Cyclosorus terminans Extract Ameliorates Insulin Resistance and Non-Alcoholic Fatty Liver Disease (NAFLD) in High-Fat Diet (HFD)-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Animal Models
2.3. Experimental Design
2.4. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Biochemical Parameter Analysis
2.7. Liver Triglyceride Determination
2.8. Glycogen Content
2.9. Gene Expression Analysis
2.10. Macroscopic and Microscopic Examination
2.11. Statistical Analysis
3. Results
3.1. EEI Reduced Body Weight of HFD-Induced Obese Rats
3.2. EEI Improved Insulin Resistance and Hyperglycemia in HFD-Induced Obese Rats
3.3. Effects of EEI on Serum Lipid Parameters and Liver Enzymes
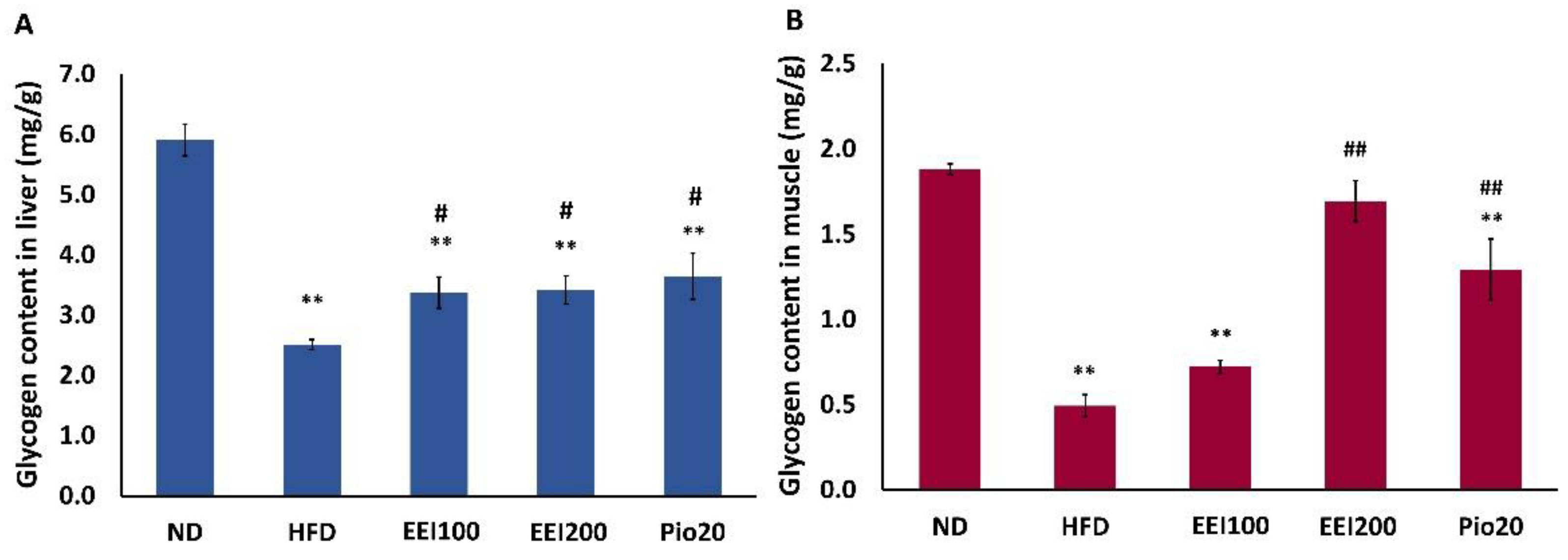
3.4. EEI Enhanced the Hepatic and Soleus Muscle Glycogen Content in HFD-Induced Obese Rats
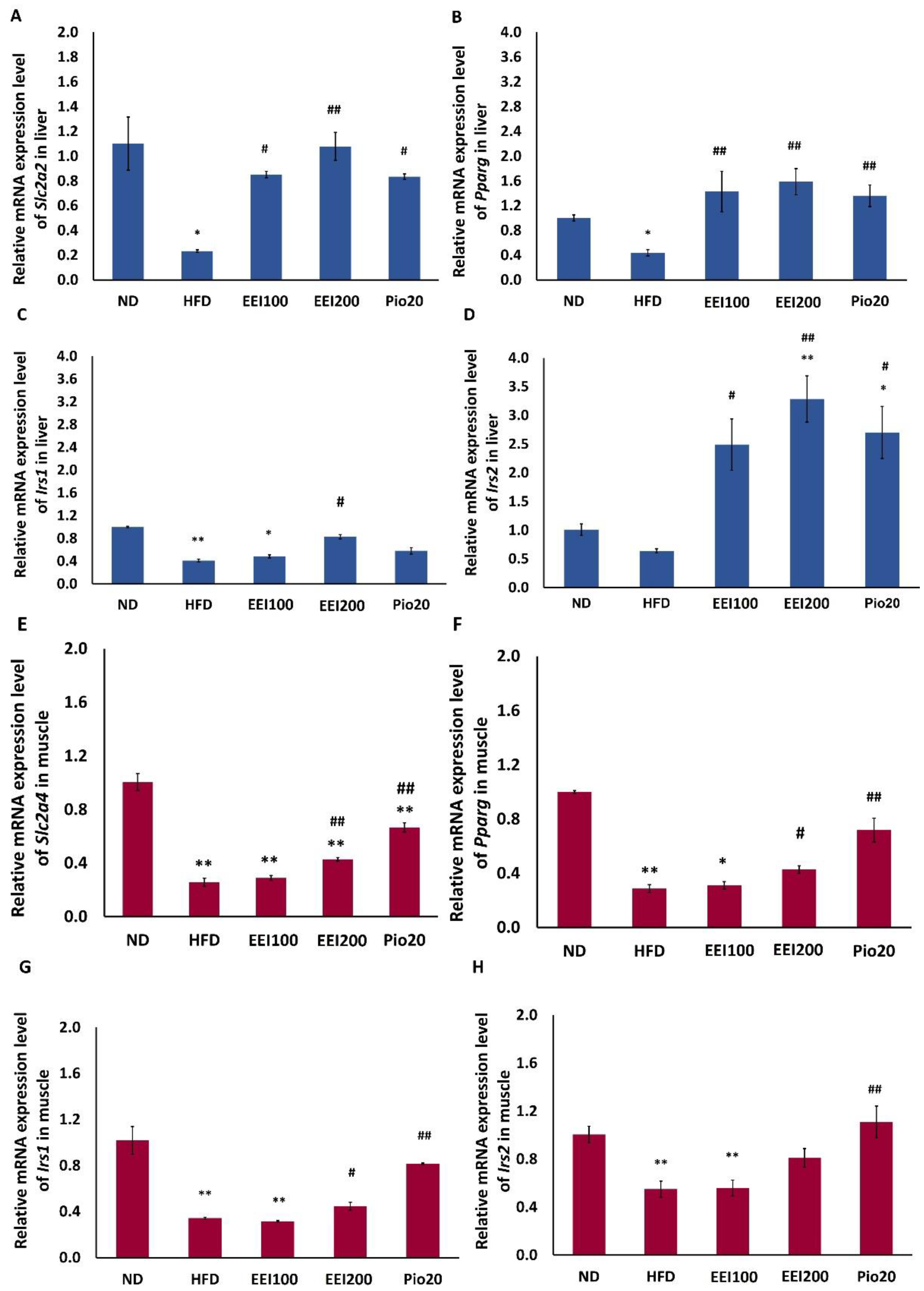
3.5. EEI Improved Glucose Transporter and Insulin Receptor Substrate Gene Expression in Liver and Soleus Muscle Tissues of HFD-Induced Obese Rats
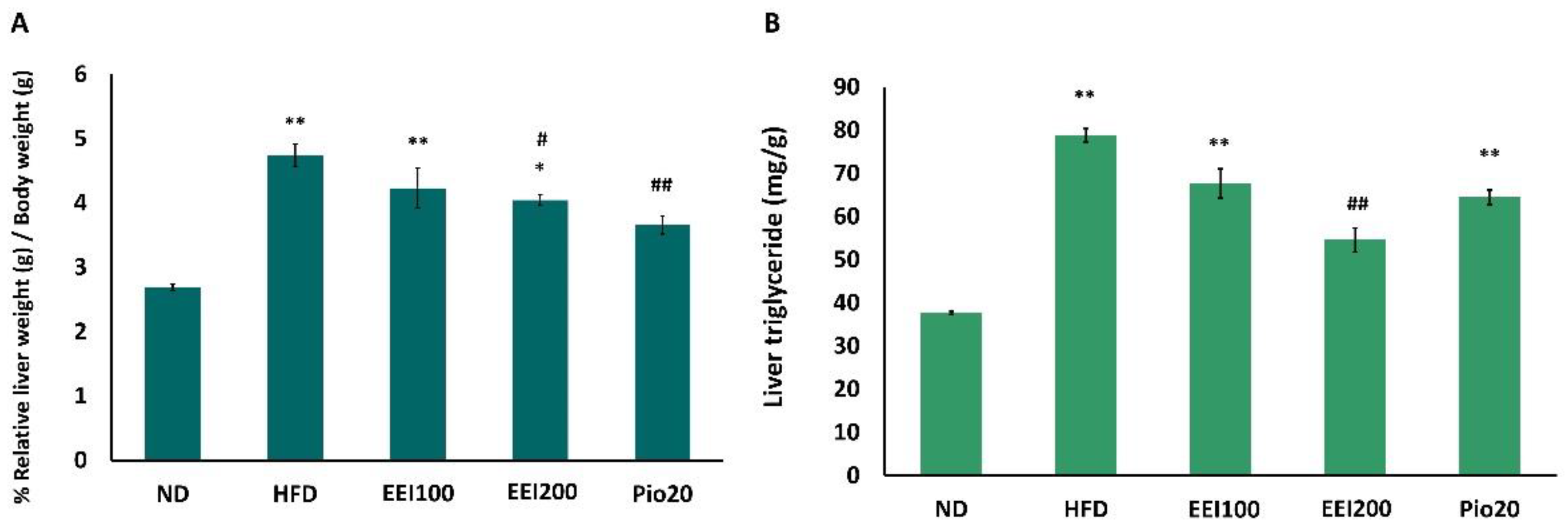
3.6. EEI Decreased Liver Weight and Triglyceride in HFD-Induced Obese Rats
3.7. EEI Ameliorated the Hepatic Histological Alteration and Fat Deposition in HFD-Induced Obese Rats
3.8. EEI Altered Hepatic Gene Expression Related to Lipid Metabolism and Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Crawford, K. Review diabetes standard of care. Nurs. Clin. N. Am. 2017, 52, 621–663. [Google Scholar] [CrossRef]
- Okur, M.E.; Karantas, I.D.; Siafaka, P. Diabetes Mellitus: A review on pathophysiology, current status of oral medications and future perspectives. Acta Pharm. Sci. 2017, 55, 61–82. [Google Scholar]
- Reaven, G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol. Metab. Clin. N. Am. 2004, 33, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, M.; Ganesh, H.K.; Vima, M.V.; John, M.; Bandgar, T.; Joshi, S.R.; Shah, S.R.; Rathi, P.M.; Joshi, A.S.; Thakkar, H.; et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Assoc. Physicians India 2009, 57, 205–210. [Google Scholar] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Amiel, S.A.; Dixon, T.; Mann, R.; Jameson, K. Hypoglycemia in type 2 diabetes. Diabet. Med. 2008, 25, 245–254. [Google Scholar] [CrossRef]
- Nanjan, M.J.; Mohammed, M.; Kumar, B.R.P.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.R.; Hul, W.V.; Mertens, I.; et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Barbosa-da-Silva, S.; Souza-Mello, V.; Magliano, D.C.; de Souza Marinho, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Singular effects of PPAR agonists on nonalcoholic fatty liver disease of diet-induced obese mice. Life Sci. 2015, 127, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concisreview. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kaewsuwan, S.; Yuenyongsawad, S.; Plubrukarn, A.; Kaewchoothong, A.; Raksawong, A.; Puttarak, P.; Apirug, C. Bioactive interruptins A and B from Cyclosorus terminans: Antibacterial, anticancer, stem cell proliferation and ROS scavenging activities. Songklanakarin J. Sci. Technol. 2015, 37, 309–317. [Google Scholar]
- Chaiwong, S.; Puttarak, P.; Sretrirutchai, S.; Kaewsuwan, S. In vitro anti-inflammatory and antioxidative activities of isolated interruptins from Cyclosorus terminans. Lat. Am. J. Pharm 2019, 38, 1677–1682. [Google Scholar]
- Kaewsuwan, S.; Plubrukan, A.; Utsintong, M.; Kim, S.H.; Jeong, J.H.; Cho, J.G.; Park, S.G.; Sung, J.H. Interruptin B induces brown adipocyte differentiation and glucose consumption in adipose-derived stem cells. Mol. Med. Rep. 2016, 13, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Songtrai, S.; Kaewsuwan, S. Anti-diabetic effect of interruptins A and B from Cyclosorus terminans on mouse hepatocytes. Thai J. Pharm. Sci. 2021; (accepted). [Google Scholar]
- Songtrai, S.; Dejyong, K.; Kaewsuwan, S. Acute oral toxicological evaluation in Wistar rats of interruptin-rich extract from Cyclosorus terminans and its in vitro anti-diabetic potential. J. Pharm. Pharmacogn. Res. 2022, 10, 800–811. [Google Scholar] [CrossRef]
- Chaiwong, S.; Puttarak, P.; Kaewsuwan, S. Anti Propionibacterium acnes activity, HPLC method validation for simultaneous analysis and extraction of coumarins from the fern Cyclosorus terminans. Lat. Am. J. Pharm 2018, 37, 1791–1797. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Nammi, S.; Kim, M.S.; Gavande, N.S.; Li, G.Q.; Roufogalis, B.D. Regulation of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme a reductase expression by Zingiber officinale in the liver of high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 389–395. [Google Scholar] [CrossRef]
- Sruthi, T.; Satyavati, D.; Reddy, C.M.; Srilatha, N. Estimation of glycogen content in liver, skeletal muscle and cardiac muscle of niddwin, a polyherbal formulation in alloxan induced diabetic rats. Pharma Innov. 2014, 2, 56–60. [Google Scholar]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Vaag, A.; Henriksen, J.E.; Beck-Nielsen, H. Decreased insulin activation of glycogen synthase in skeletal muscle in young nonobese caucasian first-degree relatives of patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1992, 89, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Guillam, M.T.; Burcelin, R.; Thorens, B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 12317–12321. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Murgia, M.; Nagaraj, N.; Treebak, J.T.; Cox, J.; Mann, M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol. Cell Proteom. 2015, 14, 841–853. [Google Scholar] [CrossRef]
- Liu, M.L.; Gibbs, E.M.; McCoid, S.C.; Milici, A.J.; Stukenbrok, H.A.; McPherson, R.K.; Treadway, J.L.; Pessin, J.E. Transgenic mice expressing the human GLUT4/muscle-fat facilitative glucose transporter protein exhibit efficient glycemic control. Proc. Natl. Acad. Sci. USA 1993, 90, 11346–11350. [Google Scholar] [CrossRef]
- Marshall, B.A.; Mueckler, M.M. Differential effects of GLUT-1 or GLUT-4 overexpression on insulin responsiveness in transgenic mice. Am. J. Physiol. 1994, 267, E738–E744. [Google Scholar] [CrossRef]
- Samuel, T.V.; Liu, X.Z.; Qu, X.; Elder, D.B.; Bilz, S.; Befroy, D.; Romanelli, J.A.; Shulman, I.G. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Abraham, M.; Unalp, A.; Wilson, L.; Lavine, J.; Doo, E.; Bass, N.M. Nonalcoholic steatohepatitis clinical research network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012, 56, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: A meta analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Kumboonruang, N. Fern Diversity at Silaphet Waterfall, Pua District, Nan Province. Master’s Thesis, Srinakharinwirot University, Bangkok, Thailand, 2009. [Google Scholar]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef]
- Ros, S.; Zafra, D.; Valles-Ortega, J.; García-Rocha, M.; Forrow, S.; Domínguez, J.; Calbó, J.; Guinovart, J.J. Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis. J. Biol. Chem. 2010, 285, 37170–37177. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef]
- Blüher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Janochova, K.; Haluzik, M.; Buzga, M. Visceral fat and insulin resistance—What we know? Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2019, 163, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Sharma, P. Non-invasive diagnosis of fibrosis in non-alcoholic fatty liver disease. J. Clin. Exp. Hepatol. 2012, 2, 145–155. [Google Scholar] [CrossRef]
- Paulauskis, J.D.; Sul, H.S. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J. Biol. Chem. 1989, 264, 574–577. [Google Scholar] [CrossRef]
- Sul, H.S.; Wang, D. Nutritional and hormonal regulation of enzymes in fat synthesis: Studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 1998, 18, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7286. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef] [PubMed]
- Louet, J.F.; Chatelain, F.; Decaux, J.F.; Park, E.A.; Kohl, C.; Pineau, T.; Girard, J.; Pegorier, J.P. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem. J. 2001, 354, 189–197. [Google Scholar] [CrossRef]
- Mascaro, C.; Acosta, E.; Ortiz, J.A.; Marrero, P.F.; Hegardt, F.G.; Haro, D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998, 273, 8560–8563. [Google Scholar] [CrossRef]
- Barrero, M.J.; Camarero, N.; Marrero, P.F.; Haro, D. Control of human carnitine palmitoyltransferase II gene transcription by peroxisome proliferator activated receptor through a partially conserved peroxisome proliferator responsive element. Biochem. J. 2003, 369, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Kleemann, R.; Pilon, A.; Percevault, F.; Koenig, W.; Staels, B.; Kooistra, T. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J. Biol. Chem. 2004, 279, 16154–16160. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, R.M.; Bauge, E.; Staels, B.; Gervois, P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology 2008, 149, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Iwamoto, T.; Kotake, S.; Momohara, S.; Yamanaka, H.; Kamatani, N. Inhibition of NK-κB signaling by fenofibrate, a peroxisome proliferator-activated receptor-α ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, 323–330. [Google Scholar]
- Hashimoto, T.; Cook, W.S.; Qi, C.; Yeldandi, A.V.; Reddy, J.K.; Rao, M.S. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J. Biol. Chem. 2000, 275, 28918–28928. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steato-hepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, R.; Plomgaard, P.; Møller, K.; Mittendorfer, B.; Pedersen, B.K. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E108–E114. [Google Scholar] [CrossRef]
- Hossain, M.; Faruque, M.O.; Kabir, G.; Hassan, N.; Sikdar, D.; Nahar, Q.; Ali, L. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. Int. J. Diabetes Mellit. 2010, 2, 165–168. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-a signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Guagnano, M.T.; Pacini, G.; Vigneri, S.; Falco, A.; Marinopiccoli, M.; Manigrasso, M.R.; Basili, S.; Davi, G. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 5321–5326. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; McKolanis, T.M.; Hegazi, R.A.; Kuller, L.H.; Kalhan, S.C. Fatty liver in type 2 diabetes mellitus: Relation to regional adiposity, fatty acids, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E906–E916. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Fernandez-Gil, P.; Hernandez-Guerra, M.; Cayon, A.; Mayorga, M.; Dominguez-Diez, A.; Fernandez-Escalante, J.C.; Pons-Romero, F. Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis? Obes. Surg 2001, 11, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Kugelmas, M.; Hill, D.B.; Vivian, B.; Marsano, L.; McClain, C.J. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Zieleniak, A.; Wojcik, M.; Wo´zniak, L.A. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ. Arch. Immunol. Ther. Exp. 2008, 56, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.K.; Connolly, T.M. Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Curr. Opin. Investig. Drugs 2008, 9, 247–255. [Google Scholar] [PubMed]
- Plutzky, J. Emerging concepts in metabolic abnormalities associated with coronary artery disease. Curr. Opin. Cardiol. 2000, 15, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Fazio, S. Re-emergence of fibrates in the management of dyslipidemia and cardiovascular risk. Curr. Atheroscler. Rep. 2000, 2, 29–35. [Google Scholar] [CrossRef] [PubMed]




| Genes | Forward Primer (5′-3′) | Gene Location | NCBI Reference Sequence | Size (bp) |
|---|---|---|---|---|
| Irs1 | F: 5′ GCCAATCTTCATCCAGTTGC 3′ | 1374–1393 | NM_012969.2 | 336 |
| R: 5′ CATCGTGAGAAGGCATAGG 3′ | 1710–1691 | |||
| Irs2 | F: 5′ AGTAAACGGAGGTGGCTACA 3′ | 2142–2161 | NM_001168633.1 | 197 |
| R: 5′ AAGCTGCTGGAAGTCAGGT 3′ | 2338–2319 | |||
| Slc2a2 | F: 5′ ATTCGCCTGGATGATTACG 3′ | 1511–1530 | XM_006232207.3 | 203 |
| R: 5′ AGTCCGCCAATGTACTGGAAG 3′ | 1713–1694 | |||
| Slc2a4 | F: 5′ ATTGGCATTCTGGTTGCCCA 3′ | 644–663 | XM_006246596.4 | 163 |
| R: 5′ GGTTCCGGATGATGTAGAGGTA 3′ | 806–785 | |||
| Srebf1 | F: 5′ GATGCCAACCAGATTCCCTAAG 3′ | 7431–7452 | NM_001276708.1 | 210 |
| R: 5′ TCAGTTGTTTCTTTGCCTTCCA 3′ | 7640–7619 | |||
| Cpt2 | F: 5′ GTGGCAAGGAGTTCCTGAAG 3′ | 1494–1513 | XM_039109299.1 | 411 |
| R: 5′ TGGTTCATCTGCTGGTATGC 3′ | 1904–1885 | |||
| Fasn | F: 5′ TCCCAGGTCTTGCCGTGC 3′ | 6224–6241 | NM_017332.2 | 260 |
| R: 5′ GCGGATGCCTAGGATGTGTGC 3′ | 6483–6463 | |||
| Tnf | F: 5′ GTAGCCCACGTCGTAGCAAA 3′ | 455–474 | HQ201305 | 347 |
| R: 5′ CCCTTCTCCAGCTGGAAGAC 3′ | 801–782 | |||
| Il6 | F: 5′ AGTTGCCTTCTTGGGACTGA 3′ | 97–116 | NM_012589.2 | 485 |
| R: 5′ GAGCATTGGAAGTTGGGGTA 3′ | 581–562 | |||
| Ppara | F: 5′ GATTCGGAAACTGCAGACCTC 3′ | 868–888 | XM_017594680.1 | 444 |
| R: 5′ TAGGAACTCTCGGGTGATGA 3′ | 1311–1292 | |||
| Pparg | F: 5′ CAAAGTAGAACCTGCATCTCC 3′ | 402–422 | XM_006237009.3 | 156 |
| R: 5′ CCTTCACAAGCATGAACTCC 3′ | 557–538 | |||
| Act | F: 5′ GTACCACTGGCATTGTGATG 3′ | 518–537 | NM_031144.3 | 541 |
| R: 5′ ATCTTCATGGTGCTAGGAGC 3′ | 1058–1039 |
| Parameters | ND | High Fat Diet | |||
|---|---|---|---|---|---|
| HFD | EEI100 (mg/kg/day) | EEI200 (mg/kg/day) | Pio20 (mg/kg) | ||
| Blood glucose (mg/dL) | 126.8 ± 2.9 | 139.4 ± 4.3 ** | 128.3 ± 2.1 # | 126.1 ± 2.9 ## | 124.1 ± 2.3 ## |
| Insulin (ng/mL) | 2.8 ± 0.6 | 5.2 ± 0.4 ** | 4.4 ± 0.8 * | 3.2 ± 0.4 # | 3.5 ± 0.1 # |
| HOMA-IR | 21.8 ± 4.3 | 43.8 ± 2.2 ** | 34.6 ± 6.6 * | 24.2 ± 2.5 ## | 26.8 ± 0.7 # |
| OGTT AUC (mg/dL × min × 104) | 4.8 ± 0.1 | 5.6 ± 0.2 ** | 5.1 ± 0.2 | 5.0 ± 0.1 *,## | 5.0 ± 0.1 ## |
| Parameters | ND | High Fat Diet | |||
|---|---|---|---|---|---|
| HFD | EEI100 (mg/kg/day) | EEI200 (mg/kg/day) | Pio20 (mg/kg/day) | ||
| Triglyceride (mg/dL) | 86.6 ± 14.8 | 82.1 ± 5.7 | 77.0 ± 4.0 | 75.3 ± 4.5 | 57.3 ± 6.1 **,## |
| Cholesterol (mg/dL) | 88.6 ± 3.3 | 114.7 ± 3.8 | 107.2 ± 2.8 ** | 106.2 ± 3.9 ** | 101.7 ± 7.0 *,# |
| LDL (mg/dL) | 33.0 ± 2.9 | 69.7 ± 3.3 ** | 61.5 ± 2.6 ** | 61.0 ± 3.2 ** | 69.9 ± 5.8 ** |
| HDL (mg/dL) | 29.3 ± 1.4 | 25.3 ± 1.5 * | 28.0 ± 0.8 | 30.6 ± 0.8 ## | 28.0 ± 1.3 |
| AST (U/L) | 109.3 ± 15.6 | 267.8 ± 44.2 ** | 201.8 ± 16.4 ** | 196.3 ± 17.4 **,# | 194.5 ± 15.8 **,# |
| ALT (U/L) | 39.1 ± 3.7 | 157.0 ± 18.0 ** | 128.6 ± 17.8 ** | 95.7 ± 14.9 **,## | 112.7 ± 9.8 **,# |
| ALP (U/L) | 77.1 ± 1.7 | 191.3 ± 17.5 ** | 162.9 ± 11.9 ** | 151.0 ± 16.3 ** | 144.9 ± 10.4 * |
| Histological Features (Score) | ND | High Fat Diet | |||
|---|---|---|---|---|---|
| HFD | EEI100 (mg/kg/day) | EEI200 (mg/kg/day) | Pio20 (mg/kg/day) | ||
| Steatosis | |||||
| Microvesicular steatosis (0–3) | 0.1 ± 0.1 | 2.8 ± 0.1 ** | 2.3 ± 0.2 | 2.2 ± 0.1 | 2.3 ± 0.2 * |
| Macrovesicular steatosis (0–3) | 0.1 ± 0.1 | 2.1 ± 0.1 ** | 1.2 ± 0.1 **,# | 1.0 ± 0.1 *,## | 1.1 ± 0.1 *,# |
| Hypertrophy (0–3) | 0.0 ± 0.0 | 3.0 ± 0.0 ** | 2.3 ± 0.5 ** | 1.2 ± 0.4 ## | 2.0 ± 0.5 ** |
| Inflammation | |||||
| Number of inflammatory foci/field (0–3) | 0.8 ± 0.1 | 2.3 ± 0.2 ** | 1.4 ± 0.3 | 1.3 ± 0.2 # | 1.3 ± 0.2 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songtrai, S.; Pratchayasakul, W.; Arunsak, B.; Chunchai, T.; Kongkaew, A.; Chattipakorn, N.; Chattipakorn, S.C.; Kaewsuwan, S. Cyclosorus terminans Extract Ameliorates Insulin Resistance and Non-Alcoholic Fatty Liver Disease (NAFLD) in High-Fat Diet (HFD)-Induced Obese Rats. Nutrients 2022, 14, 4895. https://doi.org/10.3390/nu14224895
Songtrai S, Pratchayasakul W, Arunsak B, Chunchai T, Kongkaew A, Chattipakorn N, Chattipakorn SC, Kaewsuwan S. Cyclosorus terminans Extract Ameliorates Insulin Resistance and Non-Alcoholic Fatty Liver Disease (NAFLD) in High-Fat Diet (HFD)-Induced Obese Rats. Nutrients. 2022; 14(22):4895. https://doi.org/10.3390/nu14224895
Chicago/Turabian StyleSongtrai, Sujinda, Wasana Pratchayasakul, Busarin Arunsak, Titikorn Chunchai, Aphisek Kongkaew, Nipon Chattipakorn, Siriporn C. Chattipakorn, and Sireewan Kaewsuwan. 2022. "Cyclosorus terminans Extract Ameliorates Insulin Resistance and Non-Alcoholic Fatty Liver Disease (NAFLD) in High-Fat Diet (HFD)-Induced Obese Rats" Nutrients 14, no. 22: 4895. https://doi.org/10.3390/nu14224895
APA StyleSongtrai, S., Pratchayasakul, W., Arunsak, B., Chunchai, T., Kongkaew, A., Chattipakorn, N., Chattipakorn, S. C., & Kaewsuwan, S. (2022). Cyclosorus terminans Extract Ameliorates Insulin Resistance and Non-Alcoholic Fatty Liver Disease (NAFLD) in High-Fat Diet (HFD)-Induced Obese Rats. Nutrients, 14(22), 4895. https://doi.org/10.3390/nu14224895








