Optimal Serum 25(OH)D Level and Vitamin D Intake in Young Korean Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. General and Lifestyle Characteristics
2.3. Dietary Assessment
2.4. Anthropometry, Bone Turnover Markers (BMTs), and BMD
2.5. Statistical Analysis
3. Results
3.1. The Status of Participants
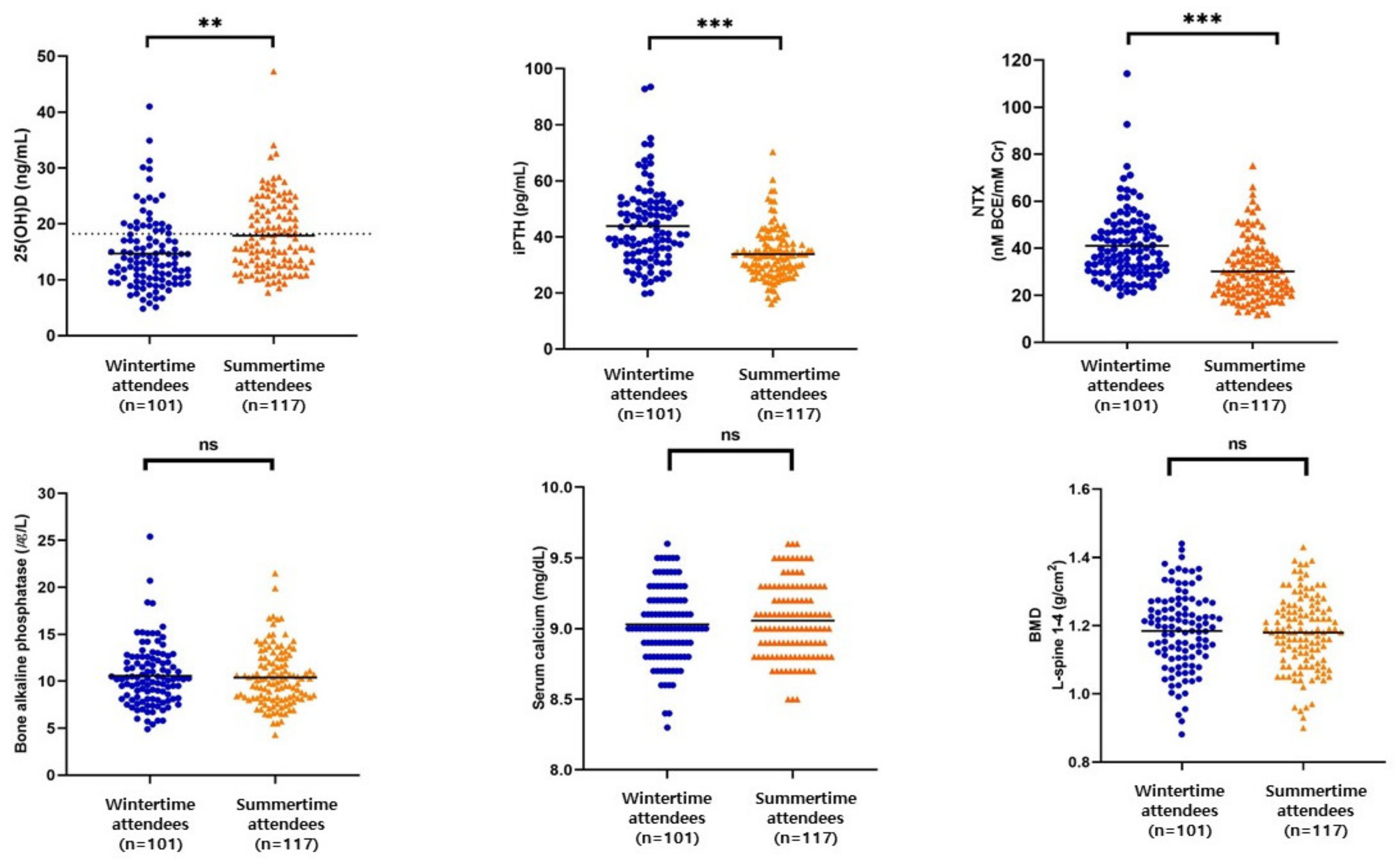
3.2. Serum Levels of 25(OH)D, Calcium, BMTs, and BMD
3.3. Seasonal Nutrient Intake
3.4. Multiple Linear Regression of Factors Affecting Serum 25(OH)D Level by Season
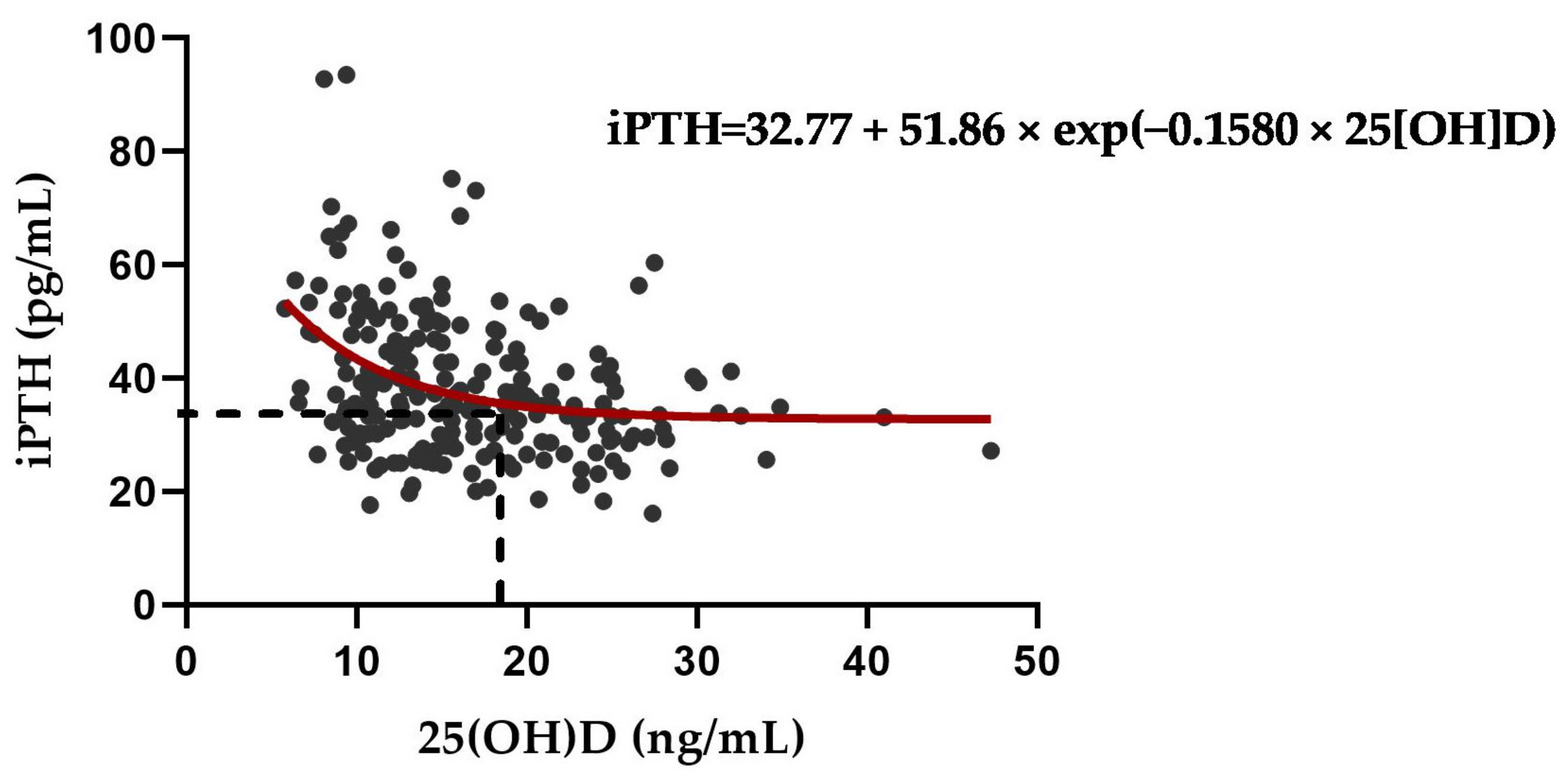
3.5. Exponential Decay for Optimal Serum 25(OH)D to Suppress PTH Elevation
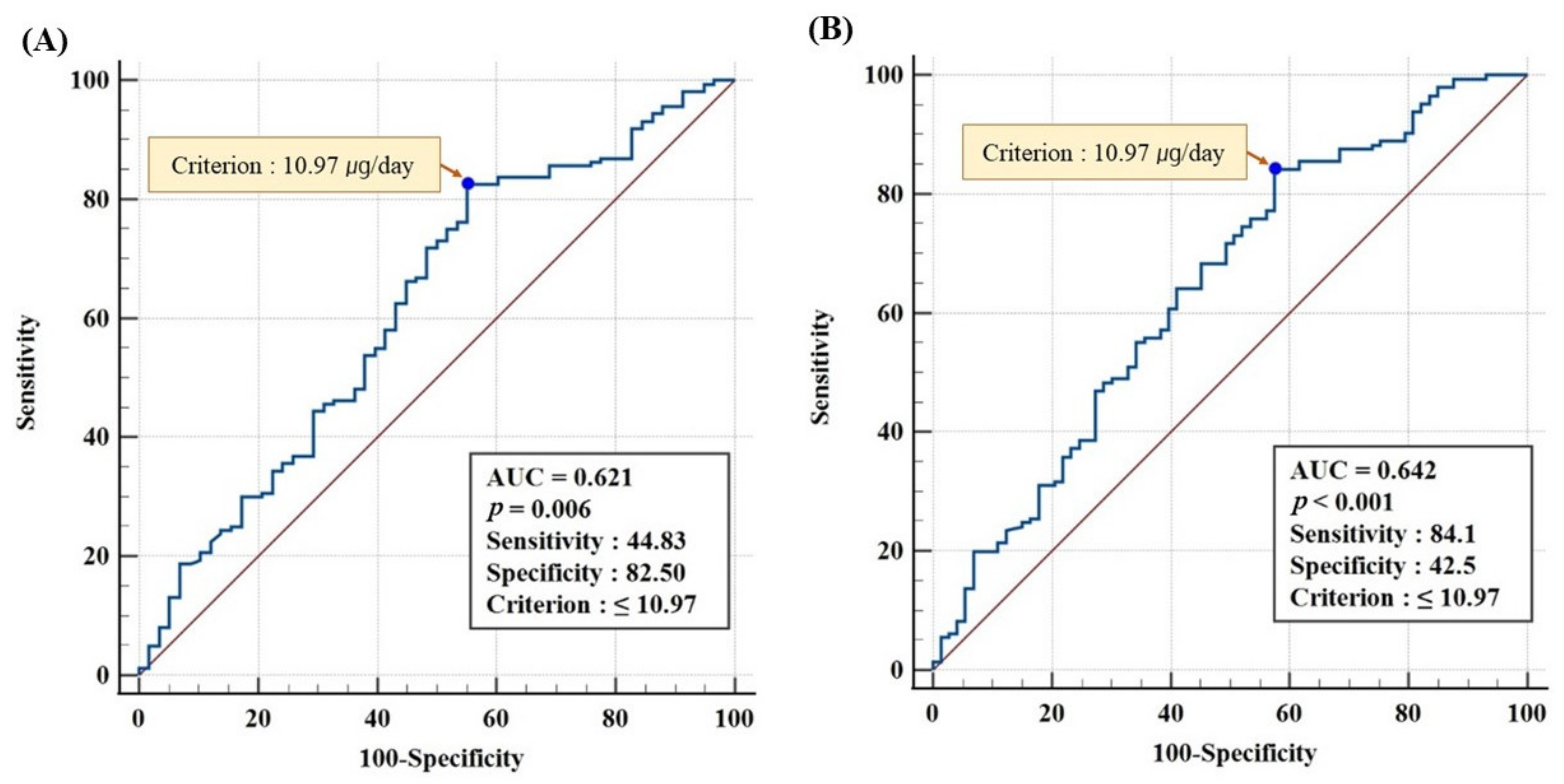
3.6. ROC Analysis for Optimal Vitamin D Intake at Serum 25(OH)D ≥20 and ≥18.44 ng/mL
3.7. Correlation between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Yu, D.; Wang, J.; Lin, S. Association between vitamin D deficiency and fragility fractures in Chinese elderly patients: A cross-sectional study. Ann. Palliat. Med. 2020, 9, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.; Can, U.; Staehelin, H.; Platz, A.; Henschkowski, J.; Michel, B.; Dawson-Hughes, B.; Theiler, R. Severe vitamin D deficiency in Swiss hip fracture patients. Bone 2008, 42, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2019, 67, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutrition: Its role in bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pang, Q. The effect of vitamin D and calcium supplementation on falls in older adults. Der Orthopäde 2017, 46, 729–736. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- You, H.; Shin, H.R.; Song, S.; Ly, S.Y. Vitamin D intake and bone mineral density in Korean adults: Analysis of the 2009–2011 Korea National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2021, 16, e19. [Google Scholar]
- Chew, C.K.; Clarke, B.L. Causes of low peak bone mass in women. Maturitas 2018, 111, 61–68. [Google Scholar] [CrossRef]
- Matkovic, V. Calcium and peak bone mass. J. Intern. Med. 1992, 231, 151–160. [Google Scholar] [CrossRef]
- Kelly, P.J.; Eisman, J.A.; Sambrook, P.N. Interaction of genetic and environmental influences on peak bone density. Osteoporos. Int. 1990, 1, 56–60. [Google Scholar] [CrossRef]
- Richelson, L.S.; Wahner, H.W.; Melton III, L.; Riggs, B.L. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. New Engl. J. Med. 1984, 311, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, L.; Yu, L.; Huang, W. Pregnancy- and lactation-associated osteoporosis with vertebral fractures: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 926. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Penckofer, S.; Fagan, M.; Giurgescu, C. Associations between Pregnancy-Related Symptoms, Serum 25(OH)D, and Physical Quality of Life in Pregnant Women. Nutrients 2022, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jukic, A.M.Z.; Song, H.; Zhang, L.; Yang, F.; Wu, S.; Yin, D.; Jiang, H. Serum Vitamin D Concentrations, Time to Pregnancy, and Pregnancy Outcomes among Preconception Couples: A Cohort Study in Shanghai, China. Nutrients 2022, 14, 3058. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cell. Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef]
- Keser, I.; Cvijetić, S.; Bituh, M.; Rumora Samarin, I.; Ilich, J.Z.; Colić Barić, I.; Rumbak, I. Vitamin D and parathyroid hormone in relation to bone health in Croatian women. Arch. Osteoporos. 2018, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.F.; Talwar, S.A.; Pollack, S.; Feuerman, M.; Yeh, J.K. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am. J. Clin. Nutr. 2006, 84, 602–609. [Google Scholar] [CrossRef]
- AlQuaiz, A.M.; Mujammami, M.; Kazi, A.; Hasanato, R.M.; Alodhayani, A.; Shaik, S.A.; Al-Daghri, N.M. Vitamin D cutoff point in relation to parathyroid hormone: A population based study in Riyadh city, Saudi Arabia. Arch. Osteoporos. 2019, 14, 22. [Google Scholar] [CrossRef]
- Shin, H.R.; Park, H.J.; Song, S.; Ly, S.Y. Dietary vitamin D intake in low ultraviolet irradiation seasons is associated with a better nutritional status of vitamin D in Korean adults according to the 2013–2014 National Health and Nutrition Examination Survey. Nutr. Res. 2022, 105, 53–65. [Google Scholar] [CrossRef]
- Asakura, K.; Etoh, N.; Imamura, H.; Michikawa, T.; Nakamura, T.; Takeda, Y.; Mori, S.; Nishiwaki, Y. Vitamin D status in Japanese adults: Relationship of serum 25-hydroxyvitamin D with simultaneously measured dietary vitamin D intake and ultraviolet ray exposure. Nutrients 2020, 12, 743. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; Brock, K.E.; Wright, J.; Ridges, L.; Small, P.; Seibel, M.J.; Conigrave, A.D.; Mason, R.S. Determinants of vitamin D status of healthy office workers in Sydney, Australia. J. Steroid Biochem. Mol. Biol. 2019, 189, 127–134. [Google Scholar] [CrossRef] [PubMed]
- The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2020; Ministry of Health and Welfare; The Korean Nutrition Society: Sejong, Republic of Korea, 2020.
- Yoo, K.-O.; Kim, M.-J.; Ly, S.Y. Association between vitamin D intake and bone mineral density in Koreans aged ≥ 50 years: Analysis of the 2009 Korea National Health and Nutrition Examination Survey using a newly established vitamin D database. Nutr. Res. Pract. 2019, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Lee, Y.-K.; Ha, Y.-C.; Kim, H.-Y. Inadequate dietary calcium and vitamin D intake in patients with osteoporotic fracture. J. Bone Metab. 2016, 23, 55–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.-K.; Chang, J.-S.; Min, Y.-K.; Byun, D.-W.; Park, Y.; Ha, Y.-C. Low calcium and vitamin D intake in Korean women over 50 years of age. J. Bone Miner. Metab. 2017, 35, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Cho, J.; Ly, S. Vitamin D intake and serum 25-hydroxyvitamin D levels in korean adults: Analysis of the 2009 Korea national health and nutrition examination survey (KNHANES IV-3) using a newly established vitamin D database. Nutrients 2016, 8, 610. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and Reliability of Korean Version of International Physical Activity Questionnaire (IPAQ) Short Form. Korean J. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- The Korean Nutrition Society. Computer Aided Nutritional Analysis Program; CAN-Pro 5.0 (Web Ver.). Available online: http://canpro5.kns.or.kr/ (accessed on 25 August 2022).
- Chen, Y.; Kinney, L.; Božović, A.; Smith, H.; Tarr, H.; Diamandis, E.P.; LeBlanc, A. Performance Evaluation of Siemens ADVIA Centaur and Roche MODULAR Analytics E170 Total 25-OH Vitamin D Assays. Clin. Biochem. 2012, 45, 1485–1490. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Guillemant, J.; Taupin, P.; Le, H.T.; Taright, N.; Allemandou, A.; Pérès, G.; Guillemant, S. Vitamin D status during puberty in French healthy aale adolescents. Osteoporos. Int. 1999, 10, 222–225. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of Vitamin D Insufficiency in an Adult Normal Population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. New Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Dharmshaktu, P.; Saha, S.; Kar, P.; Sreenivas, V.; Ramakrishnan, L.; Goswami, R. Absence of vitamin D deficiency among common outdoor workers in Delhi. Clin. Endocrinol. 2019, 91, 356–362. [Google Scholar] [CrossRef]
- Kwak, M.K.; Kim, J.H. The Radiative Characteristics of EUV-B over the Korean Peninsula and Exposure Time for Synthesizing Adequate Vitamin D. Atmosphere 2011, 21, 123–130. [Google Scholar]
- Park, S.S.; Lee, Y.G.; Kim, M.; Kim, J.; Koo, J.-H.; Kim, C.K.; Um, J.; Yoon, J. Simulation of threshold UV exposure time for vitamin D synthesis in South Korea. Adv. Meteorol. 2019, 2019, 4328151. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Neyestani, T.R. The effects of vitamin D-fortified foods on circulating 25(OH)D concentrations in adults: A systematic review and meta-analysis. Br. J. Nutr. 2022, 127, 1821–1838. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef]
- Ohta, H.; Kuroda, T.; Tsugawa, N.; Onoe, Y.; Okano, T.; Shiraki, M. Optimal vitamin D intake for preventing serum 25-hydroxyvitamin D insufficiency in young Japanese women. J. Bone Miner. Metab. 2018, 36, 620–625. [Google Scholar] [CrossRef]
- Zareef, T.A.; Jackson, R.T.; Alkahtani, A.A. Vitamin D Intake among Premenopausal Women Living in Jeddah: Food Sources and Relationship to Demographic Factors and Bone Health. Nutr. Metab. 2018, 2018, 8570986. [Google Scholar] [CrossRef]
- Holick, M.F. The role of vitamin D for bone health and fracture prevention. Curr. Osteoporos. Rep. 2006, 4, 96–102. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kim, M.-J.; Ly, S.Y. Vitamin D intake, serum 25OHD, and bone mineral density of Korean adults: Based on the Korea national health and nutrition examination survey (KNHANES, 2011). J. Nutr. Health 2016, 49, 437. [Google Scholar] [CrossRef][Green Version]
- Islam, M.Z.; Shamim, A.A.; Viljakainen, H.T.; Akhtaruzzaman, M.; Jehan, A.H.; Khan, H.U.; Al-Arif, F.A.; Lamberg-Allardt, C. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: A double-blinded, randomised, placebo-controlled 1-year intervention. Br. J. Nutr. 2010, 104, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.F.; Dhaliwal, R.; Shieh, A.; Mikhail, M.; Fazzari, M.; Ragolia, L.; Abrams, S.A. Vitamin D supplementation increases calcium absorption without a threshold effect. Am. J. Clin. Nutr. 2014, 99, 624–631. [Google Scholar] [CrossRef]
- Shapses, S.A.; Sukumar, D.; Schneider, S.H.; Schlussel, Y.; Sherrell, R.M.; Field, M.P.; Ambia-Sobhan, H. Vitamin D supplementation and calcium absorption during caloric restriction: A randomized double-blind trial. Am. J. Clin. Nutr. 2013, 97, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Korea Health Industry Development Institute. National Nutrition Statistics. Available online: https://www.khidi.or.kr/kps/dhraStat/result5?menuId=MENU01656&gubun=age3&year=2020 (accessed on 26 August 2022).
- Brown, J.P.; Albert, C.; Nassar, B.A.; Adachi, J.D.; Cole, D.; Davison, K.S.; Dooley, K.C.; Don-Wauchope, A.; Douville, P.; Hanley, D.A.; et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin. Biochem. 2009, 42, 929–942. [Google Scholar] [CrossRef]
- Macdonald, H.M.; Mavroeidi, A.; Barr, R.J.; Black, A.J.; Fraser, W.D.; Reid, D.M. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone 2008, 42, 996–1003. [Google Scholar] [CrossRef]
- Al-Yatama, F.I.; AlOtaibi, F.; Al-Bader, M.D.; Al-Shoumer, K.A. The effect of clothing on vitamin D status, bone turnover markers, and bone mineral density in young Kuwaiti females. Int. J. Endocrinol. 2019, 2019, 6794837. [Google Scholar] [CrossRef]
- Orito, S.; Kuroda, T.; Onoe, Y.; Sato, Y.; Ohta, H. Age-related distribution of bone and skeletal parameters in 1,322 Japanese young women. J. Bone Miner. Metab. 2009, 27, 698–704. [Google Scholar] [CrossRef]
- Williams, C.; Sapra, A. Osteoporosis Markers. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559306/ (accessed on 19 October 2022).
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef]
- Eastell, R.; Pigott, T.; Gossiel, F.; Naylor, K.E.; Walsh, J.S.; Peel, N.F.A. DIAGNOSIS OF ENDOCRINE DISEASE: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton III, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Calvo, M.S. Vitamin D fortification and supplementation policies to correct vitamin D insufficiency/deficiency globally. In Vitamin D; Elsevier Academic Press: London, UK, 2018; pp. 91–108. [Google Scholar]
| Wintertime Attendees | Summertime Attendees | Total Participants | p-Value | ||
|---|---|---|---|---|---|
| (n = 101) | (n = 117) | (n = 218) | |||
| Age (years) | 23.2 ± 0.25 | 23.02 ± 0.22 | 23.1 ± 0.16 | 0.527 | |
| Height (cm) | 162.0 ± 0.56 | 162.3 ± 0.51 | 162.1 ± 0.34 | 0.753 | |
| Weight (kg) | 56.2 ± 0.96 | 56.2 ± 0.85 | 56.2 ± 0.63 | 0.921 | |
| BMI (kg/m2) | 21.5 ± 0.28 | 21.5 ± 0.25 | 21.5 ± 0.18 | 0.853 | |
| UV exposure time (min) | 14.5 ± 1.82 | 19.5 ± 1.77 | 17.2 ± 1.28 | 0.052 | |
| Occupation | Student | 70 (69.3) | 84 (71.8) | 154 (70.6) | 0.921 |
| Worker | 19 (18.8) | 20 (17.1) | 39 (17.9) | ||
| Other | 12 (11.9) | 13 (11.1) | 25 (11.5) | ||
| Smoking status | Yes | 7 (6.9) | 9 (7.7) | 16 (7.3) | 0.830 |
| No | 94 (93.1) | 108 (92.3) | 202 (92.7) | ||
| Alcohol consumption | Yes | 76 (75.2) | 88 (75.2) | 164 (75.2) | 0.995 |
| No | 25 (24.8) | 29 (24.8) | 54 (24.8) | ||
| Number of ways to avoid UV exposure | Never | 11 (10.8) | 6 (5.1) | 17 (7.8) | 0.414 |
| 1 | 24 (23.8) | 29 (24.8) | 53 (24.3) | ||
| 2 | 33 (32.7) | 37 (31.6) | 70 (32.1) | ||
| ≥3 | 33 (32.7) | 45 (38.5) | 78 (35.8) | ||
| Physical activity | Low | 40 (39.6) | 57 (48.7) | 97 (44.5) | 0.401 |
| Moderate | 41 (40.6) | 40 (34.2) | 81 (37.2) | ||
| High | 20 (19.8) | 20 (17.1) | 40 (18.3) | ||
| Taking vitamin D supplements | Yes | 19 (18.8) | 36 (30.8) | 55 (25.2) | 0.043 |
| No | 82 (81.2) | 81 (69.2) | 163 (74.8) | ||
| Prevalence of 25(OH)D deficiency | Deficiency (<20 ng/mL) | 83 (82.2) | 77 (65.8) | 160 (73.4) | 0.006 |
| Sufficiency (≥20 ng/mL) | 18 (17.8) | 40 (34.2) | 58 (26.6) | ||
| Wintertime Attendees | Summertime Attendees | Total Participants | p-Value | |
|---|---|---|---|---|
| Dietary intake of nutrients (n = 218) | ||||
| Energy (kcal/day) | 1390.5 ± 37.0 | 1458.8 ± 36.9 | 1427 ± 26.2 | 0.195 |
| Calcium (mg/day) | 533.8 ± 23.0 | 572.46 ± 21.4 | 554.55 ± 15.7 | 0.220 |
| Vitamin D (µg/day) | 3.19 ± 0.36 | 3.09 ± 0.29 | 3.14 ± 0.22 | 0.827 |
| Dietary intake of vitamin D rich food groups and contribution (µg/day, % §) | ||||
| Fish and shellfish | 2.07 ± 0.35 (64.9) | 1.68 ± 0.28 (54.4) | 1.86 ± 0.22 (59.2) | 0.393 |
| Eggs | 0.64 ± 0.06 (20.1) | 0.73 ± 0.04 (23.6) | 0.69 ± 0.04 (22.0) | 0.248 |
| Meat and meat products | 0.24 ± 0.01 (7.5) | 0.33 ± 0.06 (10.7) | 0.29 ± 0.04 (9.2) | 0.196 |
| Milk and dairy products | 0.12 ± 0.03 (3.8) | 0.11 ± 0.02 (3.6) | 0.12 ± 0.02 (3.8) | 0.913 |
| Mushrooms | 0.08 ± 0.01 (2.5) | 0.10 ± 0.01 (3.2) | 0.09 ± 0.01 (2.9) | 0.321 |
| Grain and grain products | 0.04 ± 0.02 (1.3) | 0.06 ± 0.02 (1.9) | 0.05 ± 0.02 (1.6) | 0.602 |
| Total vitamin D intake (µg/day) | ||||
| With supplements † (n = 55) | 24.7 ± 3.22 | 19.2 ± 2.70 | 21.1 ± 2.10 | 0.218 |
| Without supplements ‡ (n = 163) | 3.32 ± 0.41 | 3.17 ± 0.36 | 3.25 ± 0.27 | 0.789 |
| Overall (n = 218) | 7.34 ± 1.07 | 8.10 ± 1.10 | 7.75 ± 0.77 | 0.998 |
| Season | Variable | Regression Coefficient | 95% CI | p Value |
|---|---|---|---|---|
| Total participants | Vitamin D intake (with supplement, µg/1000 kcal) | 0.170 | 0.078, 0.263 | <0.001 |
| Way to avoid UV exposure | −1.577 | −2.453, −0.702 | <0.001 | |
| UV exposure time (min) | 0.056 | 0.012, 0.101 | 0.013 | |
| Wintertime attendees | Vitamin D intake (with supplement, µg/1000 kcal) | 0.170 | 0.030,0.310 | 0.018 |
| Summertime attendees | Way to avoid UV exposure | −2.559 | −3.758, −1.360 | <0.001 |
| Vitamin D intake (with supplement, µg/1000 kcal) | 0.147 | 0.028, 0.266 | 0.016 | |
| UV exposure time (min) | 0.059 | 0.003, 0.115 | 0.039 |
| Season | Variables | iPTH | NTX | BAP | Serum Calcium | BMD (L1-4) |
|---|---|---|---|---|---|---|
| Total participants | 25(OH)D | −0.302 *** | −0.095 | −0.070 | 0.092 | −0.045 |
| iPTH | 0.132 | −0.056 | −0.134 * | 0.053 | ||
| NTX | 0.480 *** | 0.025 | −0.087 | |||
| BAP | 0.037 | −0.133 | ||||
| Serum calcium | −0.010 | |||||
| Wintertime attendees | 25(OH)D | −0.335 *** | −0.072 | −0.109 | −0.037 | 0.011 |
| iPTH | −0.058 | −0.074 | −0.202 * | 0.068 | ||
| NTX | 0.589 *** | −0.063 | −0.151 | |||
| BAP | −0.083 | −0.187 | ||||
| Serum calcium | 0.013 | |||||
| Summertime attendees | 25(OH)D | −0.134 | 0.036 | −0.056 | 0.183 * | −0.086 |
| iPTH | 0.052 | −0.033 | −0.018 | 0.015 | ||
| NTX | 0.490 *** | 0.169 | −0.053 | |||
| BAP | 0.116 | −0.094 | ||||
| Serum calcium | −0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.R.; Park, H.J.; Ly, S.Y. Optimal Serum 25(OH)D Level and Vitamin D Intake in Young Korean Women. Nutrients 2022, 14, 4845. https://doi.org/10.3390/nu14224845
Shin HR, Park HJ, Ly SY. Optimal Serum 25(OH)D Level and Vitamin D Intake in Young Korean Women. Nutrients. 2022; 14(22):4845. https://doi.org/10.3390/nu14224845
Chicago/Turabian StyleShin, Hye Ran, Hyeon Ju Park, and Sun Yung Ly. 2022. "Optimal Serum 25(OH)D Level and Vitamin D Intake in Young Korean Women" Nutrients 14, no. 22: 4845. https://doi.org/10.3390/nu14224845
APA StyleShin, H. R., Park, H. J., & Ly, S. Y. (2022). Optimal Serum 25(OH)D Level and Vitamin D Intake in Young Korean Women. Nutrients, 14(22), 4845. https://doi.org/10.3390/nu14224845







