Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Laboratory Analysis
2.3. Statistical Analysis
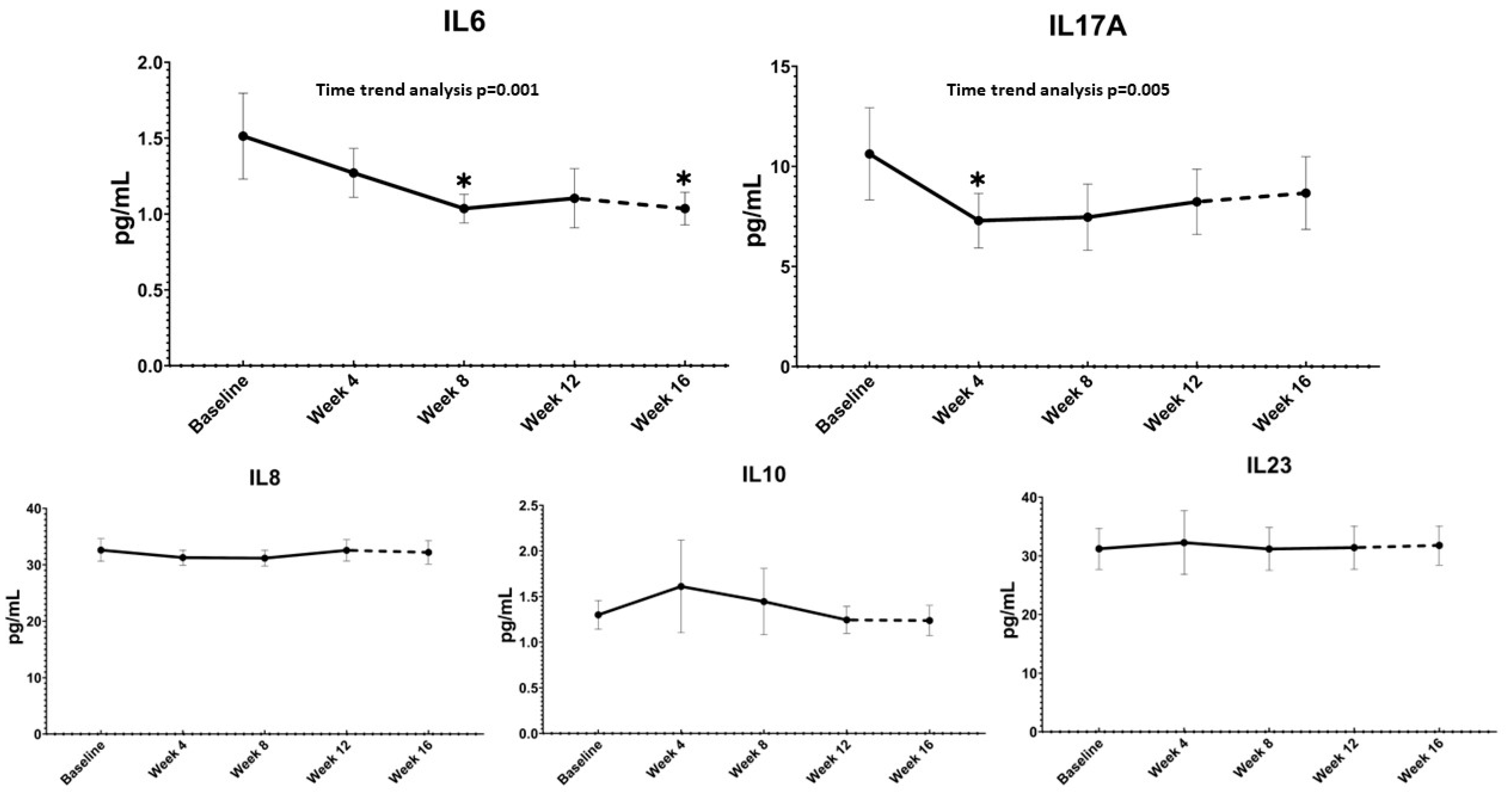
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckett, E. More Than Bone Health: The Many Roles for Vitamin D. Nutrients 2020, 12, 2388. [Google Scholar] [CrossRef] [PubMed]
- Bleizgys, A. Vitamin D Dosing: Basic Principles and a Brief Algorithm (2021 Update). Nutrients 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: Individual Participant Data Meta-Analysis. Health Technol. Assess 2019, 23, 1–44. [Google Scholar] [CrossRef]
- Coussens, A.K.; Wilkinson, R.J.; Hanifa, Y.; Nikolayevskyy, V.; Elkington, P.T.; Islam, K.; Timms, P.M.; Venton, T.R.; Bothamley, G.H.; Packe, G.E.; et al. Vitamin D Accelerates Resolution of Inflammatory Responses during Tuberculosis Treatment. Proc. Natl. Acad. Sci. USA 2012, 109, 15449–15454. [Google Scholar] [CrossRef]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 736665. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Rafiee, P.; Haghighi, S.; Razeghi Jahromi, S.; Djalali, M.; Moradi-Tabriz, H.; Mahmoudi, M.; Togha, M. The Effects of Vitamin D3 Supplementation on TGF-β and IL-17 Serum Levels in Migraineurs: Post Hoc Analysis of a Randomized Clinical Trial. J. Pharm. Health Care Sci. 2021, 7, 9. [Google Scholar] [CrossRef]
- Toghianifar, N.; Ashtari, F.; Zarkesh-Esfahani, S.H.; Mansourian, M. Effect of High Dose Vitamin D Intake on Interleukin-17 Levels in Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Neuroimmunol. 2015, 285, 125–128. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and Immunologic Effects of High- vs Low-Dose Cholecalciferol in Multiple Sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of Vitamin D Supplementation on Non-Skeletal Disorders: A Systematic Review of Meta-Analyses and Randomised Trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Fassio, A.; Adami, G.; Rossini, M.; Giollo, A.; Caimmi, C.; Bixio, R.; Viapiana, O.; Milleri, S.; Gatti, M.; Gatti, D. Pharmacokinetics of Oral Cholecalciferol in Healthy Subjects with Vitamin D Deficiency: A Randomized Open-Label Study. Nutrients 2020, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Gatti, D.; Rossini, M.; Benini, C.; Fracassi, E.; Bertoldo, E.; Viapiana, O.; Milleri, S.; Gatti, M.; Adami, G. Pharmacodynamics of Oral Cholecalciferol in Healthy Individuals with Vitamin D Deficiency: A Randomized Open-Label Study. Nutrients 2021, 13, 2293. [Google Scholar] [CrossRef] [PubMed]
- DIBASE, Summary of Product 2017. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000972_036635_RCP.pdf&sys=m0b1l3 (accessed on 31 August 2022).
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proença, L.; Mendes, J.J.; Botelho, J. Vitamin D and Periodontitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, E2177. [Google Scholar] [CrossRef]
- Gisondi, P.; Rossini, M.; Di Cesare, A.; Idolazzi, L.; Farina, S.; Beltrami, G.; Peris, K.; Girolomoni, G. Vitamin D Status in Patients with Chronic Plaque Psoriasis. Br. J. Dermatol. 2012, 166, 505–510. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Vitamin D and Marine Omega 3 Fatty Acid Supplementation and Incident Autoimmune Disease: VITAL Randomized Controlled Trial. Available online: https://www.bmj.com/content/376/bmj-2021-066452 (accessed on 1 September 2022).
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D Supplementation and Total Cancer Incidence and Mortality: A Meta-Analysis of Randomized Controlled Trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Valvano, M.; Magistroni, M.; Mancusi, A.; D’Ascenzo, D.; Longo, S.; Stefanelli, G.; Vernia, F.; Viscido, A.; Necozione, S.; Latella, G. The Usefulness of Serum Vitamin D Levels in the Assessment of IBD Activity and Response to Biologics. Nutrients 2021, 13, 323. [Google Scholar] [CrossRef]
- Rossini, M.; Maddali Bongi, S.; La Montagna, G.; Minisola, G.; Malavolta, N.; Bernini, L.; Cacace, E.; Sinigaglia, L.; Di Munno, O.; Adami, S. Vitamin D Deficiency in Rheumatoid Arthritis: Prevalence, Determinants and Associations with Disease Activity and Disability. Arthritis Res. Ther. 2010, 12, R216. [Google Scholar] [CrossRef] [PubMed]
- Elolemy, G.; Hassan, W.; Nasr, M.; Baraka, E. Hypovitaminosis D in Patients with Ankylosing Spondylitis: Frequency and Consequences. Curr. Rheumatol. Rev. 2021, 17, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 10659. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 Biology into Effective Treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Adami, G.; Rossini, M.; Bogliolo, L.; Cantatore, F.P.; Varenna, M.; Malavolta, N.; Del Puente, A.; Muratore, M.; Orsolini, G.; Gatti, D.; et al. An Exploratory Study on the Role of Vitamin D Supplementation in Improving Pain and Disease Activity in Rheumatoid Arthritis. Mod. Rheumatol. 2019, 29, 1059–1062. [Google Scholar] [CrossRef]
- Giannini, S.; Giusti, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; Sinigaglia, L. The Immunologic Profile of Vitamin D and Its Role in Different Immune-Mediated Diseases: An Expert Opinion. Nutrients 2022, 14, 473. [Google Scholar] [CrossRef]
- Nonn, L.; Peng, L.; Feldman, D.; Peehl, D.M. Inhibition of P38 by Vitamin D Reduces Interleukin-6 Production in Normal Prostate Cells via Mitogen-Activated Protein Kinase Phosphatase 5: Implications for Prostate Cancer Prevention by Vitamin D. Cancer Res. 2006, 66, 4516–4524. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, J.; Zheng, C.; Wu, J.; Cheng, Y.; Zhu, S.; Lin, C.; Cao, Q.; Zhu, J.; Jin, T. 1,25-Dihydroxyvitamin D3-Induced Dendritic Cells Suppress Experimental Autoimmune Encephalomyelitis by Increasing Proportions of the Regulatory Lymphocytes and Reducing T Helper Type 1 and Type 17 Cells. Immunology 2017, 152, 414–424. [Google Scholar] [CrossRef]
- Carvalho, J.T.G.; Schneider, M.; Cuppari, L.; Grabulosa, C.C.; Aoike, D.T.; Redublo, B.M.Q.; Batista, M.C.; Cendoroglo, M.; Maria Moyses, R.; Dalboni, M.A. Cholecalciferol Decreases Inflammation and Improves Vitamin D Regulatory Enzymes in Lymphocytes in the Uremic Environment: A Randomized Controlled Pilot Trial. PLoS ONE 2017, 12, e0179540. [Google Scholar] [CrossRef]
- Corrado, A.; Rotondo, C.; Sanpaolo, E.R.; Altomare, A.; Maruotti, N.; Cici, D.; Cantatore, F.P. 1,25OH-Vitamin D3 and IL-17 Inhibition Modulate Pro-Fibrotic Cytokines Production in Peripheral Blood Mononuclear Cells of Patients with Systemic Sclerosis. Int. J. Med. Sci. 2022, 19, 867–877. [Google Scholar] [CrossRef]
- Rolf, L.; Smolders, J.; van den Ouweland, J.; Hupperts, R.; Damoiseaux, J. Correlation of Different Cellular Assays to Analyze T Cell-Related Cytokine Profiles in Vitamin D3-Supplemented Patients with Multiple Sclerosis. Mol. Immunol. 2019, 105, 198–204. [Google Scholar] [CrossRef]
- Beilfuss, J.; Berg, V.; Sneve, M.; Jorde, R.; Kamycheva, E. Effects of a 1-Year Supplementation with Cholecalciferol on Interleukin-6, Tumor Necrosis Factor-Alpha and Insulin Resistance in Overweight and Obese Subjects. Cytokine 2012, 60, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Woźniewicz, M.; Walkowiak, J.; Bogdański, P.; Jeszka, J.; Stelmach-Mardas, M. The Effect of Vitamin D Supplementation on Selected Inflammatory Biomarkers in Obese and Overweight Subjects: A Systematic Review with Meta-Analysis. Eur. J. Nutr. 2016, 55, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Taylor, A.J.; Plant, K.; Dahl, A.; Lau, E.; Hill, M.; Sims, D.; Heger, A.; Emberson, J.; Armitage, J.; Clarke, R.; et al. Genomic Response to Vitamin D Supplementation in the Setting of a Randomized, Placebo-Controlled Trial. eBioMedicine 2018, 31, 133–142. [Google Scholar] [CrossRef]
- Motamed, S.; Nikooyeh, B.; Kashanian, M.; Hollis, B.W.; Neyestani, T.R. Efficacy of Two Different Doses of Oral Vitamin D Supplementation on Inflammatory Biomarkers and Maternal and Neonatal Outcomes. Matern. Child Nutr. 2019, 15, e12867. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D Status and Ill Health: A Systematic Review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Ward, G.; Simpson, A.; Boscato, L.; Hickman, P.E. The Investigation of Interferences in Immunoassay. Clin. Biochem. 2017, 50, 1306–1311. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Viljoen, A. Analytical Error and Interference in Immunoassay: Minimizing Risk. Ann. Clin. Biochem. 2011, 48, 418–432. [Google Scholar] [CrossRef]
| Baseline Characteristics | All Subjects (n = 75) | Group A (n = 25) | Group B (n = 25) | Group C (n = 25) |
|---|---|---|---|---|
| Sex | ||||
| Male; N (%) | 31 (41.3%) | 12 (48%) | 7 (28%) | 12 (48%) |
| Female; N (%) | 44 (58.7%) | 13 (52%) | 18 (72%) | 13 (52%) |
| Age (years); Years (SD) | 34.1 (10.2) | 30.2 (10) | 36.7 (8.8) | 35.4 (11) |
| Weight (Kg); Mean (SD) | 66.7 (12.4) | 65.2 (13.5) | 67.4 (9.8) | 67.6 (13.7) |
| BMI (Kg/m2); Mean (SD) | 23.1 (2.6) | 22.6 (2.9) | 23.4 (2.1) | 23.2 (2.8) |
| All Subjects (n = 73) | Group A (n = 24) | Group B (n = 25) | Group C (n = 24) | |
|---|---|---|---|---|
| IL-6 (pg/mL) | ||||
| Baseline | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.99 (0.81–2.43) | 1.12 (0.81–1.57) | 2.33 (1.04–5.85) | 0.84 (0.81–3.02) |
| Min-Max | 0.81–6.81 | 0.81–2.38 | 0.82–6.81 | 0.81–4.64 |
| Week 4 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.92 (0. 81–1.84) | 1.40 (0.81–1.70) | 1.01 (0.81–1.64) | 1.19 (0.81–2.56) |
| Min-Max | 0.81–3.72 | 0.81–3.72 | 0.81–3.27 | 0.81–3.11 |
| Week 8 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.81 (0. 81–1.53) | 0.81 (0.81–1.17) | 0.89 (0.81–1.66) | 0.9 (0.81–2.28) |
| Min-Max | 0.81–2.55 | 0.81–2.55 | 0.81–1.89 | 0.81–2.51 |
| Week 12 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.81 (0. 81–1.18) | 0.81 (0.81–1.10) | 0.86 (0.81–1.57) | 0.95 (0.81–1.37) |
| Min-Max | 0.81–1.79 | 0.81–1.54 | 0.81–1.79 | 0.81–1.74 |
| IL-17A (pg/mL) | ||||
| Baseline | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 5.57 (4.45–13.38) | 5.54 (4.17–8.52) | 5.61 (3.87–10.60) | 9.92 (4.28–16.83) |
| Min-Max | 2.67–20.45 | 2.67–20.45 | 3.3–12.25 | 2.91–20.36 |
| Week 4 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 4.05 (2.32–11.43) | 3.71 (2.46–12.91) | 3.81 (2.3–8.79) | 7.03 (2.34–15.16) |
| Min-Max | 2.3–18.40 | 2.3–17.88 | 2.3–9.95 | 2.3–18.40 |
| Week 8 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 3.85 (2.30–10.48) | 3.85 (2.3–11.87) | 3.43 (2.3–4.63) | 6.18 (2.93–18.52) |
| Min-Max | 2.3–21.01 | 2.3–16.37 | 2.3–5.65 | 2.3–21.01 |
| Week 12 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 5.54 (2.50–13.66) | 5.88 (2.69–11.87) | 3.52 (2.3–15.17) | 9.41 (2.59–18.09) |
| Min-Max | 2.3–18.76 | 2.3–15.44 | 2.3–18.64 | 2.3–18.76 |
| IL-23 (pg/mL) | ||||
| Baseline | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 37.76 (20–45.45) | 37.76 (20–46.54) | 45.91 (24.62–57.36) | 34.11 (20–38.86) |
| Min-Max | 20–60.4 | 20–48.7 | 20–60.4 | 20–52.32 |
| Week 4 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 35.57 (20–41.28) | 35.57 (20–42.15) | 37.76 (23.71–36.48) | 35.57 (20–40.69) |
| Min-Max | 20–129.65 | 20–129.65 | 20–37.03 | 20–45.08 |
| Week 8 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 36.30 (20–42.63) | 36.3 (20–37.03) | 39.33 (23.89–53.67) | 38.13 (20–50.39) |
| Min-Max | 20–56.77 | 20–37.76 | 20–56.77 | 20–55.35 |
| Week 12 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 36.29 (20–40.32) | 35.66 (20–39.95) | 39.07 (24.55–39.77) | 34.11 (20–48.74) |
| Min-Max | 20–55.35 | 20–40.68 | 20–39.95 | 20–55.35 |
| IL-8 (pg/mL) | ||||
| Baseline | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 29 (29–34.27) | 29 (29–32.19) | 32.67 (29–52.29) | 29 (29–32.73) |
| Min-Max | 29–63.35 | 29–62.43 | 29–63.35 | 29–43.91 |
| Week 4 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 29 (29-39.17) | 35.33 (29–49.96) | 29 (29–39.86) | 29 (29–34.90) |
| Min-Max | 29–51.61 | 29–51.61 | 29–43.91 | 29–43.01 |
| Week 8 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 29 (29–35.34) | 29 (29–33.25) | 29 (29–32.98) | 33.76 (29–41.22) |
| Min-Max | 29–49.96 | 29–36.34 | 29–38.31 | 29–49.96 |
| Week 12 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 29 (29–41.69) | 29 (29–29) | 33.34 (29–42.58) | 39.01 (29–55.61) |
| Min-Max | 29–57.93 | 29–24.02 | 29–45.69 | 29–57.93 |
| IL-10 (pmol/L) | ||||
| Baseline | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 1.52 (0.98–1.81) | 1.27 (0.98–1.95) | 1.94 (1.13–2.44) | 1.25 (0.98–1.59) |
| Min-Max | 0.98–2.78 | 0.98–2.78 | 0.98–2.68 | 0.98–2.62 |
| Week 4 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.98 (0.98–1.82) | 0.98 (0.98–1.89) | 1.18 (0.98–2.48) | 1.31 (0.98–2.06) |
| Min-Max | 0.98–2.73 | 0.98–2.41 | 0.98–2.73 | 0.98–2.46 |
| Week 8 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 1.02 (0.98–2.28) | 1.02 (0.98–1.36) | 1.31 (0.98–4.66) | 1.56 (0.98–2.47) |
| Min-Max | 0.98–6.34 | 0.98–6.34 | 0.98–5.67 | 0.98–2.58 |
| Week 12 | ||||
| N | 73 | 24 | 25 | 24 |
| Median (IQR) | 0.98 (0.98–1.15) | 1.01 (0.98–2.32) | 0.98 (0.98–3.00) | 0.98 (0.98–1.24) |
| Min-Max | 0.98–3.86 | 0.98–3.86 | 0.98–3.64 | 0.98–1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fassio, A.; Gatti, D.; Rossini, M.; Bertelle, D.; Bixio, R.; Viapiana, O.; Milleri, S.; Benini, C.; Pistillo, F.; Zanetti, G.; et al. Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency. Nutrients 2022, 14, 4823. https://doi.org/10.3390/nu14224823
Fassio A, Gatti D, Rossini M, Bertelle D, Bixio R, Viapiana O, Milleri S, Benini C, Pistillo F, Zanetti G, et al. Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency. Nutrients. 2022; 14(22):4823. https://doi.org/10.3390/nu14224823
Chicago/Turabian StyleFassio, Angelo, Davide Gatti, Maurizio Rossini, Davide Bertelle, Riccardo Bixio, Ombretta Viapiana, Stefano Milleri, Camilla Benini, Francesca Pistillo, Giulia Zanetti, and et al. 2022. "Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency" Nutrients 14, no. 22: 4823. https://doi.org/10.3390/nu14224823
APA StyleFassio, A., Gatti, D., Rossini, M., Bertelle, D., Bixio, R., Viapiana, O., Milleri, S., Benini, C., Pistillo, F., Zanetti, G., & Adami, G. (2022). Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency. Nutrients, 14(22), 4823. https://doi.org/10.3390/nu14224823







