Changes in Vitamin D Status in Korean Adults during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participant Characteristics
3.2. Comparison of 25(OH)D Levels before and after the COVID-19 Pandemic
3.3. Changes in Vitamin D Status after the COVID-19 Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization: Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 21 September 2021).
- Ministry of Health and Welfare of South Korea. Coronavirus Disease-19, Republic of Korea. Available online: http://ncov.mohw.go.kr/en/ (accessed on 21 September 2022).
- Coronavirus Infectious Disease-19 Press Release: From May 2nd (Mon), the Mandatory Wearing of an Outdoor Mask Has Been Adjusted. Available online: http://ncov.mohw.go.kr/tcmBoardView.do?contSeq=371325 (accessed on 21 September 2022).
- Meoli, M.; Muggli, F.; Lava, S.A.G.; Bianchetti, M.G.; Agostoni, C.; Kocher, C.; Bührer, T.W.; Ciliberti, L.; Simonetti, G.D.; Milani, G.P. Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study. Nutrients 2021, 13, 1467. [Google Scholar] [CrossRef]
- Lim, S.; Lim, H.; Després, J.P. Collateral Damage of the COVID-19 Pandemic on Nutritional Quality and Physical Activity: Perspective from South Korea. Obesity 2020, 28, 1788–1790. [Google Scholar] [CrossRef]
- Naja, F.; Hamadeh, R. Nutrition amid the COVID-19 pandemic: A multi-level framework for action. Eur. J. Clin. Nutr. 2020, 74, 1117–1121. [Google Scholar] [CrossRef]
- Beyazgül, G.; Bağ, Ö.; Yurtseven, İ.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G.; Çelik, F.; Nalbantoğlu, Ö.; Özkan, B. How Vitamin D Levels of Children Changed During COVID-19 Pandemic: A Comparison of Pre-pandemic and Pandemic Periods. J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188–195. [Google Scholar] [CrossRef]
- Kang, H.M.; Jeong, D.C.; Suh, B.K.; Ahn, M.B. The Impact of the Coronavirus Disease-2019 Pandemic on Childhood Obesity and Vitamin D Status. J. Korean Med. Sci. 2021, 36, e21. [Google Scholar] [CrossRef]
- Lippi, G.; Ferrari, A.; Targher, G. Is COVID-19 lockdown associated with vitamin D deficiency? Eur. J. Public Health 2021, 31, 278–279. [Google Scholar]
- Ferrari, D.; Locatelli, M.; Faraldi, M.; Lombardi, G. Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy. Biology 2021, 10, 237. [Google Scholar] [CrossRef]
- Raposo, L.; Martins, S.; Ferreira, D.; Guimarães, J.T.; Santos, A.C. Vitamin D, parathyroid hormone and metabolic syndrome—The PORMETS study. BMC Endocr. Disord. 2017, 17, 71. [Google Scholar] [CrossRef]
- Park, J.-H.; Hong, I.Y.; Chung, J.W.; Choi, H.S. Vitamin D status in South Korean population: Seven-year trend from the KNHANES. Medicine 2018, 97, e11032. [Google Scholar] [CrossRef]
- Anna, L.; Se Hwi, K.; Chung Mo, N.; Young-Jin, K.; Soo-Ho, J.; Kyoung-Ryul, L. Prevalence of Vitamin D Deficiency and Insufficiency in Korean Children and Adolescents and Associated Factors. Lab. Med. Online 2016, 6, 70–78. [Google Scholar]
- Klingberg, E.; Oleröd, G.; Konar, J.; Petzold, M.; Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 2015, 49, 800–808. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef]
- Bleakley, A.S.; Licciardi, P.V.; Binks, M.J. Vitamin D Modulation of the Innate Immune Response to Paediatric Respiratory Pathogens Associated with Acute Lower Respiratory Infections. Nutrients 2021, 13, 276. [Google Scholar] [CrossRef]
- Greer, R.M.; Portelli, S.L.; Hung, B.S.; Cleghorn, G.J.; McMahon, S.K.; Batch, J.A.; Conwell, L.S. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatr Diabetes 2013, 14, 31–41. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J. Serum vitamin D status and metabolic syndrome: A systematic review and dose-response meta-analysis. Nutr. Res. Pract. 2021, 15, 329–345. [Google Scholar] [CrossRef]
- Melguizo-Rodriguez, L.; Costela-Ruiz, V.J.; Garcia-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Hall, S.C.; Fischer, K.D.; Agrawal, D.K. The impact of vitamin D on asthmatic human airway smooth muscle. Expert Rev. Respir. Med. 2016, 10, 127–135. [Google Scholar] [CrossRef]
- Tian, H.Q.; Cheng, L. The role of vitamin D in allergic rhinitis. Asia Pac. Allergy 2017, 7, 65–73. [Google Scholar] [CrossRef]
- Kim, G.; Bae, J.-H. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M.; Briguglio, M.; Lombardi, G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem. Funct. 2021, 39, 35–47. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.K. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- Korea Meteorological Agency Meteorological Annual Report: Sunshine Time Trend. Available online: https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1402 (accessed on 3 October 2022).
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 2015, 6, 7000. [Google Scholar] [CrossRef]
- Pfeifer, G.M. Do Low Vitamin D Levels Increase COVID-19 Risk? Am. J. Nurs. 2020, 120, 16. [Google Scholar]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Zemb, P.; Bergman, P.; Camargo, C.A., Jr.; Cavalier, E.; Cormier, C.; Courbebaisse, M.; Hollis, B.; Joulia, F.; Minisola, S.; Pilz, S.; et al. Vitamin D deficiency and the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 133–134. [Google Scholar] [CrossRef]
- BBC News: Covid and Vitamin D: ‘Not Enough Evidence’ for Treatment. Available online: https://www.bbc.com/news/health-55333063 (accessed on 3 October 2022).
- BBC News: Covid: Free Vitamin D Pills for 2.5 Million Vulnerable in England. Available online: https://www.bbc.com/news/health-55108613 (accessed on 3 October 2022).
- Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Mielech, A.; Markiewicz-Żukowska, R.; Mielcarek, K.; Moskwa, J.; Naliwajko, S.K.; Soroczyńska, J.; Gromkowska-Kępka, K.J.; et al. Consumption of Food Supplements during the Three COVID-19 Waves in Poland-Focus on Zinc and Vitamin D. Nutrients 2021, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Edaily: Corona Consumption Market Continued This Year… Milk Kit and Healthy Food Smile. Available online: https://www.edaily.co.kr/news/read?newsId=02355046629279504&mediaCodeNo=257&OutLnkChk=Y (accessed on 3 October 2022).
- iHerb Meeting Increased Worldwide Demand for Vitamin D and C. Available online: https://siswa.blog/f-https-www.iherb.com/pressreleases/increasd-demand-for-vitamins/1098 (accessed on 3 October 2022).
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin C in the Treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Quispe, C.; Martorell, M.; Docea, A.O.; Salehi, B.; Calina, D.; Reiner, Ž.; Sharifi-Rad, J. Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022, 92, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali-Schöll, I.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2022, 77, 1373–1388. [Google Scholar] [CrossRef]
- Korea Ministry of Culture, Sports and Tourism 2020 National Life Sports Survey. Available online: https://www.mcst.go.kr/kor/s_policy/dept/deptView.jsp?pCurrentPage=3&pType=07&pTab=01&pSeq=1442&pDataCD=0417000000&pSearchType=01&pSearchWord= (accessed on 3 October 2022).
| Before COVID-19 Lockdown (n = 1483) | During COVID-19 Lockdown (n = 1483) | |||||
|---|---|---|---|---|---|---|
| Male (n = 820) | Female (n = 663) | p-Value | Male (n = 820) | Female (n = 663) | p-Value | |
| Age (year) | <0.001 | <0.001 | ||||
| 19–34 | 50 (6.1) | 92 (13.9) | 38 (4.6) | 76 (11.5) | ||
| 35–49 | 346 (42.2) | 337 (50.8) | 294 (35.9) | 294 (44.3) | ||
| 50–64 | 372 (45.4) | 211 (31.8) | 423 (51.6) | 259 (39.1) | ||
| ≥65 | 52 (6.3) | 23 (3.5) | 65 (7.9) | 34 (5.1) | ||
| Weight (kg) | 74.8 ± 11.4 | 58.7 ± 9.0 | <0.001 | 74.4 ± 11.3 | 58.9 ± 9.3 | <0.001 |
| BMI (kg/m2) | 25.3 ± 3.3 | 23.1 ± 3.5 | 0.065 | 25.2 ± 3.3 | 23.2 ± 3.6 | 0.008 |
| Smoking | <0.001 | <0.001 | ||||
| Non-smoker | 216 (26.5) | 640 (97.1) | 217 (26.5) | 639 (96.7) | ||
| Ex-smoker | 335 (41.2) | 11 (1.7) | 352 (43.0) | 12 (1.8) | ||
| Current smoker | 263 (32.3) | 8 (1.2) | 249 (30.4) | 10 (1.5) | ||
| Physical Activity | 0.001 | 0.005 | ||||
| Sedentary | 163 (20.6) | 112 (17.6) | 155 (19.7) | 106 (16.7) | ||
| Light | 485 (61.3) | 408 (64.1) | 483 (61.4) | 423 (66.6) | ||
| Moderate | 83 (10.5) | 96 (15.1) | 90 (11.4) | 84 (13.2) | ||
| Vigorous | 49 (6.2) | 17 (2.7) | 53 (6.7) | 18 (2.8) | ||
| Very vigorous | 11 (1.4) | 4 (0.6) | 6 (0.8) | 4 (0.6) | ||
| Blood pressure (mmHg) | ||||||
| Systolic | 126.0 ± 13.8 | 119.3 ± 14.6 | 0.089 | 126.2 ± 13.5 | 120.4 ± 14.8 | 0.006 |
| Diastolic | 77.2 ± 10.1 | 72.0 ± 10.1 | 0.905 | 79.9 ± 10.0 | 72.9 ± 10.4 | 0.392 |
| Season | 0.003 | 0.002 | ||||
| Spring | 139 (17.0) | 83 (12.5) | 140 (17.1) | 83 (12.5) | ||
| Summer | 265 (32.3) | 206 (31.1) | 264 (32.2) | 206 (31.1) | ||
| Autumn | 390 (47.5) | 365 (55.0) | 390 (47.5) | 365 (55.0) | ||
| Winter | 26 (3.2) | 9 (1.4) | 26 (3.2) | 9 (1.4) | ||
| Past History | <0.001 | <0.001 | ||||
| Osteoporosis or Osteopenia | 8 (1.0) | 18 (2.7) | 9 (1.1) | 28 (4.2) | ||
| HRT or contraceptive pills | 0 (0.0) | 11 (1.7) | 0 (0.0) | 12 (1.8) | ||
| Chronic liver disease | 22 (2.7) | 5 (0.8) | 26 (3.2) | 5 (0.8) | ||
| Chronic renal disease | 4 (0.5) | 1 (0.2) | 5 (0.6) | 2 (0.3) | ||
| Total (n = 1482) | Male (n = 820) | Female (n = 662) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before COVID-19 Lockdown | During COVID-19 Lockdown | p-Value | Before COVID-19 Lockdown | During COVID-19 Lockdown | p-Value | Before COVID-19 Lockdown | During COVID-19 Lockdown | p-Value | |
| 25(OH)D | |||||||||
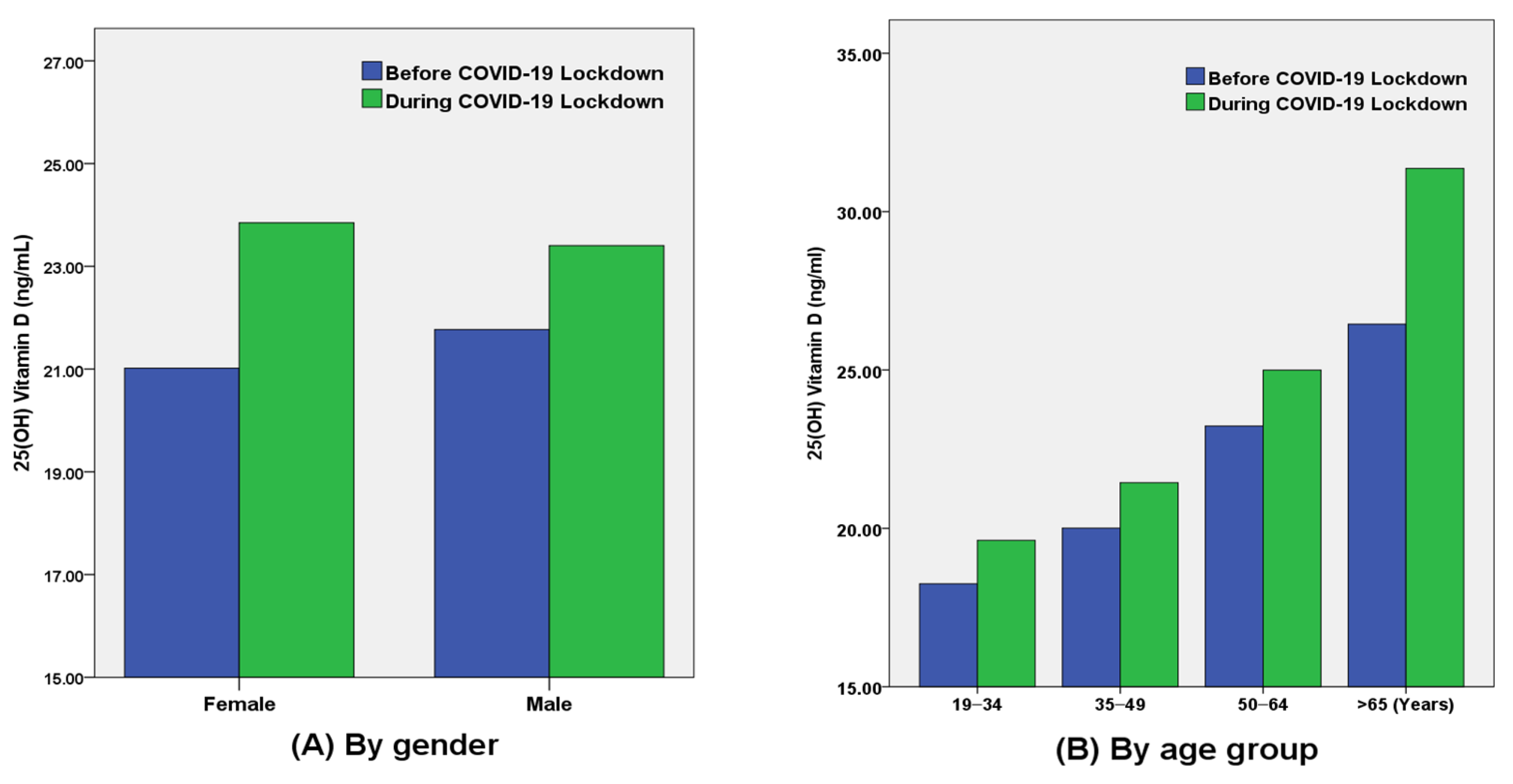
| Total (ng/mL) | 21.4 ± 10.2 | 23.6 ± 11.8 | <0.001 | 21.8 ± 8.9 | 23.4 ± 10.6 | <0.001 | 21.0 ± 11.6 | 23.9 ± 13.1 | <0.001 |
| 19–34 years | 18.3 ± 8.6 | 20.1 ± 9.6 | 0.027 | 19.2 ± 7.2 | 20.2 ± 7.8 | 0.291 | 17.8 ± 9.3 | 20.0 ± 10.6 | 0.051 |
| 35–49 years | 20.0 ± 9.1 | 21.9 ± 10.5 | <0.001 | 20.4 ± 8.0 | 22.1 ± 9.2 | <0.001 | 19.6 ± 10.2 | 21.8 ± 11.8 | <0.001 |
| 50–64 years | 23.2 ± 11.1 | 25.5 ± 12.5 | <0.001 | 22.9 ± 9.5 | 24.4 ± 11.6 | 0.006 | 23.9 ± 13.6 | 27.3 ± 13.8 | <0.001 |
| ≥65 years | 26.5 ± 10.9 | 31.1 ± 15.1 | 0.005 | 25.4 ± 10.3 | 28.1 ± 12.3 | 0.141 | 28.8 ± 12.3 | 37.7 ± 18.6 | 0.008 |
| Season | 25(OH)D (ng/mL) Before COVID-19 Lockdown | 25(OH)D (ng/mL) During COVID-19 Lockdown | p-Value |
|---|---|---|---|
| Spring (March–May) | 20.32 ± 11.26 | 25.76 ± 15.55 | <0.001 |
| Summer (June–August) | 22.35 ± 9.31 | 24.22 ± 10.56 | <0.001 |
| Autumn (September–November) | 21.26 ± 10.35 | 22.69 ± 11.22 | <0.001 |
| Winter (December, January–February) | 20.42 ± 11.84 | 21.27 ± 9.96 | 0.709 |
| Before COVID-19 Lockdown | During COVID-19 Lockdown | ||||
|---|---|---|---|---|---|
| n (%) | n (%) | p-Value | |||
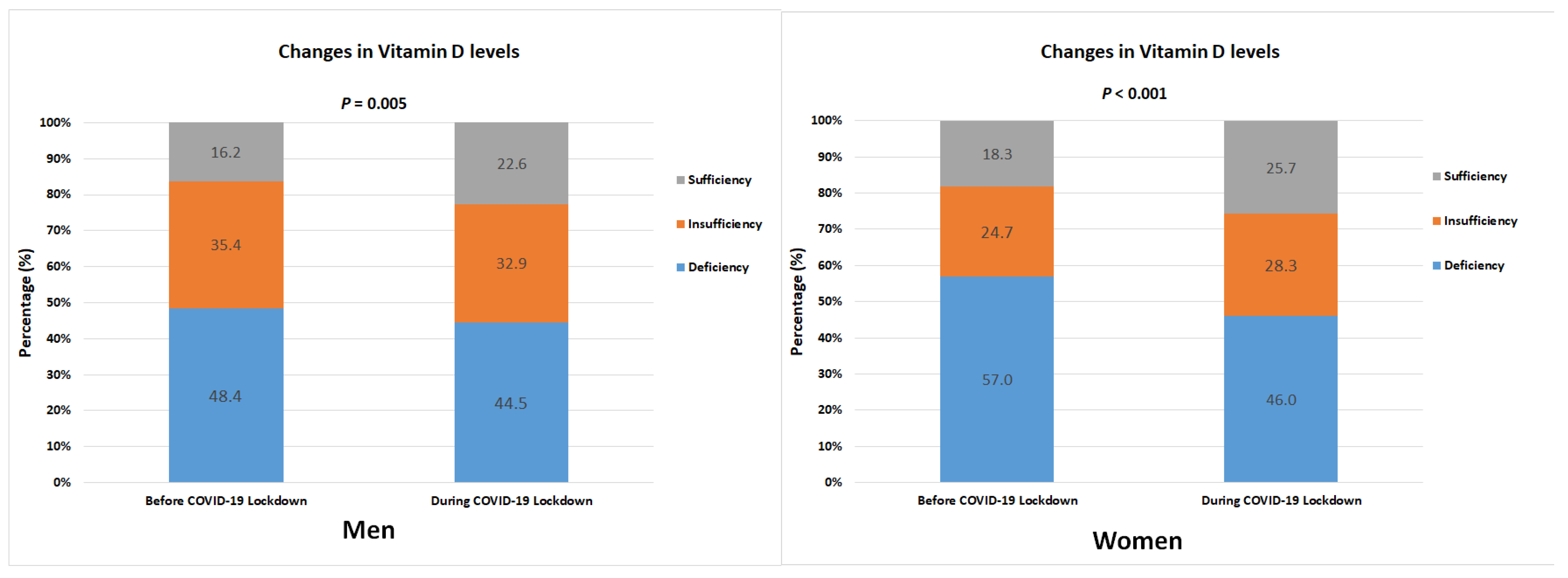
| Male | 820 | 811 | 0.005 | ||
| 25(OH)D | Deficiency | 397 (48.4) | 361 (44.5) | ||
| Insufficiency | 290 (35.4) | 267 (32.9) | |||
| Sufficiency | 133 (16.2) | 183 (22.6) | |||
| Female | 663 | 654 | <0.001 | ||
| 25(OH)D | Deficiency | 378 (57.0) | 301 (46.0) | ||
| Insufficiency | 164 (24.7) | 185 (28.3) | |||
| Sufficiency | 121 (18.3) | 168 (25.7) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-Y.; Kang, S.-G. Changes in Vitamin D Status in Korean Adults during the COVID-19 Pandemic. Nutrients 2022, 14, 4863. https://doi.org/10.3390/nu14224863
Kwon J-Y, Kang S-G. Changes in Vitamin D Status in Korean Adults during the COVID-19 Pandemic. Nutrients. 2022; 14(22):4863. https://doi.org/10.3390/nu14224863
Chicago/Turabian StyleKwon, Ji-Young, and Sung-Goo Kang. 2022. "Changes in Vitamin D Status in Korean Adults during the COVID-19 Pandemic" Nutrients 14, no. 22: 4863. https://doi.org/10.3390/nu14224863
APA StyleKwon, J.-Y., & Kang, S.-G. (2022). Changes in Vitamin D Status in Korean Adults during the COVID-19 Pandemic. Nutrients, 14(22), 4863. https://doi.org/10.3390/nu14224863







