J-Shaped Association of Tomato Intake with New-Onset Hypertension in General Adults: A Nationwide Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Nutrient Intakes
2.3. Assessments of Covariates
2.4. Assessment of Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
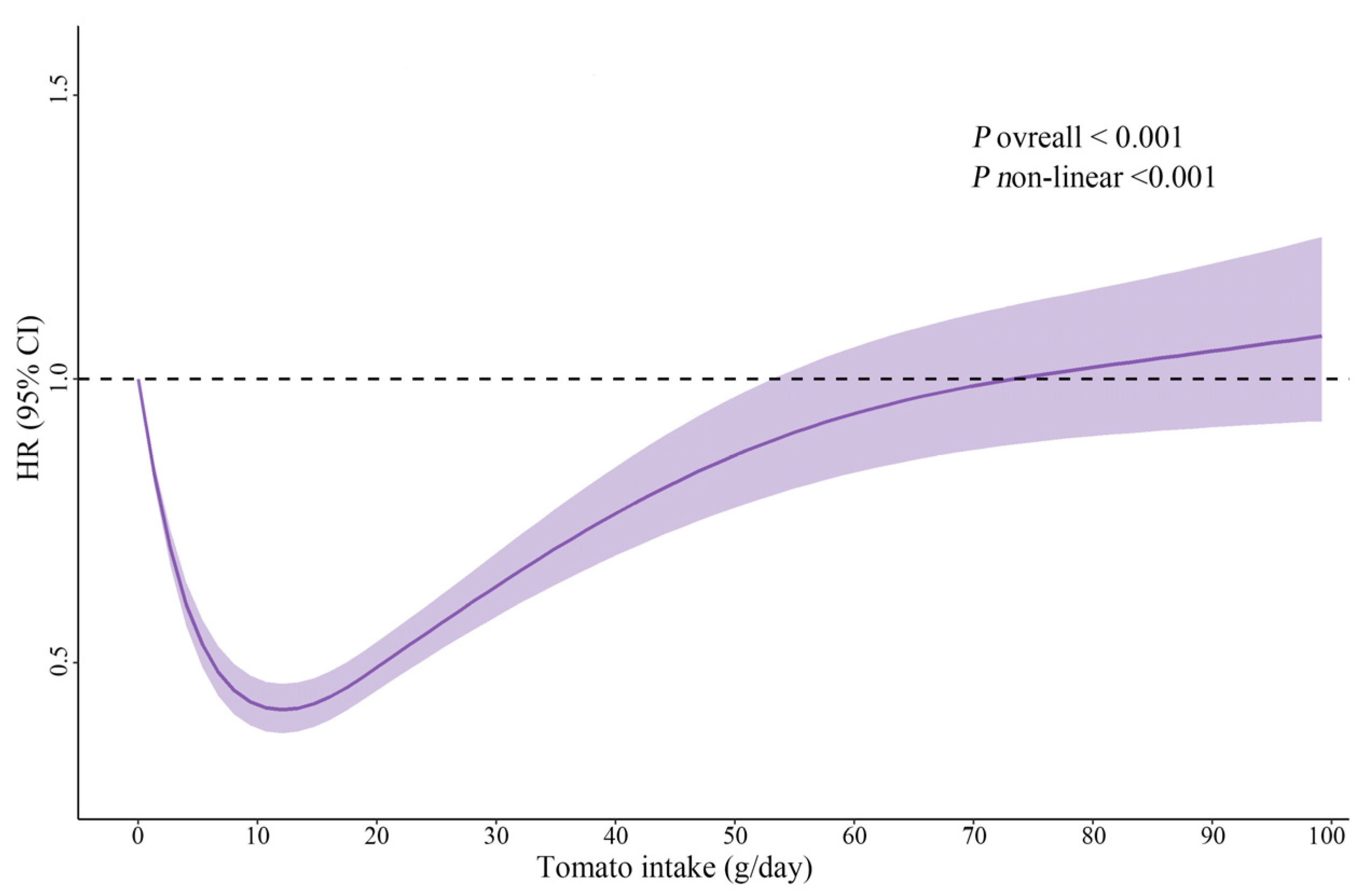
3.2. Association between Dietary Tomato Intake and New-Onset Hypertension
3.3. Sensitivity Analyses
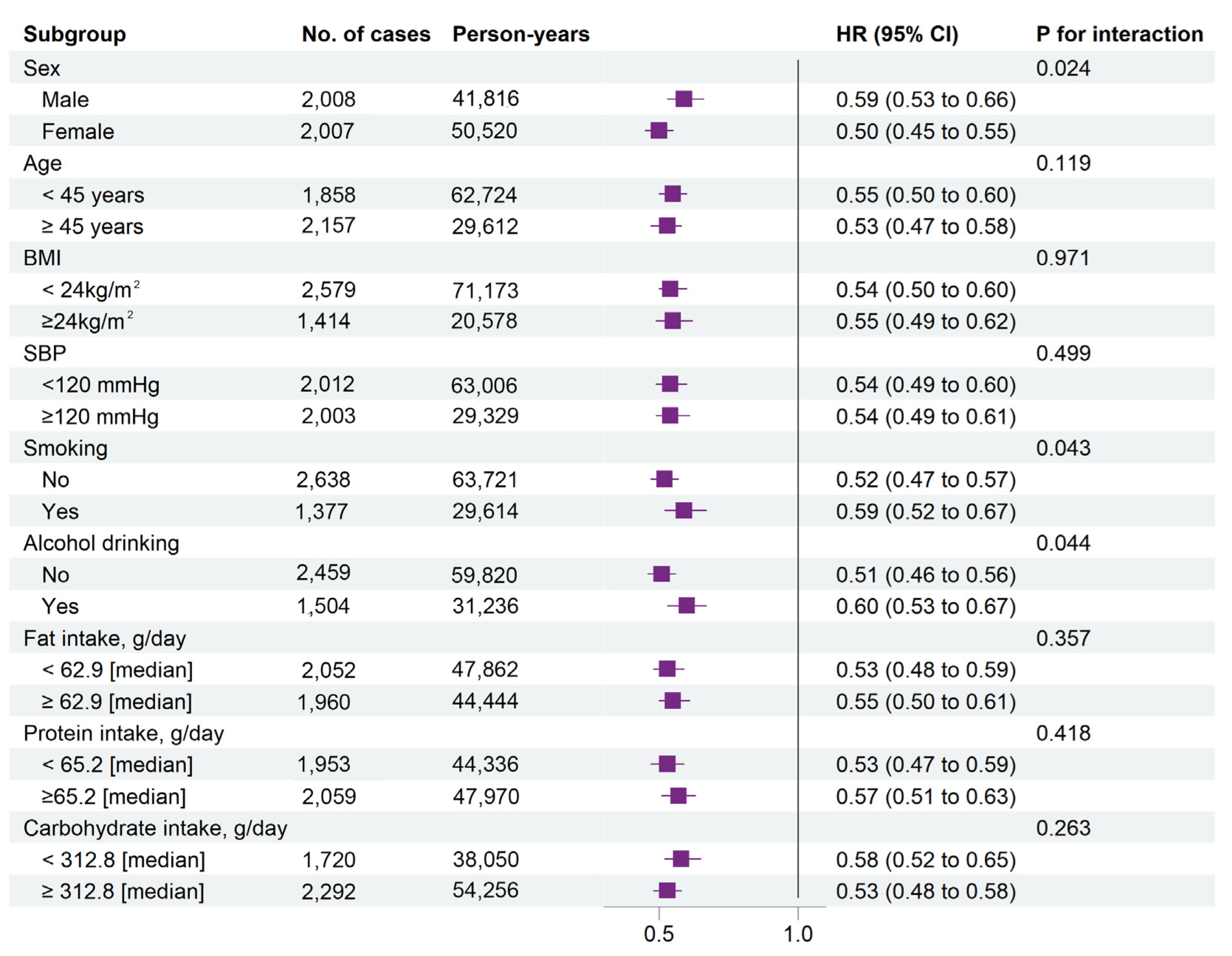
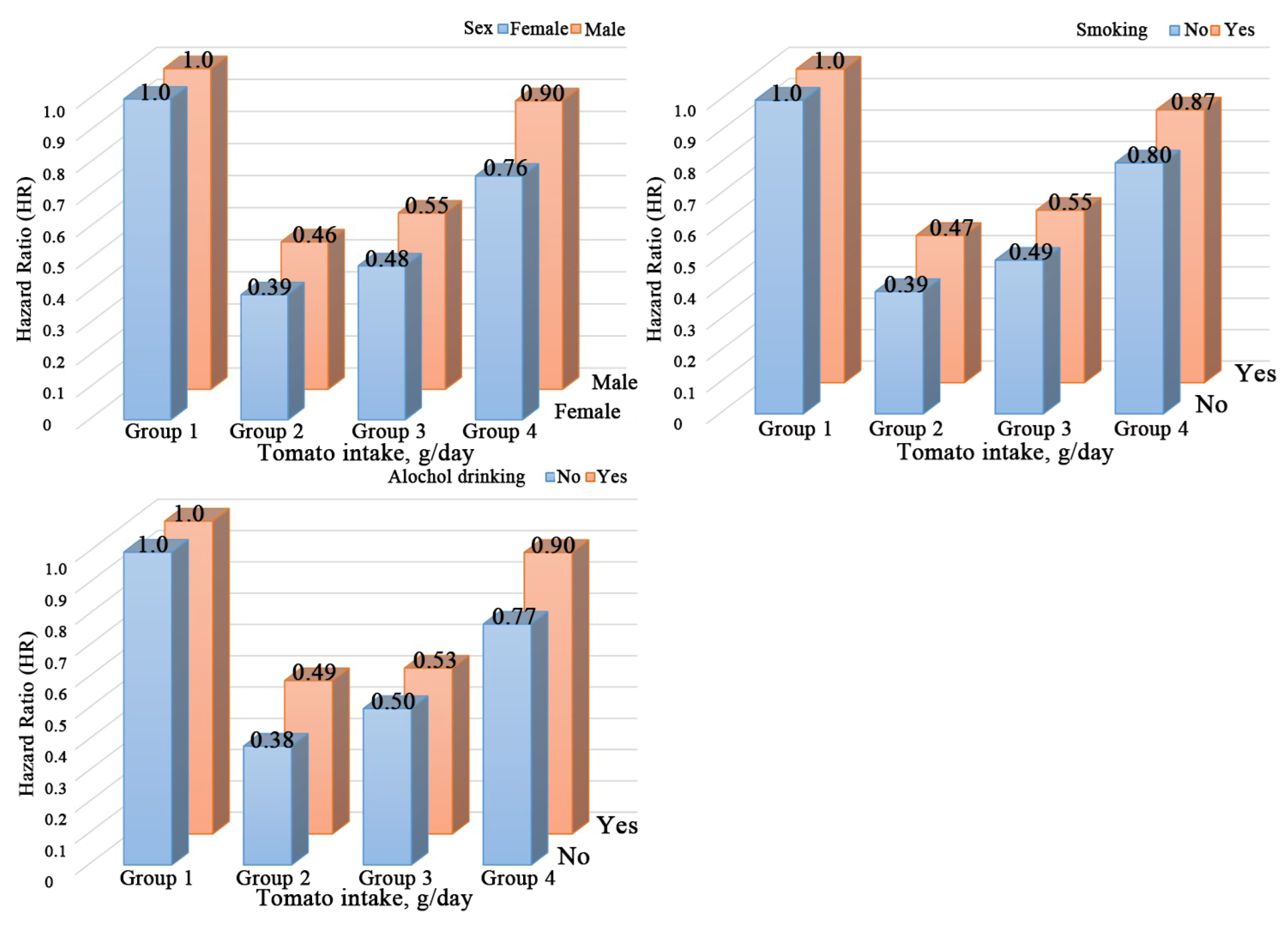
3.4. Stratified Analyses by Potential Effect Modifiers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Delgado-valero, B.; Cachofeiro, V.; Martínez-martínez, E. Fibrosis, the bad actor in cardiorenal syndromes: Mechanisms involved. Cells 2021, 10, 1824. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, L.; Wang, X.; Hao, G.; Zhang, Z.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C.; et al. Status of Hypertension in China: Results from the China Hypertension Survey, 2012–2015. Circulation 2018, 137, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Savica, V.; Bellinghieri, G.; Kopple, J.D. The effect of nutrition on blood pressure. Annu. Rev. Nutr. 2010, 30, 365–401. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Campos, H. Dietary therapy in hypertension. N. Engl. J. Med. 2010, 362, 2102–2112. [Google Scholar] [CrossRef]
- Liu, M.-w.; Yu, H.-j.; Yuan, S.; Song, Y.; Tang, B.-w.; Cao, Z.-k.; Yang, X.-h.; Towne, S.D., Jr.; He, Q.-q. Association between fruit and vegetable intake and the risk of hypertension among Chinese adults: A longitudinal study. Eur. J. Nutr. 2018, 57, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, M.R.; Asbaghi, O.; Nazarin, B.; Naeini, F.; Kaviani, M.; Moradi, S.; Askari, G.; Nourian, M.; Ashtary-Larky, D. Lycopene Supplementation and Blood Pressure: Systematic review and meta-analyses of randomized trials. J. Herb. Med. 2022, 31, 100521. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun-Waterhouse, D.; Chen, Y.; Li, F.; Li, D. Epigenetic mechanisms underlying the benefits of flavonoids in cardiovascular health and diseases: Are long non-coding RNAs rising stars? Crit. Rev. Food Sci. Nutr. 2022, 62, 3855–3872. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef]
- Shidfar, F.; Froghifar, N.; Vafa, M.; Rajab, A.; Hosseini, S.; Shidfar, S.; Gohari, M. The effects of tomato consumption on serum glucose, apolipoprotein B, apolipoprotein A-I, homocysteine and blood pressure in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Wood, N.; Johnson, R.B. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J. Clin. Periodontol. 2004, 31, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Luu, H.N.; Wang, R.; Adams-Haduch, J.; Jin, A.; Koh, W.-P.; Yuan, J.-M. Association between Dietary Tomato Intake and the Risk of Hepatocellular Carcinoma: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, B.; Li, S.; Wang, S.; Xia, D.; Meng, H. Association of dietary tomato intake with bladder cancer risk in a prospective cohort of 101,683 individuals with 12.5 years of follow-up. Aging 2021, 13, 17629–17637. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, S.; Zhu, Y. Dietary Intake of Tomato and Lycopene and Risk of All-Cause and Cause-Specific Mortality: Results from a Prospective Study. Front. Nutr. 2021, 8, 684859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. 1), 2–7. [Google Scholar] [CrossRef]
- Zhai, F.; Guo, X.; Popkin, B.M.; Ma, L.; Wang, Q.; Shuigao, W.Y.; Ge, J.A.K. Evaluation of the 24-Hour Individual Recall Method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; George, E.S.; Banach, M. Tomato and lycopene consumption is inversely associated with total and cause-specific mortality: A population-based cohort study, on behalf of the International Lipid Expert Panel (ILEP). Br. J. Nutr. 2020, 124, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Roseman, C.; Truedsson, L.; Kapetanovic, M.C. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res. Ther. 2012, 14, R170. [Google Scholar] [CrossRef]
- Nagao, T.; Nogawa, K.; Sakata, K.; Morimoto, H.; Morita, K.; Watanabe, Y.; Suwazono, Y. Effects of Alcohol Consumption and Smoking on the Onset of Hypertension in a Long-Term Longitudinal Study in a Male Workers’ Cohort. Int. J. Environ. Res. Public Health 2021, 18, 11781. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Smith, G.D.; Mehta, N.; Clarke, T.-K.; Lohoff, F.W. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PLoS Med. 2020, 17, e1003410. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Sahlin, E.; Savage, G.; Lister, C. Investigation of the antioxidant properties of tomatoes after processing. J. Food Compos. Anal. 2004, 17, 635–647. [Google Scholar] [CrossRef]
- Han, G.-M.; Liu, P. Higher serum lycopene is associated with reduced prevalence of hypertension in overweight or obese adults. Eur. J. Integr. Med. 2017, 13, 34–40. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow(®): The first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar] [CrossRef]
- Uddin, M.; Biswas, D.; Ghosh, A.; O’Kennedy, N.; Duttaroy, A.K. Consumption of Fruitflow(®) lowers blood pressure in pre-hypertensive males: A randomised, placebo controlled, double blind, cross-over study. Int. J. Food Sci. Nutr. 2018, 69, 494–502. [Google Scholar] [CrossRef]
- Rattanavipanon, W.; Nithiphongwarakul, C.; Sirisuwansith, P.; Chaiyasothi, T.; Thakkinstian, A.; Nathisuwan, S.; Pathomwichaiwat, T. Effect of tomato, lycopene and related products on blood pressure: A systematic review and network meta-analysis. Phytomedicine 2021, 88, 153512. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, L.; Zeng, G.; Zhu, H.; Sun, J.; Gao, P.; Yuan, J.; Lan, X.; Li, S.; Zhao, Y.; et al. Effects of Cuisine-Based Chinese Heart-Healthy Diet in Lowering Blood Pressure Among Adults in China: Multicenter, Single-Blind, Randomized, Parallel Controlled Feeding Trial. Circulation 2022, 146, 303–315. [Google Scholar] [CrossRef]
- Yao, M.; McCrory, M.A.; Ma, G.; Tucker, K.L.; Gao, S.; Fuss, P.; Roberts, S.B. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am. J. Clin. Nutr. 2003, 77, 1409–1416. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef]
- Xue, H.; Yang, M.; Liu, Y.; Duan, R.; Cheng, G.; Zhang, X. Relative validity of a 2-day 24-hour dietary recall compared with a 2-day weighed dietary record among adults in South China. Nutr. Diet. 2017, 74, 298–307. [Google Scholar] [CrossRef]
| Characteristics | Tomato Intake (g/day) | p | ||||
|---|---|---|---|---|---|---|
| Total | Group 1 | Group 2 | Group 3 | Group 4 | ||
| Participants, n | 11,460 | 7075 | 1382 | 1490 | 1513 | |
| Age, years | 41.7 (13.9) | 42.2 (14.3) | 39.9 (12.4) | 40.4 (13.2) | 42.1 (13.7) | <0.001 |
| Man, (n %) | 5234 (45.7) | 3281 (46.4) | 587 (42.5) | 659 (44.2) | 707 (46.7) | 0.029 |
| BMI, kg/m2 | 22.5 (3.2) | 22.4 (3.2) | 22.1 (2.8) | 22.6 (3.2) | 23.0 (3.5) | <0.001 |
| SBP, mmHg | 114.1 (11.5) | 114.3 (11.6) | 111.8 (11.0) | 113.1 (11.3) | 116.1 (11.0) | <0.001 |
| DBP, mmHg | 74.2 (7.8) | 74.2 (7.9) | 73.1 (8.0) | 73.9 (7.9) | 75.7 (7.2) | <0.001 |
| Married, (n %) | 9571 (88.8) | 5837 (88.1) | 1203 (91.5) | 1258 (89.4) | 1273 (89.0) | 0.004 |
| Education level, (n %) | <0.001 | |||||
| Middle school or below | 7933 (70.6) | 5269 (76.1) | 945 (70.1) | 899 (61.4) | 820 (54.8) | |
| High school | 2458 (21.9) | 1277 (18.4) | 337 (25.0) | 414 (28.3) | 430 (28.7) | |
| College or above | 842 (7.5) | 379 (5.5) | 67 (5.0%) | 150 (10.3) | 246 (16.4) | |
| Former or current smoker (n %) | 3502 (100.0) | 2207 (100.0) | 431 (100.0) | 432 (100.0) | 432 (100.0) | 0.103 |
| Alcohol consumer (n %) | 3932 (34.7) | 2371 (34.0) | 459 (33.6) | 533 (36.0) | 569 (37.7) | 0.023 |
| Urban residence (n %) | 4410 (38.5) | 2184 (30.9) | 575 (41.6) | 726 (48.7) | 925 (61.1) | <0.001 |
| Physical activity METs-h/week, median (IQR) | 88.9 (18.0;206.5) | 94.0 (16.9;216.0) | 99.4 (28.2;223.4) | 81.7 (19.9;186.2) | 78.3 (16.6;164.4) | <0.001 |
| Energy, kcal/day | 2143.8 (564.3) | 2165.6 (593.9) | 2124.3 (456.5) | 2091.5 (496.2) | 2111.2 (569.3) | <0.001 |
| Total carbohydrate, % of energy | 56.8 (11.3) | 58.0 (11.6) | 56.4 (9.1) | 54.8 (10.0) | 53.5 (11.8) | <0.001 |
| Total fat, % of energy | 29.8 (10.5) | 28.7 (10.8) | 30.2 (8.5) | 31.7 (9.4) | 32.3 (10.9) | <0.001 |
| Total protein, % of energy | 12.7 (2.7) | 12.5 (2.7) | 12.7 (2.3) | 13.0 (2.6) | 13.6 (3.2) | <0.001 |
| Vegetable intake, g/day | 301.1 (136.3) | 296.6 (143.4) | 293.9 (109.3) | 285.0 (106.7) | 344.5 (142.1) | <0.001 |
| Fruit intake, g/day | 43.1 (87.6) | 32.4 (76.4) | 40.5 (66.2) | 57.7 (87.8) | 81.3 (130.6) | <0.001 |
| Tomato Intake, g/day | No. of Cases/Person-Years | Crude Model | Adjusted Model 1 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | ptrend | HR (95% CI) | p | ptrend | ||
| Four categories | |||||||
| Group 1 | 2797/51,308 | Ref | Ref | ||||
| Group 2 | 381/16,980 | 0.39 (0.35, 0.44) | <0.001 | <0.001 | 0.42 (0.37, 0.47) | <0.001 | <0.001 |
| Group 3 | 407/14,384 | 0.51 (0.46, 0.56) | <0.001 | 0.51 (0.46, 0.57) | <0.001 | ||
| Group 4 | 430/9663 | 0.82 (0.74, 0.91) | <0.001 | 0.82 (0.74, 0.92) | <0.001 | ||
| Two categories | |||||||
| Non-consumers | 2700/51,212 | Ref | Ref | ||||
| Consumers | 1152/40,961 | 0.52 (0.48, 0.55) | <0.001 | 0.53 (0.49, 0.57) | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Tian, Z.; Liang, Y.; Chen, H.; Fan, Z.; Liu, Z.; Dai, S.; Liu, M.; Kuang, H.; Yang, Y. J-Shaped Association of Tomato Intake with New-Onset Hypertension in General Adults: A Nationwide Prospective Cohort Study. Nutrients 2022, 14, 4813. https://doi.org/10.3390/nu14224813
Zhao D, Tian Z, Liang Y, Chen H, Fan Z, Liu Z, Dai S, Liu M, Kuang H, Yang Y. J-Shaped Association of Tomato Intake with New-Onset Hypertension in General Adults: A Nationwide Prospective Cohort Study. Nutrients. 2022; 14(22):4813. https://doi.org/10.3390/nu14224813
Chicago/Turabian StyleZhao, Dan, Zezhong Tian, Ying Liang, Hong Chen, Zhiying Fan, Zhihao Liu, Suming Dai, Meitong Liu, Huiying Kuang, and Yan Yang. 2022. "J-Shaped Association of Tomato Intake with New-Onset Hypertension in General Adults: A Nationwide Prospective Cohort Study" Nutrients 14, no. 22: 4813. https://doi.org/10.3390/nu14224813
APA StyleZhao, D., Tian, Z., Liang, Y., Chen, H., Fan, Z., Liu, Z., Dai, S., Liu, M., Kuang, H., & Yang, Y. (2022). J-Shaped Association of Tomato Intake with New-Onset Hypertension in General Adults: A Nationwide Prospective Cohort Study. Nutrients, 14(22), 4813. https://doi.org/10.3390/nu14224813






