusakabe, Y.; Miura, H.; Ninomiya, Y.; Hino, A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem. Biophys. Res. Commun. 2001, 283, 236–242. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef]
- Liao, J.; Schultz, P.G. Three sweet receptor genes are clustered in human chromosome 1. Mamm Genome 2003, 14, 291–301. [Google Scholar] [CrossRef]
- Kim, U.K.; Wooding, S.; Riaz, N.; Jorde, L.B.; Drayna, D. Variation in the human TAS1R taste receptor genes. Chem. Senses 2006, 31, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Staszewski, L.; Tang, H.; Adler, E.; Zoller, M.; Li, X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 14258–14263. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Vigues, S.; Hobbs, J.R.; Conn, G.L.; Munger, S.D. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr. Biol. 2005, 15, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Eny, K.M.; Wolever, T.M.; Corey, P.N.; El-Sohemy, A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 2010, 92, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.V.; Agnes, G.; Vitolo, M.R.; Mattevi, V.S.; Campagnolo, P.D.B.; Almeida, S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet. Mol. Biol. 2017, 40, 415–420. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Panduro, A.; Martinez-Lopez, E.; Roman, S. Sweet Taste Receptor TAS1R2 Polymorphism (Val191Val) Is Associated with a Higher Carbohydrate Intake and Hypertriglyceridemia among the Population of West Mexico. Nutrients 2016, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.V.; Reed, D.R.; Mennella, J.A. Individual Differences Among Children in Sucrose Detection Thresholds: Relationship With Age, Gender, and Bitter Taste Genotype. Nurs. Res. 2016, 65, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.D.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.S.; An, J.; Gordon, S.D.; Zhu, G.; MacGregor, S.; Lawlor, D.A.; et al. New insight into human sweet taste: A genome-wide association study of the perception and intake of sweet substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Calò, C.; Padiglia, A.; Zonza, A.; Corrias, L.; Contu, P.; Tepper, B.J.; Barbarossa, I.T. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol. Behav. 2011, 104, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H. A comparison of different methods used to assess sensitivity to the taste of phenylthiocarbamide (PTC). Chem. Senses 1980, 5, 247–256. [Google Scholar] [CrossRef]
- Glanville, E.V.; Kaplan, A.R. Taste Perception and the Menstrual Cycle. Nature 1965, 205, 930–931. [Google Scholar] [CrossRef]
- Tepper, B.J.; Christensen, C.M.; Cao, J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001, 73, 571–577. [Google Scholar] [CrossRef]
- Melis, M.; Aragoni, M.C.; Arca, M.; Cabras, T.; Caltagirone, C.; Castagnola, M.; Crnjar, R.; Messana, I.; Tepper, B.J.; Barbarossa, I.T. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS ONE 2013, 8, e59810. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Arca, M.; Aragoni, M.C.; Cabras, T.; Caltagirone, C.; Castagnola, M.; Crnjar, R.; Messana, I.; Tepper, B.J.; Tomassini Barbarossa, I. Dose-Dependent Effects of L-Arginine on PROP Bitterness Intensity and Latency and Characteristics of the Chemical Interaction between PROP and L-Arginine. PLoS ONE 2015, 10, e0131104. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; Shaffer, G.S.; Gilmore, M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses 1993, 18, 683–702. [Google Scholar] [CrossRef]
- Barbarossa, I.T.; Melis, M.; Mattes, M.Z.; Calò, C.; Muroni, P.; Crnjar, R.; Tepper, B.J. The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol. Behav. 2015, 138, 6–12. [Google Scholar] [CrossRef]
- Zhao, L.; Kirkmeyer, S.V.; Tepper, B.J. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol. Behav. 2003, 78, 625–633. [Google Scholar] [CrossRef]
- Nolden, A.A.; McGeary, J.E.; Hayes, J.E. Predominant Qualities Evoked by Quinine, Sucrose, and Capsaicin Associate With PROP Bitterness, but not TAS2R38 Genotype. Chem. Senses 2020, 45, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.I.; Chung, J.W.; Kim, Y.K.; Chung, S.C.; Kho, H.S. The relationship between phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Arch. Oral Biol. 2006, 51, 427–432. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.J.; Feller, R.P. Age and sex effects on taste of sucrose, NaCl, citric acid and caffeine. Neurobiol. Aging 1981, 2, 315–318. [Google Scholar] [CrossRef]
- Papantoni, A.; Shearrer, G.E.; Sadler, J.R.; Stice, E.; Burger, K.S. Longitudinal Associations Between Taste Sensitivity, Taste Liking, Dietary Intake and BMI in Adolescents. Front. Psychol. 2021, 12, 597704. [Google Scholar] [CrossRef] [PubMed]
- Laeng, B.; Berridge, K.C.; Butter, C.M. Pleasantness of a Sweet Taste during Hunger and Satiety: Effects of Gender and “Sweet Tooth”. Appetite 1993, 21, 247–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serrano, J.; Seflova, J.; Park, J.; Pribadi, M.; Sanematsu, K.; Shigemura, N.; Serna, V.; Yi, F.; Mari, A.; Procko, E.; et al. The Ile191Val is a partial loss-of-function variant of the TAS1R2 sweet-taste receptor and is associated with reduced glucose excursions in humans. Mol. Metab. 2021, 54, 101339. [Google Scholar] [CrossRef]
- Serrano, J.; Yi, F.; Smith, J.; Pratley, R.E.; Kyriazis, G.A. The Ile191Val Variant of the TAS1R2 Subunit of Sweet Taste Receptors Is Associated With Reduced HbA(1c) in a Human Cohort With Variable Levels of Glucose Homeostasis. Front. Nutr. 2022, 9, 896205. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Banos, R.; Botella, C.; de la Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G.; et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS ONE 2017, 12, e0171204. [Google Scholar]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.J. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Guild, A.A. Olfactory acuity in normal and obese human subjects: Diurnal variations and the effect of d-amphetamine sulphate. J. Laryngol. Otol. 1956, 70, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nagasawa, M.; Yamada, S.; Hara, A.; Mogami, H.; Nikolaev, V.O.; Lohse, M.J.; Shigemura, N.; Ninomiya, Y.; Kojima, I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 2009, 4, e5106. [Google Scholar] [CrossRef] [PubMed]


| Male (n = 41) | Female (n = 66) | p-Value | |
|---|---|---|---|
| Age (y) | 24.71 ± 0.66 | 24.28 ± 0.52 | 0.613 |
| BMI (kg/m2) | 22.95 ± 0.48 * | 20.99± 0.37 | 0.0015 |
| TAS2R38 gene | |||
| Genotype n (%) | |||
| PAV/PAV | 10 (24.4) | 12 (19.7) | 0.510 |
| PAV/AVI | 18 (43.9) | 32 (48.5) | |
| AVI/AVI | 9 (22.0) | 17 (27.3) | |
| Rare | 4 (9.8) | 3 (4.5) | |
| Haplotype n (%) | |||
| PAV | 38 (47.5) | 58 (43.9) | 0.489 |
| AVI | 38 (47.5) | 71 (53.8) | |
| Rare | 4 (5.0) | 3 (2.3) | |
| TAS1R2 gene | |||
| rs35874116 | |||
| Genotype n (%) | |||
| TT | 20 (48.8) | 22 (33.3) | 0.061 |
| CT | 19 (46.3) | 35 (53.0) | |
| CC | 2 (4.9) | 9 (13.6) | |
| Allele n (%) | |||
| T | 59 (72.0) | 79 (59.8) | 0.086 |
| C | 23 (28.0) | 53 (40.2) | |
| rs12033832 | |||
| Genotype n (%) | |||
| GG | 22 (53.7) | 31(47.0) | 1 |
| GA | 14 (34.1) | 32 (48.5) | |
| AA | 5 (12.2) | 3 (4.5) | |
| Allele n (%) | |||
| G | 58 (70.7) | 94 (71.2) | 1 |
| A | 24 (29.3) | 38 (28.8) | |
| TAS1R3 gene | |||
| rs307355 | |||
| Genotype n (%) | |||
| CC | 36 (87.8) | 58 (87.9) | 1 |
| CT | 5 (12.2) | 8 (12.1) | |
| TT | 0 | 0 | |
| Allele | |||
| C | 77 (93.9) | 124 (93.9) | 1 |
| T | 5 (6.1) | 8 (6.1) | |
| rs35744813 | |||
| Genotype n (%) | |||
| CC | 41 (100) | 66 (100) | - |
| CT | 0 | 0 | |
| TT | 0 | 0 | |
| Allele n (%) | |||
| C | 82 (100) | 132 (100) | - |
| T | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, M.; Mastinu, M.; Naciri, L.C.; Muroni, P.; Tomassini Barbarossa, I. Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of Fungiform Papillae in a Genetically Homogeneous Sardinian Cohort. Nutrients 2022, 14, 4903. https://doi.org/10.3390/nu14224903
Melis M, Mastinu M, Naciri LC, Muroni P, Tomassini Barbarossa I. Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of Fungiform Papillae in a Genetically Homogeneous Sardinian Cohort. Nutrients. 2022; 14(22):4903. https://doi.org/10.3390/nu14224903
Chicago/Turabian StyleMelis, Melania, Mariano Mastinu, Lala Chaimae Naciri, Patrizia Muroni, and Iole Tomassini Barbarossa. 2022. "Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of Fungiform Papillae in a Genetically Homogeneous Sardinian Cohort" Nutrients 14, no. 22: 4903. https://doi.org/10.3390/nu14224903
APA StyleMelis, M., Mastinu, M., Naciri, L. C., Muroni, P., & Tomassini Barbarossa, I. (2022). Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of Fungiform Papillae in a Genetically Homogeneous Sardinian Cohort. Nutrients, 14(22), 4903. https://doi.org/10.3390/nu14224903









