Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Gut Microbiota Data Sampling, DNA Extraction, Sequencing, and Data Analysis
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Clinical Characteristics
3.2. Nutritional Intake and Food Group Intake
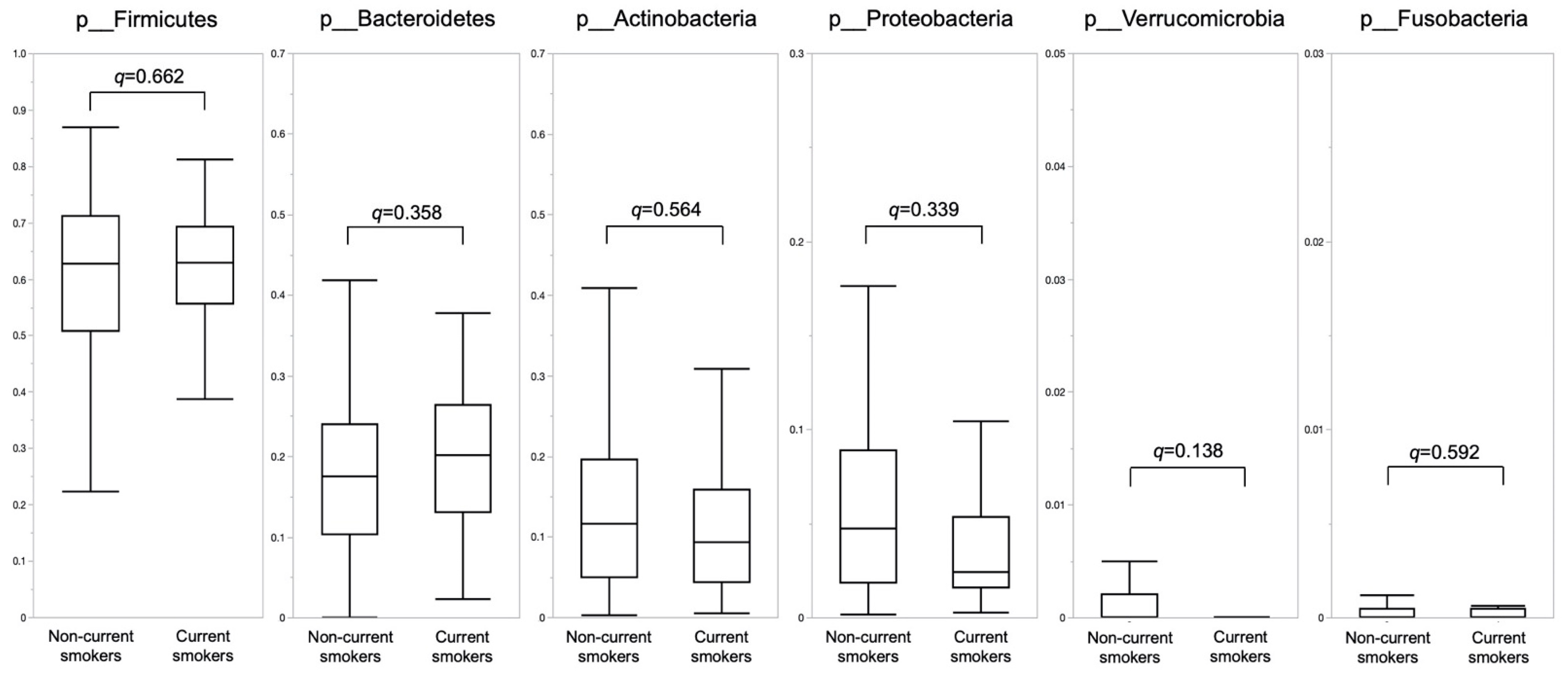
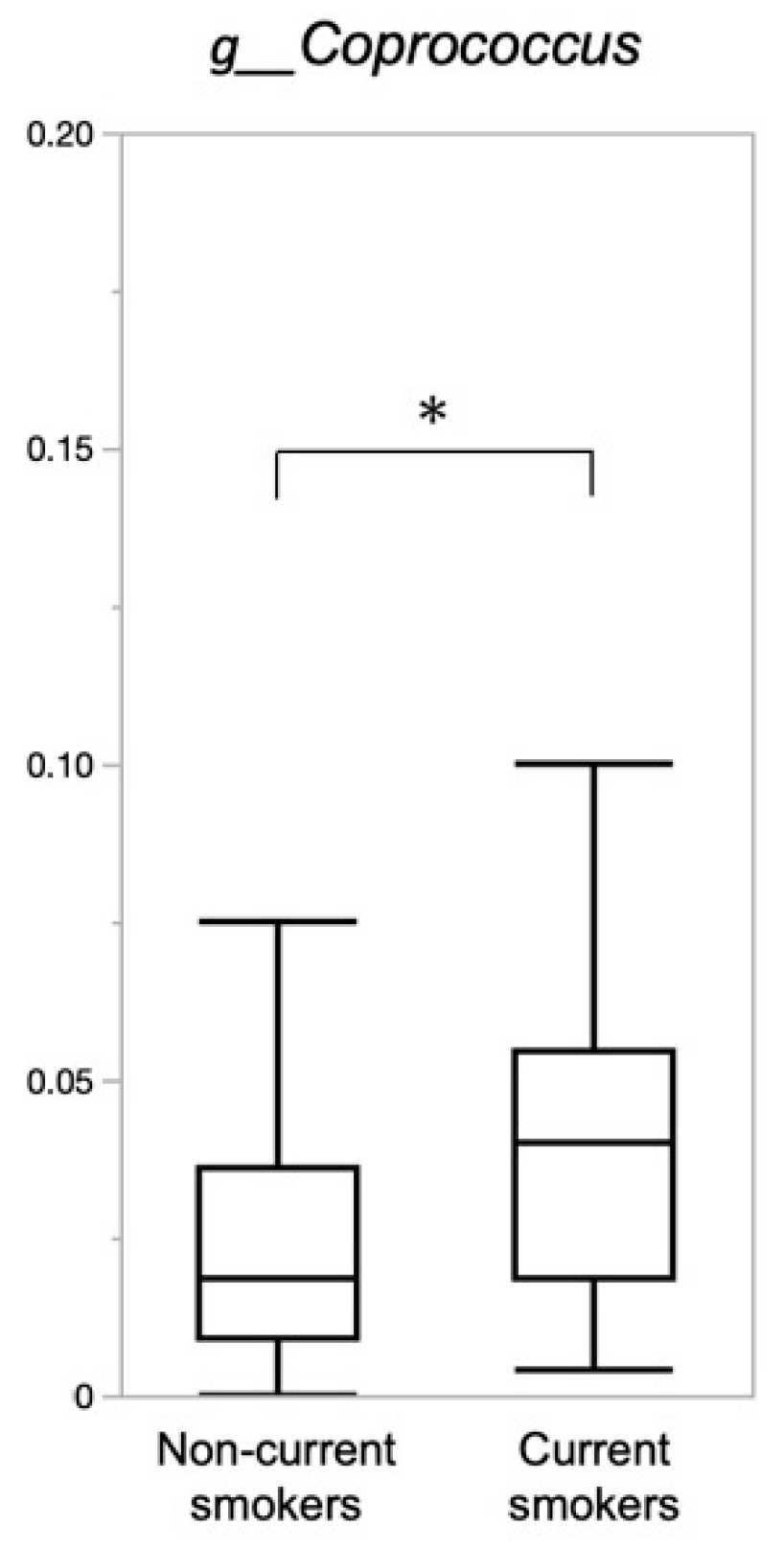
3.3. Composition of Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.A. Smoking and Type 2 Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 399–403. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Hunerdosse, D.; Kerege, A.; Playdon, M.; Samek, A.M.; Eckel, R.H. Novel and Reversible Mechanisms of Smoking-Induced Insulin Resistance in Humans. Diabetes 2012, 61, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Garbin, U.; Fratta Pasini, A.; Stranieri, C.; Cominacini, M.; Pasini, A.; Manfro, S.; Lugoboni, F.; Mozzini, C.; Guidi, G.; Faccini, G.; et al. Cigarette Smoking Blocks the Protective Expression of Nrf2/ARE Pathway in Peripheral Mononuclear Cells of Young Heavy Smokers Favouring Inflammation. PLoS ONE 2009, 4, e8225. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Schächinger, V.; Minners, J. Long-Term Cigarette Smoking Impairs Endothelium-Dependent Coronary Arterial Vasodilator Function. Circulation 1995, 92, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chaudhry, Z.; Lee, C.-C.; Bone, R.N.; Kanojia, S.; Maddatu, J.; Sohn, P.; Weaver, S.A.; Robertson, M.A.; Petrache, I.; et al. Cigarette Smoke Exposure Impairs β-Cell Function through Activation of Oxidative Stress and Ceramide Accumulation. Mol. Metab. 2020, 37, 100975. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.-J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L.; Kim, H.-N.; Lee, J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal Microbiota Dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Hu, Y.; Zong, G.; Liu, G.; Wang, M.; Rosner, B.; Pan, A.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Sun, Q. Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality. N. Engl. J. Med. 2018, 379, 623–632. [Google Scholar] [CrossRef]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking Cessation Induces Profound Changes in the Composition of the Intestinal Microbiota in Humans. PLoS ONE 2013, 8, e59260. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking Cessation Alters Intestinal Microbiota: Insights from Quantitative Investigations on Human Fecal Samples Using FISH. Inflamm. Bowel. Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Alkerwi, A.; Baydarlioglu, B.; Sauvageot, N.; Stranges, S.; Lemmens, P.; Shivappa, N.; Hébert, J.R. Smoking Status Is Inversely Associated with Overall Diet Quality: Findings from the ORISCAV-LUX Study. Clin. Nutr. 2017, 36, 1275–1282. [Google Scholar] [CrossRef]
- Anker, J.J.; Nakajima, M.; Raatz, S.; Allen, S.; al’Absi, M. Tobacco Withdrawal Increases Junk Food Intake: The Role of the Endogenous Opioid System. Drug Alcohol. Depend. 2021, 225, 108819. [Google Scholar] [CrossRef] [PubMed]
- MacLean, R.R.; Cowan, A.; Vernarelli, J.A. More to Gain: Dietary Energy Density Is Related to Smoking Status in US Adults. BMC Public Health 2018, 18, 365. [Google Scholar] [CrossRef]
- de Leon, J.; Rendon, D.M.; Baca-Garcia, E.; Aizpuru, F.; Gonzalez-Pinto, A.; Anitua, C.; Diaz, F.J. Association between Smoking and Alcohol Use in the General Population: Stable and Unstable Odds Ratios across Two Years in Two Different Countries. Alcohol Alcohol. 2007, 42, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lohse, T.; Rohrmann, S.; Bopp, M.; Faeh, D. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PLoS ONE 2016, 11, e0148563. [Google Scholar] [CrossRef]
- Berube, L.; Duffy, V.B.; Hayes, J.E.; Hoffman, H.J.; Rawal, S. Associations between Chronic Cigarette Smoking and Taste Function: Results from the 2013-2014 National Health and Nutrition Examination Survey. Physiol. Behav. 2021, 240, 113554. [Google Scholar] [CrossRef]
- Glennon, S.-G.; Huedo-Medina, T.; Rawal, S.; Hoffman, H.J.; Litt, M.D.; Duffy, V.B. Chronic Cigarette Smoking Associates Directly and Indirectly with Self-Reported Olfactory Alterations: Analysis of the 2011-2014 National Health and Nutrition Examination Survey. Nicotine Tob. Res. 2019, 21, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.; Pagoto, S.; McChargue, D.; Hedeker, D.; Werth, J. Altered Reward Value of Carbohydrate Snacks for Female Smokers Withdrawn from Nicotine. Pharmacol. Biochem. Behav. 2003, 76, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Criscitelli, K.; Avena, N.M. The Neurobiological and Behavioral Overlaps of Nicotine and Food Addiction. Prev. Med. 2016, 92, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Anastasiou, C.A.; Zachari, K.; Sidiropoulou, M.; Katsaounou, P.; Tenta, R. Acute Effect of Smoking and Smoking Abstinence on Energy Intake and Appetite-Related Hormones Blood Concentrations. Physiol. Behav. 2018, 184, 78–82. [Google Scholar] [CrossRef]
- Kroemer, N.B.; Wuttig, F.; Bidlingmaier, M.; Zimmermann, U.S.; Smolka, M.N. Nicotine Enhances Modulation of Food-Cue Reactivity by Leptin and Ghrelin in the Ventromedial Prefrontal Cortex. Addiction Biol. 2015, 20, 832–844. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Osaka, T.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Intake of Sucrose Affects Gut Dysbiosis in Patients with Type 2 Diabetes. J. Diabetes Investig. 2020, 11, 1623–1634. [Google Scholar] [CrossRef]
- Kondo, Y.; Hashimoto, Y.; Hamaguchi, M.; Ando, S.; Kaji, A.; Sakai, R.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; et al. Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 3816. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Takahashi, F.; Kawano, R.; Hamaguchi, M.; Fukui, M. Effect of Exercise Habit on Skeletal Muscle Mass Varies with Protein Intake in Elderly Patients with Type 2 Diabetes: A Retrospective Cohort Study. Nutrients 2020, 12, 3220. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Okubo, H.; Hosoi, Y.; Horiguchi, H.; Oguma, E.; Kayama, F. Dietary Glycemic Index and Load in Relation to Metabolic Risk Factors in Japanese Female Farmers with Traditional Dietary Habits. Am. J. Clin. Nutr. 2006, 83, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, H.J.M.; Brodsky, J.B.; Bernstein, D.P. Estimating Ideal Body Weight--a New Formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef]
- Kaji, A.; Hashimoto, Y.; Sakai, R.; Okada, H.; Hamaguchi, M.; Ushigome, E.; Majima, S.; Yamazaki, M.; Fukui, M. Frequent Usage of Convenience Stores Is Associated with Low Diet Quality. Nutrients 2019, 11, 1212. [Google Scholar] [CrossRef]
- Inoue, R.; Ohue-Kitano, R.; Tsukahara, T.; Tanaka, M.; Masuda, S.; Inoue, T.; Yamakage, H.; Kusakabe, T.; Hasegawa, K.; Shimatsu, A.; et al. Prediction of Functional Profiles of Gut Microbiota from 16S RRNA Metagenomic Data Provides a More Robust Evaluation of Gut Dysbiosis Occurring in Japanese Type 2 Diabetic Patients. J. Clin. Biochem. Nutr. 2017, 61, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Kamada, K.; Ishikawa, T.; Inoue, R.; Okuda, K.; Tsujimoto, Y.; et al. Changes in the Gut Microbiota Are Associated with Hypertension, Hyperlipidemia, and Type 2 Diabetes Mellitus in Japanese Subjects. Nutrients 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, e00021-18. [Google Scholar] [CrossRef]
- Hu, X.; Fan, Y.; Li, H.; Zhou, R.; Zhao, X.; Sun, Y.; Zhang, S. Impacts of Cigarette Smoking Status on Metabolomic and Gut Microbiota Profile in Male Patients With Coronary Artery Disease: A Multi-Omics Study. Front. Cardiovasc. Med. 2021, 8, 766739. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ma, Z.; Jiao, M.; Wang, Y.; Li, A.; Ding, S. Effects of Smoking on Inflammatory Markers in a Healthy Population as Analyzed via the Gut Microbiota. Front. Cell Infect. Microbiol. 2021, 11, 633242. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with Active Crohn’s Disease Have a Clinically Relevant Dysbiosis of the Gastrointestinal Microbiota. Inflamm. Bowel. Dis. 2012, 18, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Opstelten, J.L.; Plassais, J.; van Mil, S.W.C.; Achouri, E.; Pichaud, M.; Siersema, P.D.; Oldenburg, B.; Cervino, A.C.L. Gut Microbial Diversity Is Reduced in Smokers with Crohn’s Disease. Inflamm. Bowel. Dis. 2016, 22, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Harlan, L.C.; Mattson, M.E. Food and Nutrient Intake Differences between Smokers and Non-Smokers in the US. Am. J. Public Health 1990, 80, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Marangon, K.; Herbeth, B.; Lecomte, E.; Paul-Dauphin, A.; Grolier, P.; Chancerelle, Y.; Artur, Y.; Siest, G. Diet, Antioxidant Status, and Smoking Habits in French Men. Am. J. Clin. Nutr. 1998, 67, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Chéruel, F.; Jarlier, M.; Sancho-Garnier, H. Effect of Cigarette Smoke on Gustatory Sensitivity, Evaluation of the Deficit and of the Recovery Time-Course after Smoking Cessation. Tob. Induc. Dis. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.-J.; Farley, A.; Lycett, D.; Lahmek, P.; Aveyard, P. Weight Gain in Smokers after Quitting Cigarettes: Meta-Analysis. BMJ 2012, 345, e4439. [Google Scholar] [CrossRef]
- Sun, S.; Araki, Y.; Hanzawa, F.; Umeki, M.; Kojima, T.; Nishimura, N.; Ikeda, S.; Mochizuki, S.; Oda, H. High Sucrose Diet-Induced Dysbiosis of Gut Microbiota Promotes Fatty Liver and Hyperlipidemia in Rats. J. Nutr. Biochem. 2021, 93, 108621. [Google Scholar] [CrossRef]
- Antinozzi, M.; Giffi, M.; Sini, N.; Gallè, F.; Valeriani, F.; de Vito, C.; Liguori, G.; Romano Spica, V.; Cattaruzza, M.S. Cigarette Smoking and Human Gut Microbiota in Healthy Adults: A Systematic Review. Biomedicines 2022, 10, 510. [Google Scholar] [CrossRef]
- Stewart, C.J.; Auchtung, T.A.; Ajami, N.J.; Velasquez, K.; Smith, D.P.; de La Garza, R.; Salas, R.; Petrosino, J.F. Effects of Tobacco Smoke and Electronic Cigarette Vapor Exposure on the Oral and Gut Microbiota in Humans: A Pilot Study. PeerJ 2018, 6, e4693. [Google Scholar] [CrossRef]
- Curtis, K.; Stewart, C.J.; Robinson, M.; Molfese, D.L.; Gosnell, S.N.; Kosten, T.R.; Petrosino, J.F.; de La Garza, R.; Salas, R. Insular Resting State Functional Connectivity Is Associated with Gut Microbiota Diversity. Eur. J. Neurosci. 2019, 50, 2446–2452. [Google Scholar] [CrossRef]
- Prakash, A.; Peters, B.A.; Cobbs, E.; Beggs, D.; Choi, H.; Li, H.; Hayes, R.B.; Ahn, J. Tobacco Smoking and the Fecal Microbiome in a Large, Multi-Ethnic Cohort. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Sugimoto, M.; Inoue, R.; Ohno, M.; Ban, H.; Nishida, A.; Inatomi, O.; Takahashi, S.; Naito, Y.; Andoh, A. Influence of Potassium-Competitive Acid Blocker on the Gut Microbiome of Helicobacter Pylori-Negative Healthy Individuals. Gut 2017, 66, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Biswas, K.; Roy, S.; Banerjee, R.K.; Bandyopadhyay, U. Smoking and the Pathogenesis of Gastroduodenal Ulcer--Recent Mechanistic Update. Mol. Cell Biochem. 2003, 253, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and Microbiome in Oral, Airway, Gut and Some Systemic Diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, Z.; Li, M.D. Effect of Cigarette Smoke on Gut Microbiota: State of Knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-Analysis of Alcohol Induced Gut Dysbiosis and the Resulting Behavioral Impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Kjølbæk, L.; Brahe, L.K.; Astrup, A.; Larsen, L.H.; Sanz, Y. Impact of Dietary Fiber and Fat on Gut Microbiota Re-Modeling and Metabolic Health. Trends Food Sci. Technol. 2016, 57, 201–212. [Google Scholar] [CrossRef]
- Tomoda, K.; Kubo, K.; Asahara, T.; Nomoto, K.; Nishii, Y.; Yamamoto, Y.; Yoshikawa, M.; Kimura, H. Suppressed Anti-Oxidant Capacity Due to a Cellulose-Free Diet Declines Further by Cigarette Smoke in Mice. J. Toxicol. Sci. 2012, 37, 575–585. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Kawasaki, Y.; Hu, H.; Kuwahara, K.; Yamamoto, M.; Uehara, A.; Honda, T.; Yamamoto, S.; Nakagawa, T.; Miyamoto, T.; et al. Smoking Cessation, Weight Gain, and the Trajectory of Estimated Risk of Coronary Heart Disease: 8-Year Follow-up From a Prospective Cohort Study. Nicotine Tob. Res. 2021, 23, 85–91. [Google Scholar] [CrossRef]
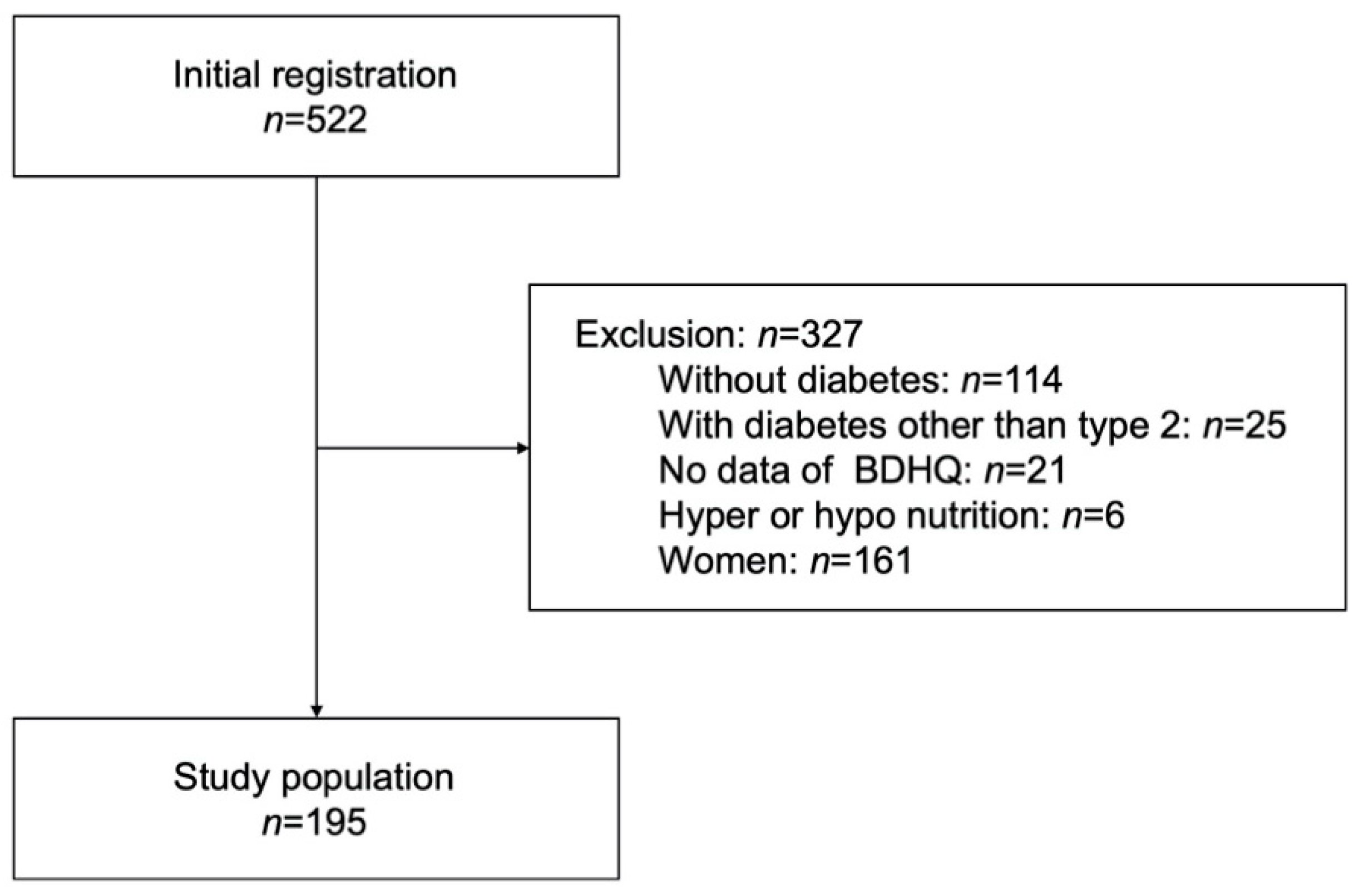
| Non-Current Smokers n = 164 | Current Smokers n = 31 | p Value | |
|---|---|---|---|
| Age (years) | 67.8 (10.9) | 63.9 (9.5) | 0.023 |
| Duration of diabetes (years) | 15.5 (9.7) | 12.7 (8.5) | 0.155 |
| Body mass index (kg/m2) | 23.9 (3.4) | 23.5 (3.5) | 0.652 |
| Smoking amount (cigarettes/day) | 18.2 (17.7) | 20.3 (10.4) | 0.134 |
| Smoking duration (years) | 18.9 (17.3) | 43.1 (9.6) | <0.001 |
| Exercise (−/+) | 73/91 | 20/11 | 0.041 |
| Habitual alcohol intake (−/+) | 86/78 | 11/20 | 0.083 |
| Systolic blood pressure (mmHg) | 134.3 (17.5) | 129.4 (21.0) | 0.070 |
| Diastolic blood pressure (mmHg) | 78.9 (10.5) | 80.2 (12.7) | 0.799 |
| Insulin (−/+) | 127/37 | 21/10 | 0.247 |
| Sulfonylureas (−/+) | 116/48 | 29/2 | 0.008 |
| Glinides (−/+) | 153/11 | 30/1 | 0.460 |
| Thiazolidines (−/+) | 165/9 | 31/0 | 0.182 |
| Biguanides (−/+) | 100/64 | 20/11 | 0.710 |
| Glucagon-like peptide-1 receptor agonist (−/+) | 142/22 | 26/5 | 0.688 |
| Dipeptidyl peptidase-4 inhibitors (−/+) | 65/99 | 18/13 | 0.057 |
| Sodium-glucose cotransporter inhibitors (−/+) | 135/29 | 26/5 | 0.834 |
| α-glucosidase inhibitors (−/+) | 144/20 | 28/3 | 0.690 |
| Proton pump inhibitors (−/+) | 130/34 | 24/7 | 0.817 |
| Hemoglobin A1c (mmol/mol) | 56.4 (12.4) | 59.8 (18.7) | 0.834 |
| Hemoglobin A1c (%) | 7.3 (1.1) | 7.6 (1.7) | 0.834 |
| HDL cholesterol (mmol/L) | 1.5 (0.4) | 1.5 (0.5) | 0.942 |
| Triglycerides (mmol/L) | 1.5 (0.9) | 1.7 (1.1) | 0.482 |
| Uric acid (mmol/L) | 324.9 (76.1) | 300.5 (82.0) | 0.159 |
| Creatinine (μmol/L) | 83.6 (37.2) | 80.0 (28.3) | 0.474 |
| eGFR (mL/min/1.73m2) | 68.6 (19.7) | 73.1 (22.7) | 0.372 |
| Non-Current Smokers n = 164 | Current Smokers n = 31 | p Value | |
|---|---|---|---|
| Total energy (kcal/day) | 1874.6 (539.0) | 2016.3 (645.6) | 0.191 |
| Energy (kcal/IBW/day) | 30.6 (8.9) | 32.6 (10.6) | 0.323 |
| Total fat (g/day) | 57.8 (20.2) | 58.5 (22.2) | 0.986 |
| Fat (g/IBW/day) | 0.9 (0.3) | 0.9 (0.4) | 0.873 |
| Fat per energy (%) | 27.8 (6.4) | 26.3 (5.9) | 0.255 |
| Total protein (g/day) | 75.1 (25.5) | 76.2 (31.0) | 0.972 |
| Protein (g/IBW/day) | 1.2 (0.4) | 1.2 (0.5) | 0.827 |
| Protein per energy (%) | 16.1 (3.1) | 15.1 (3.2) | 0.092 |
| Total carbohydrate (g/day) | 237.9 (80.4) | 248.1 (92.2) | 0.795 |
| Carbohydrate (g/IBW/day) | 3.9 (1.3) | 4.0 (1.5) | 0.970 |
| Carbohydrate per energy (%) | 50.9 (9.4) | 49.3 (8.3) | 0.209 |
| Dietary fiber (g/day) | 12.4 (5.0) | 12.4 (5.6) | 0.747 |
| Sucrose (g/day) | 11.8 (8.1) | 13.8 (9.0) | 0.242 |
| Salt (g/day) | 11.4 (3.4) | 11.9 (4.0) | 0.677 |
| Alcohol (g/day) | 11.4 (23.1) | 23.4 (30.0) | 0.040 |
| Vitamin A (μgRAE/day) | 774.7 (542.2) | 941.7 (945.0) | 0.525 |
| Vitamin B1 (mg/day) | 0.8 (0.3) | 0.8 (0.3) | 0.989 |
| Vitamin B2 (mg/day) | 1.4 (0.5) | 1.5 (0.6) | 0.928 |
| Vitamin B6 (mg/day) | 1.3 (0.5) | 1.4 (0.7) | 0.757 |
| Vitamin B12 (μg/day) | 11.5 (6.5) | 11.4 (7.1) | 0.635 |
| Vitamin C (mg/day) | 120.5 (60.4) | 120.6 (76.8) | 0.644 |
| Vitamin D (μg/day) | 17.4 (10.5) | 14.9 (9.4) | 0.144 |
| Vitamin E (mg/day) | 8.0 (2.9) | 7.8 (3.1) | 0.593 |
| Non-Current Smokers n = 164 | Current Smokers n = 31 | p Value | |
|---|---|---|---|
| Cereals (g/day) | 390.8 (172.5) | 419.7 (191.5) | 0.631 |
| Potatoes (g/day) | 35.3 (34.0) | 38.2 (38.4) | 0.736 |
| Sugar and sweeteners (g/day) | 4.1 (4.0) | 6.5 (6.4) | 0.034 |
| Pulses (g/day) | 64.0 (52.7) | 57.7 (38.3) | 0.816 |
| Green and yellow vegetables (g/day) | 118.7 (83.3) | 113.6 (83.4) | 0.649 |
| Other vegetables (g/day) | 181.0 (119.1) | 198.8 (139.2) | 0.670 |
| Fruits (g/day) | 124.7 (111.6) | 84.6 (81.2) | 0.044 |
| Fish and shellfish (g/day) | 93.6 (57.6) | 85.5 (53.0) | 0.457 |
| Meat (g/day) | 72.0 (45.1) | 86.8 (61.7) | 0.346 |
| Eggs (g/day) | 47.5 (30.9) | 41.8 (26.2) | 0.415 |
| Milk (g/day) | 166.0 (121.9) | 181.1 (160.4) | 0.879 |
| Fat and oil (g/day) | 11.3 (6.7) | 12.1 (5.9) | 0.267 |
| Snacks (g/day) | 40.4 (37.7) | 40.2 (41.3) | 0.765 |
| Alcoholic and non-alcoholic beverages (g/day) | 809.5 (465.0) | 835.3 (504.1) | 0.704 |
| Seasonings (g/day) | 224.9 (121.9) | 253.4 (148.0) | 0.287 |
| g__Coprococcus | ||
|---|---|---|
| Standardized Coefficient β | p Value | |
| Current smoking | 0.253 | <0.001 |
| Age (years) | −0.068 | 0.353 |
| Exercise | −0.050 | 0.495 |
| Alcohol intake (g/day) | −0.039 | 0.593 |
| Sugar and sweeteners intake (g/day) | −0.055 | 0.443 |
| Fruits intake (g/day) | 0.060 | 0.404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, Y.; Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 4800. https://doi.org/10.3390/nu14224800
Kondo Y, Hashimoto Y, Hamaguchi M, Kaji A, Sakai R, Inoue R, Kashiwagi S, Mizushima K, Uchiyama K, Takagi T, et al. Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus. Nutrients. 2022; 14(22):4800. https://doi.org/10.3390/nu14224800
Chicago/Turabian StyleKondo, Yuriko, Yoshitaka Hashimoto, Masahide Hamaguchi, Ayumi Kaji, Ryosuke Sakai, Ryo Inoue, Saori Kashiwagi, Katsura Mizushima, Kazuhiko Uchiyama, Tomohisa Takagi, and et al. 2022. "Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus" Nutrients 14, no. 22: 4800. https://doi.org/10.3390/nu14224800
APA StyleKondo, Y., Hashimoto, Y., Hamaguchi, M., Kaji, A., Sakai, R., Inoue, R., Kashiwagi, S., Mizushima, K., Uchiyama, K., Takagi, T., Naito, Y., & Fukui, M. (2022). Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus. Nutrients, 14(22), 4800. https://doi.org/10.3390/nu14224800










