Avicularin Attenuates Lead-Induced Impairment of Hepatic Glucose Metabolism by Inhibiting the ER Stress-Mediated Inflammatory Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Ethics
2.3. Experimental Design
2.4. Biochemical Analysis
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
3.1. Avi Rescues Pb-Induced Liver Dysfunction
3.2. Effects of Avi on Pb-Induced Insulin Resistance
3.3. Effects of Avi on the Abnormal Activities of Pb-Induced Glucose Metabolism in the Liver
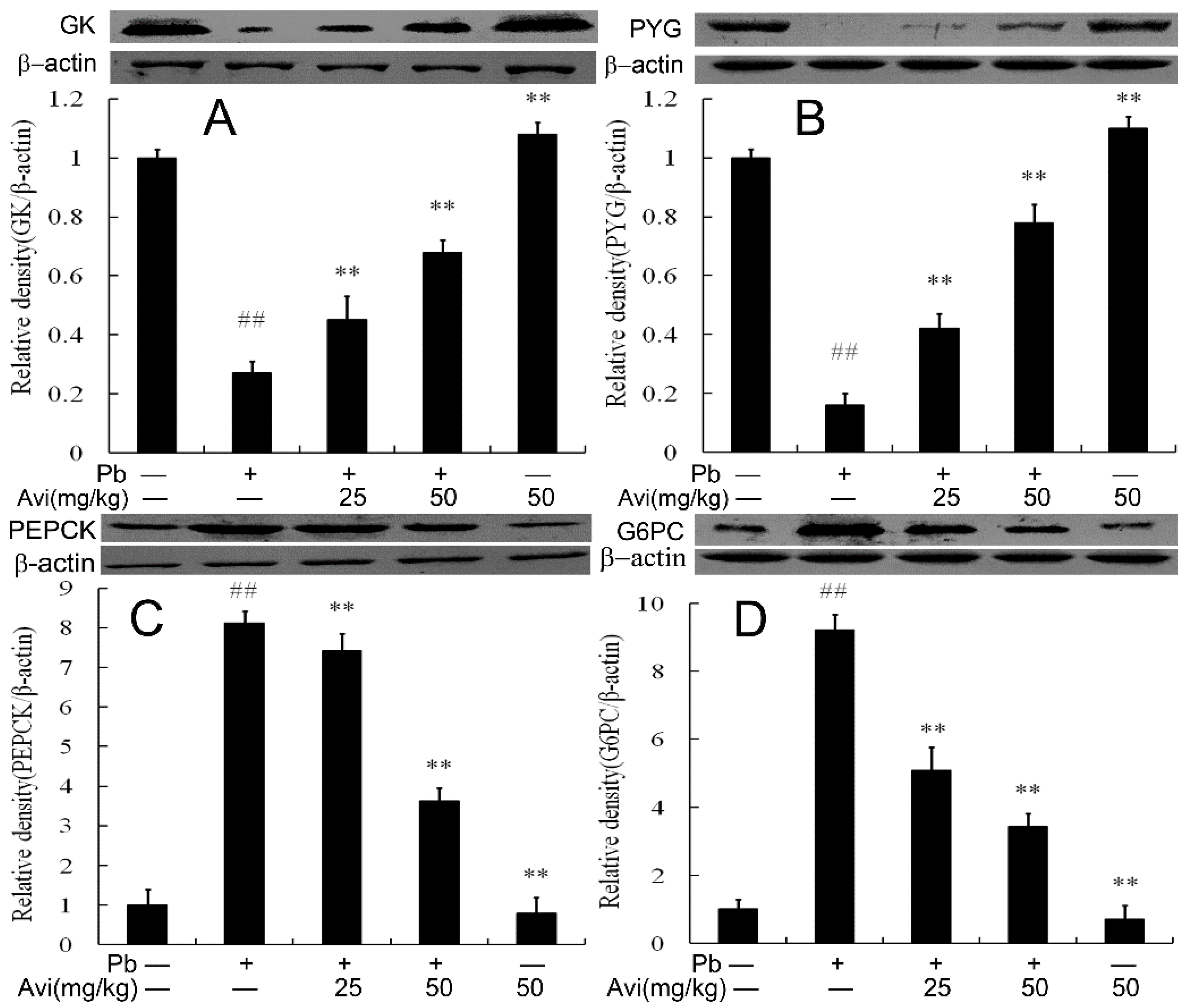
3.4. Avi Regulated the Expression Levels of Glucose Metabolism Enzymes in the Liver
3.5. Avi Suppressed Hepatic Inflammation
3.6. Avi Suppresses the ERS Pathway in the Liver
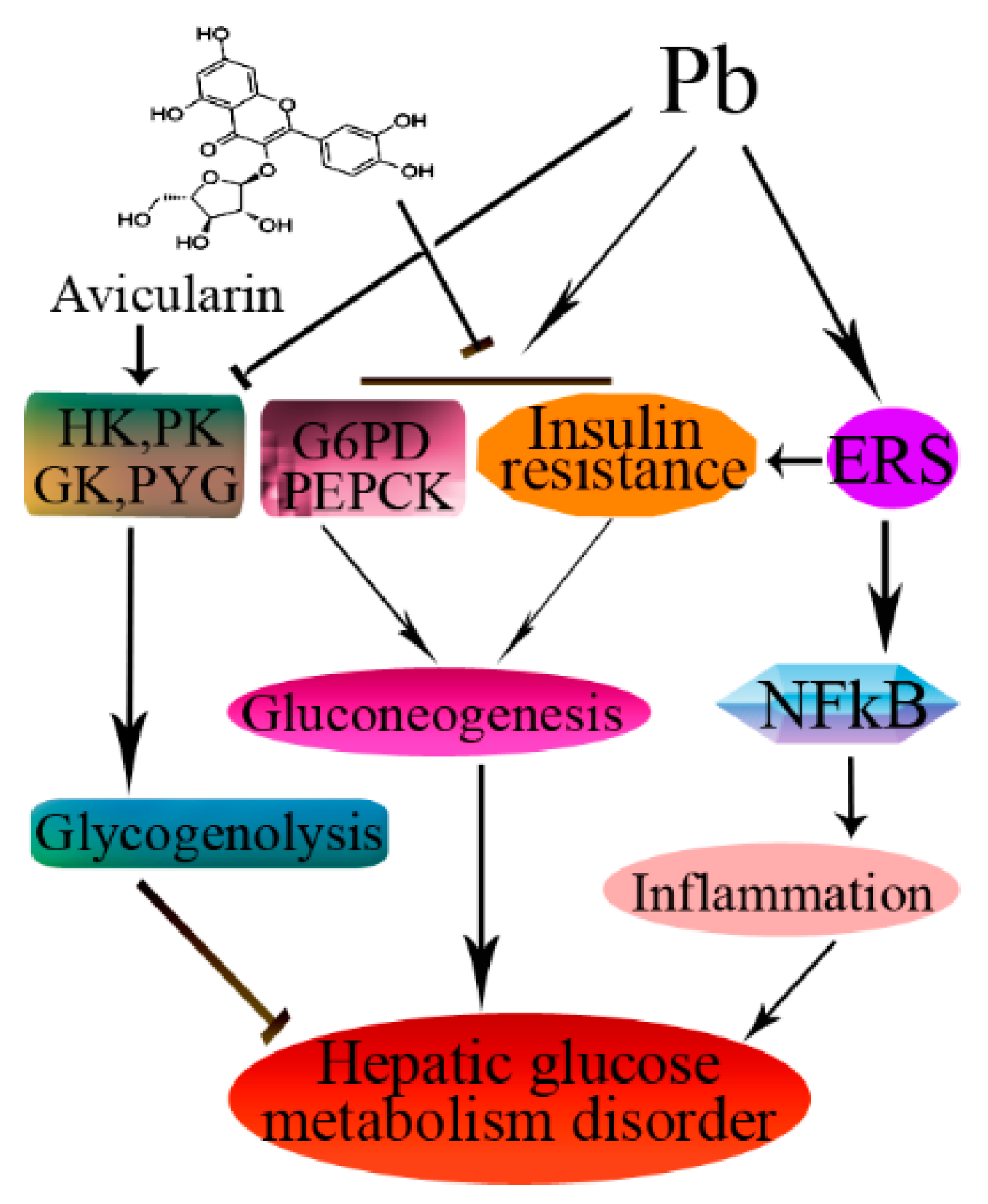
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviation
References
- White, D.; Cory-Slechta, A.; Gilbert, E.; Tiffany-Castiglioni, E.; Zawia, H.; Virgolini, M.; Rossi-George, A.; Lasley, M.; Qian, C.; Basha, R. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 2007, 225, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.B.; Hafida, S.; Stemmer, P.; Adhami, A.; Leff, T. Lead (Pb) exposure promotes diabetes in obese rodents. J. Trace Elem. Med. Biol. 2017, 39, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Baeeri, M.; Bahadar, H.; Soltany-Rezaee-Rad, M.; Gholami, M.; Abdollahi, M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ. Toxicol. Pharmacol. 2015, 39, 16–26. [Google Scholar] [CrossRef]
- Alya, A.; Ines, D.B.; Montassar, L.; Najoua, G.; Saloua, E.F. Oxidative stress, biochemical alterations, and hyperlipidemia in female rats induced by lead chronic toxicity during puberty and post puberty periods. Iran. J. Basic Med. Sci. 2015, 18, 1034–1043. [Google Scholar] [PubMed]
- Liu, C.M.; Yang, H.X.; Ma, J.Q.; Yang, W.; Feng, Z.J.; Sun, J.; Cheng, C.; Li, J.; Jiang, H. Role of AMPK pathway in lead-induced endoplasmic reticulum stress in kidney and in paeonol-induced protection in mice. Food Chem. Toxicol. 2018, 122, 87–94. [Google Scholar] [CrossRef]
- Asgary, S.; Movahedian, A.; Keshvari, M.; Taleghani, M.; Sahebkar, A.; Sarrafzadegan, N. Serum levels of lead, mercury and cadmium in relation to coronary artery disease in the elderly: A cross-sectional study. Chemosphere 2017, 180, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Gąssowska, M.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Chibowska, K.; Goschorska, M.; Lubkowska, A.; Chlubek, D. Glycogen metabolism in brain and neurons-astrocytes metabolic cooperation can be altered by pre- and neonatal lead (Pb) exposure. Toxicology 2017, 390, 146–158. [Google Scholar] [CrossRef]
- Yun, S.; Wu, Y.; Niu, R.; Feng, C.; Wang, J. Effects of lead exposure on brain glucose metabolism and insulin signaling pathway in the hippocampus of rats. Toxicol. Lett. 2019, 310, 23–30. [Google Scholar] [CrossRef]
- Xia, J.; Lu, L.; Jin, C.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp. Biochem. Physiol. Part C 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Wan, H.; Wang, B.; Cui, Y.; Wang, Y.; Zhang, K.; Chen, C.; Xia, F.; Ye, L.; Wang, L.; Wang, N.; et al. Low-level lead exposure promotes hepatic gluconeogenesis and contributes to the elevation of fasting glucose level. Chemosphere 2021, 276, 130111. [Google Scholar] [CrossRef]
- Liu, C.M.; Ma, J.Q.; Sun, J.M.; Feng, Z.J.; Cheng, C.; Yang, W.; Jiang, H. Association of changes in ER stress-mediated signaling pathway with lead-induced insulin resistance and apoptosis in rats and their prevention by A-type dimeric epigallocatechin-3-gallate. Food Chem. Toxicol. 2017, 110, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Shen, M.L.; Zhou, J.J.; Wang, X.Y.; Xia, J.Z.; Fu, Z.W.; Jin, Y.X. Chronic exposure to low doses of Pb induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels of mice. Environ. Toxicol. 2019, 34, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qiu, Z.; Ouyang, W.; Miao, J.; Xiong, P.; Mao, D.; Feng, K.; Li, M.; Luo, M.; Xiao, H.; et al. Hepatic transcriptome and proteome analyses provide new insights into the regulator mechanism of dietary avicularin in diabetic mice. Food Res. Int. 2019, 125, 108570. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, Y.; Dong, X.; Xu, W.; Ma, Y. Network pharmacology and reverse molecular docking-based prediction of the molecular targets and pathways for avicularin against cancer. Comb. Chem. High Throughput Screen. 2019, 22, 4–12. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K. Therapeutic importance of avicularin for the treatment of liver inflammatory disorders. Metabolism 2021, 116, 154549. [Google Scholar] [CrossRef]
- Amadi, J.A.; Amadi, P.U.; Njoku, U.C.; Onitchi, C.L. Lettuce-avicularin treatment reverses insulin resistance through stimulation of glycolytic kinases and insulin signaling molecules. Iran. J. Basic Med. Sci. 2021, 24, 232–239. [Google Scholar]
- Patel, D.K. Medicinal importance of avicularin as potential anti-inflammatory agents for the treatment of liver disorders: Therapeutic assessment and biological importance in the medicine. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, S296. [Google Scholar] [CrossRef]
- Samant, N.P.; Gupta, G.L. Avicularin attenuates memory impairment in rats with amyloid beta-induced Alzheimer’s disease. Neurotox. Res. 2022, 40, 140–153. [Google Scholar] [CrossRef]
- Beier, E.E.; Inzana, J.A.; Sheu, T.J.; Shu, L.; Puzas, J.E.; Mooney, R.A. Effects of combined exposure to lead and high-fat diet on bone quality in juvenile male mice. Environ. Health Perspect. 2015, 123, 935–943. [Google Scholar] [CrossRef]
- Hayes, J.M.; Kantsadi, A.L.; Leonidas, D.D. Natural products and their derivatives as inhibitors of glycogen phosphorylase: Potential treatment for type 2 diabetes. Phytochem. Rev. 2014, 13, 471–498. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Liu, B.; Zou, Y.; Sun, M.; Li, Z.; Yang, R.; Xu, X.; Zou, L.; Li, G.; et al. P2Y12 shRNA normalizes inflammatory dysfunctional hepatic glucokinase activity in type 2 diabetic rats. Biomed. Pharmacother. 2020, 132, 110803. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, W.; Oldham, W.M.; Priolo, C.; Pandey, A.K.; Loscalzo, J. Immunometabolic endothelial phenotype: Integrating inflammation and glucose metabolism. Circ. Res. 2021, 126, 9–29. [Google Scholar]
- Shen, Z.; Xu, Y.; Jiang, X.; Wang, Z.; Guo, Y.; Pan, W.; Hou, J. Avicularin relieves depressive-like behaviors induced by chronic unpredictable mild stress in mice. Med. Sci. Monit. 2019, 25, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Sun, M.; Yin, W.; Yang, L.; Kong, L. Avicularin suppresses cartilage extracellular matrix degradation and inflammation via TRAF6/MAPK activation. Phytomedicine 2021, 91, 153657. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, H.; Zheng, M.; Liu, X.; Yu, J. Protective effect of avicularin on rheumatoid arthritis and its associated mechanisms. Exp. Ther. Med. 2018, 16, 5343–5349. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Du, L. Avicularin reduces the expression of mediators of inflammation and oxidative stress in bradykinin-treated MG-63 human osteoblastic osteosarcoma cells. Med. Sci. Monit. 2020, 26, e921957-1. [Google Scholar] [CrossRef]
- Xiong, W.; Fei, M.; Wu, C.; Wang, W.; Luo, R.; Shen, L.; Zhang, Z. Atorvastatin inhibits endoplasmic reticulum stress through AMPK signaling pathway in atherosclerosis in mice. Exp. Ther. Med. 2020, 19, 2266–2272. [Google Scholar] [CrossRef]
- Ji, L.; Gu, H. The anti-obesity effects of rhein on improving insulin resistance (IR) and blood lipid levels are involved in endoplasmic reticulum stress (ERs), inflammation, and oxidative stress in vivo and vitro. Bioengineered 2021, 12, 5797–5813. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Q.; Cheng, B.; Xin, Y.; Wang, J.; Li, J.; Li, C.; Yang, N. Ghrelin Alleviates endoplasmic reticulum stress in MC3T3E1 cells by inhibiting AMPK phosphorylation. Oxid. Med. Cell. Longev. 2021, 2021, 9940355. [Google Scholar] [CrossRef]
- Bobrovnikova-Marjon, E.; Hatzivassiliou, G.; Grigoriadou, C.; Romero, M.; Cavener, D.R.; Thompson, C.B.; Diehl, J.A. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 16314–16319. [Google Scholar] [CrossRef]
- Fu, S.N.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Z.; Hu, Y.; Lu, Y.; Li, D.; Liu, J.; Liao, S.; Hu, M.; Wang, Y.; Zhang, D.; et al. Sustained ER stress promotes hyperglycemia by increasing glucagon action through the deubiquitinating enzyme USP14. Proc. Natl. Acad. Sci. USA 2019, 116, 21732–21738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vera, L.; Fischer, W.H.; Montminy, M. The CREB Coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 2009, 460, 534–537. [Google Scholar] [CrossRef] [PubMed]



| ALT (U/L) | AST (U/L) | FBG (mM/L) | IS (mM/L) | HOMA-IR | |
|---|---|---|---|---|---|
| Control | 25.21 ± 1.07 | 37.42 ± 2.51 | 3.64 ± 0.04 | 0.66 ± 0.02 | 0.11 ± 0.01 |
| Pb | 86.62 ± 1.76 # | 98.42 ± 4.50 # | 4.75 ± 0.08 # | 1.67 ± 0.04 # | 0.35 ± 0.01 # |
| Pb + Avi (25mg/kg) | 73.10 ± 1.72 * | 79.02 ± 2.17 * | 4.02 ± 0.07 * | 1.31 ± 0.03 * | 0.24 ± 0.01 * |
| Pb + Avi (50mg/kg) | 57.09 ± 1.67 * | 71.56 ± 2.87 * | 3.69 ± 0.04 * | 1.08 ± 0.02 * | 0.18 ± 0.01 * |
| Avi (50mg/kg) | 43.04 ± 1.75 * | 42.94 ± 4.16 * | 3.63 ± 0.03 * | 0.66 ± 0.03 * | 0.11 ± 0.02 * |
| GK (U/g.prot) | HK (U/g.prot) | PK (U/g.prot) | PEPCK (U/g.prot) | D6PD (U/g.prot) | |
|---|---|---|---|---|---|
| Control | 1.81 ± 0.04 | 231.21 ± 11.74 | 170.85 ± 2.37 | 0.53 ± 0.04 | 0.59 ± 0.04 |
| Pb | 0.62 ± 0.01 # | 131.41 ± 13.23 # | 62.28 ± 4.96 # | 1.04 ± 0.02 # | 1.68 ± 0.06 # |
| Pb + Avi (25mg/kg) | 0.88 ± 0.03 * | 168.11 ± 5.94 * | 119.84 ± 8.53 * | 0.84 ± 0.02 * | 1.31 ± 0.03 * |
| Pb + Avi (50mg/kg) | 1.06 ± 0.02 * | 188.22 ± 9.31 * | 140.80 ± 5.76 * | 0.72 ± 0.03 * | 1.06 ± 0.05 * |
| Avi (50mg/kg) | 1.82 ± 0.02 * | 206.12 ± 5.94 * | 163.93 ± 13.60 * | 0.56 ± 0.02 * | 0.73 ± 0.03 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Shi, J.-X.; Cheng, C.; Jiang, H.; Ruan, H.-N.; Li, J.; Liu, C.-M. Avicularin Attenuates Lead-Induced Impairment of Hepatic Glucose Metabolism by Inhibiting the ER Stress-Mediated Inflammatory Pathway. Nutrients 2022, 14, 4806. https://doi.org/10.3390/nu14224806
Qiu T, Shi J-X, Cheng C, Jiang H, Ruan H-N, Li J, Liu C-M. Avicularin Attenuates Lead-Induced Impairment of Hepatic Glucose Metabolism by Inhibiting the ER Stress-Mediated Inflammatory Pathway. Nutrients. 2022; 14(22):4806. https://doi.org/10.3390/nu14224806
Chicago/Turabian StyleQiu, Ting, Jia-Xue Shi, Chao Cheng, Hong Jiang, Hai-Nan Ruan, Jun Li, and Chan-Min Liu. 2022. "Avicularin Attenuates Lead-Induced Impairment of Hepatic Glucose Metabolism by Inhibiting the ER Stress-Mediated Inflammatory Pathway" Nutrients 14, no. 22: 4806. https://doi.org/10.3390/nu14224806
APA StyleQiu, T., Shi, J.-X., Cheng, C., Jiang, H., Ruan, H.-N., Li, J., & Liu, C.-M. (2022). Avicularin Attenuates Lead-Induced Impairment of Hepatic Glucose Metabolism by Inhibiting the ER Stress-Mediated Inflammatory Pathway. Nutrients, 14(22), 4806. https://doi.org/10.3390/nu14224806






