Abstract
Autoimmune thyroid disease (AITD) is the most prevalent autoimmune disease all over the world and the most frequent cause of hypothyroidism in areas of iodine sufficiency. The pathogenesis of AITD is multifactorial and depends on complex interactions between genetic and environmental factors, with epigenetics being the crucial link. Iron deficiency (ID) can reduce the activities of thyroid peroxidase and 5′-deiodinase, inhibit binding of triiodothyronine to its nuclear receptor, and cause slower utilization of T3 from the serum pool. Moreover, ID can disturb the functioning of the immune system, increasing the risk of autoimmune disorders. ID can be responsible for residual symptoms that may persist in patients with AITD, even if their thyrometabolic status has been controlled. The human lifestyle in the 21st century is inevitably associated with exposure to chemical compounds, pathogens, and stress, which implies an increased risk of autoimmune disorders and thyroid dysfunction. To summarize, in our paper we discuss how iron deficiency can impair the functions of the immune system, cause epigenetic changes in human DNA, and potentiate tissue damage by chemicals acting as thyroid disruptors.
1. Introduction
Autoimmune thyroid disease (AITD), chronic autoimmune thyroiditis, also known as chronic lymphocytic and Hashimoto’s thyroiditis, was first described by Hashimoto in 1912. Hashimoto reported four patients with goiter, lymphocytic infiltration on histopathological examination, parenchymal cell atrophy, fibrosis, and eosinophilic inclusions in some follicular cells [1]. Until now, several variants of Hashimoto’s thyroiditis have been described, which are different from the original disease reported by Hashimoto [2]. Classically, the disease manifests with painless enlargement of the thyroid gland (goitrous form) with or without hypothyroidism in young or middle-aged women. The atrophic form is less common and is usually diagnosed with serological tests in patients with hypothyroidism and normal or atrophic thyroid. AITD is the most prevalent autoimmune disease all over the world and the most frequent cause of hypothyroidism in areas of iodine sufficiency [3]. AITD results in hypothyroidism in 20–30% of patients [4,5]. AITD affects 0.3–1.5/1000 subjects/year and is 4–10 times more frequent in women than in men [(3.5–5/1000 subjects/year in women versus 0.6–1.0/1000 subjects in men [6]. AITD is diagnosed based on increased serum levels of antithyroid antibodies and/or hypoechogenicity of the thyroid gland at ultrasound [4,5]. Studies indicate that anti-TPO and/or anti-Tg antibodies are found in 2–17% of women of childbearing age.
Iron is essential for proper functioning of all cells in the human body. It is involved in the transfer of oxygen and electrons and is crucial for the activity of numerous enzymes [7]. Two percent of human genes encode iron-binding proteins and 6.5% of all enzymes are directly dependent on iron [8].
Iron deficiency (ID) is among the most prevalent nutritional deficiencies, affecting as many as 2 billion people all over the world, mostly pregnant women and children [9]. Its prevalence correlates with socioeconomic status –iron deficiency is estimated to affect 4–18% of the population in the United States and Northern and Western Europe, 9–50% of the population in Eastern Europe, 64% in Asia, 54% in Southern Asia, and 62% in Latin America [10]. It is the underlying cause of anemia in 42% and 50% of anemia cases in children and women, respectively. ID is also among the five most frequent causes of disability in women from 35 countries [11].
Several chronic diseases are frequently associated with iron deficiency anemia, notably chronic kidney disease, chronic heart failure, cancer, and inflammatory bowel disease [10]. Moreover, iron deficiency anemia (IDA) is an independent risk factor for increased perioperative mortality [12].
ID becomes more prevalent in patients with a higher NYHA (New York Heart Association) class, increases the mortality and morbidity of patients with HfrEF (heart failure with reduced ejection fraction), and impairs exercise tolerance [13]. It can impair brain functions. Iron is essential for the synthesis of neurotransmitters [14], including for the activity of tryptophan hydroxylase (involved in serotonin production) and tyrosine hydroxylase (involved in norepinephrine and dopamine production). ID results in irreversible disorders of dopamine release in young rats [15]. Georgieff was the first to show that ID impairs hippocampal metabolism, dendritogenesis and development [16]. Other studies have reported that behavioral changes are associated with hippocampal dysfunction, including spatial learning disorders [17] and impaired trace conditioning [18]. ID can increase the risk of psychiatric disorders, including depression and psychotic disorders [19].
As mentioned above, iron deficiency is the most common nutritional deficiency worldwide, with particularly high prevalence in women of reproductive age. Due to high opportunities for iron deficiency prevention and easy iron supplementation, iron metabolism should be investigated in relation to AITD–as the disease can cause not only hypothyroidism but also infertility and can increase the frequency of obstetric complications in euthyroid women [20].
The aim of this paper is to discuss the effects of iron deficiency on the development of autoimmune thyroid disease based on the available evidence.
2. Material and Methods
The study presents an analysis of data in the currently available literature. We examined electronic databases, including: MEDLINE and Pubmed. The search terms we used included: “iron deficiency”; “iron deficiency anemia”; “iron and thyroid disease”; “iron and autoimmune diseases”; “iron deficiency and AITD”; “iron and obesity”; “iron and diabetes”; “iron and immunology”; “iron metabolism and environmental pollution”.
3. Etiology of AITD
3.1. AITD Is Associated with Other Autoimmune Disorders
The pathogenesis of AITD is multifactorial and depends on complex interactions between genetic and environmental factors leading to the breakdown of immune tolerance [21]. Genetic factors are estimated to contribute to the disease in 70%, while environmental factors, associated with the activation of the innate immune system, in 20–30% [22]. In numerous cases AITD appears to be the first manifestation of the antiphospholipid syndrome (APS) [23]. Studies confirm that AITD patients are more likely to suffer from Huntington’s disease, multiple sclerosis, celiac disease, diabetes, sickle-cell anemia, sarcoidosis, alopecia areata, rheumatoid arthritis, polymyalgia, articular psoriasis, scleroderma, and HCV cryoglobulinemia [24].
3.2. Impact of Genetic Factors on the Development of AITD
Genes responsible for the development of AITD are genes that encode immune regulatory factors and are involved in the complex processes of ensuring robust immune responses against appropriate foreign antigens while maintaining tolerance to self-antigens [21]. AITD may develop as a result of congenital alterations in the development of central tolerance in the thymus and Treg-dependent peripheral tolerance, which can lead to abnormal co-stimulation of T cells and precursor cells (PCs) in the immunological synapse [21].
AITD develops in 20–30% of siblings of patients with AITD, with the sibling risk ratio of 16.9. Concordance rate for AITD in monozygotic twins is estimated to be 29–55% [25]. Simmonds et al. found that AITD susceptibility is associated with genes: IL2RA, HLA, PTPN22, and CTLA4 [26,27]. Other genes that increase the risk of AITD include: FCR3, TSH-R, HLA-1, FOXE3, GDCG4p14, and RNASET2, expressed on CD4+ and CD8+ cells; 7 out of 11 AITD susceptibility genes are involved in maintaining proper functions of T cells, which speaks for their key role in the development of AITD [5]. The first reported monogenic predisposition to AITD is splice site variant in the thyroglobulin gene (TG c. 1076-1G > C) [20,28].
3.3. Impact of Viral Infections, including COVID-19, on the Development of AITD
Viruses can activate innate immunity and lead to the development of AITD. An association was confirmed between HTLV1, HSV, Rubella, Mumps, EBV, HIV, HCV, Parvovirus, and Enterovirus infections and AITD [29,30]. ID can impair and distort the body’s immune response to viral infections. Enhanced lymphocyte metabolism requires higher amounts of iron, and mice with ID presented attenuated T- and B-cell response to Adenovirus and Vaccinia virus [31].
One of the hypotheses that explain the increased incidence of autoimmune disorders in the 21st century says that excessive hygiene lowers the threshold for immune activation [32] in response to pathogens. Numerous autoimmune diseases are triggered by infection. Reducing inflammation in autoimmune diseases can restore the immune balance and induce remission. Infections may result in disruption of the immune regulation, which may cause autoantigen mimicry. Infection can also affect target organs and increase their susceptibility to autoimmune disorders. The dual role of infection means that it is both an indicator of autoimmunity and a promoter of disease progression and escalation of the autoimmune process to the autoimmune disease. Molecular mimicry, antigen expression, and tissue modifications can lead to antigen-specific signals that initiate autoimmune reactions. Other effects include induction of the pathogen-associated molecular pattern (PAMP) and Toll-like receptors involved in the progression from the autoimmune reaction to the autoimmune disease. Infection can shift the body’s normal immune response toward a pathological process that results in full-blown disease. Effective apoptosis is a mechanism that enables the body to eliminate damaged cells without inducing inflammatory state. Infection and other environmental factors can impair apoptosis and increase the risk of autoimmune process. Damaged cells that are not cleared by macrophages and monocytes remain in tissues as a potential source of autoimmune process. Impaired apoptosis can lead to autoimmune lymphoproliferative disorders associated with CD95 (fas)-fas-ligand mutation. Iron deficiency can impair ferroptosis–a type of iron-dependent cell death.
There is a connection between the effects of COVID-19 infection and thyroiditis, which involves a mechanism of direct effects of the virus on thyrocytes, with initiation of inflammation in the thyroid gland, and possible indirect effect on the hypothalamic–pituitary–thyroid axis [33]. About 80% of patients who died of COVID-19 had functional ID due to iron sequestration as well as increased IL-6 and hepcidin levels [34]. Studies have confirmed that functional ID was associated with longer duration of hospital stay, fivefold increase in the risk of admission to intensive care unit (ICU), and eightfold increase in the risk of mechanical ventilation [34]. COVID-19 virus replication relies on the host intracellular proteins dependent on iron availability [35], including RNA reductase. Therefore, immunity to COVID-19 may directly depend on intracellular iron stores.
3.4. How the Inflamamtory Infiltration Develops in AITD
AITD is characterized by lymphocytic infiltration of the thyroid gland, caused mostly by T lymphocytes, which leads to the thyroid fibrosis and atrophy [36]. The CD4/CD8 cell ratio is changing due to the decrease in CD8 + count. Active CD8 and/or CD4 infiltration is often observed in the inflammatory infiltration, with evident predominance of CD4 [37]. Active T lymphocytes themselves show higher levels of HLA-DR expression. Moreover, a decrease in Treg [38] is seen, with aberration of Helios and PD-1 expression [38,39] and activation of the PD-1/PDL-1 signaling pathway. An important role is played by Th17, Th22 and recently discovered thyroid specific T follicular helper cells (Tfh) [40]. Th17 cells are abundant in the thyroid gland, producing Il-17 [40,41]. A study involving activation of dendritic cells (DCs) by exosomes has shown that exosomes of AITD patients were activated mostly by CD4+ monocytes and C11+ dendritic cells. They were different from the control group–they could present antigens for DCs and bind Toll-like receptors, causing the activation of DCs via the Nf-kappaB signaling pathway, which might be the cause of altered differentiation of CD4+ and subsequent thyroid inflammation [42].
The thyroid autoimmune process is strongly affected by cytokines. Patients with a high degree of lymphocytic infiltration and hypoechoic pattern of the thyroid are observed to have high levels of IFN-gamma [43]. INF gamma and TNF alpha, produced by Th1 cells, stimulate thyrocytes to release CXCL10 [43,44]. CXCL10, binding to chemokine receptor (C-X-X- motif receptor CXCR3) on Th1 cells, makes Th1 cells migrate to target tissues and promote inflammation. Tfh cells with CXCR5 expression, which produce IL-21, are necessary for activation of B cells and formation of germinal centers in the thyroid [44]. It is intrathyroidal B lymphocytes that are responsible for the production of antibodies. In vivo studies have shown that antithyroid antibodies come from the thyroid, juxta thyroid lymph nodes, and other germinal centers in the bone marrow.
3.5. ID and Production of Anti-TPO Antibodies
Iron deficiency can trigger inflammatory processes in the thyroid gland, including the development of thyroid antibodies. The pathogenesis of AITD involves interactions between the patients’ serum and a number of antigens. Thyroglobulin (Tg) gene product represents 80% of the total thyroidal protein. Having been synthetized, Tg can pass to the systemic circulation, where it is exposed to the immune system [3]. Thyroid peroxidase (TPO) antibodies can recognize predominantly conformational epitopes in the immunodominant region of the antigen, which contains two overlapping regions, A and B, that become the major target for anti-TPO antibodies [45].
Anti-TPO antibodies stimulate two types of cytotoxicity: antibody-dependent cytotoxicity and complement-dependent cytotoxicity, which results in thyroid atrophy [46]. The relationship between IDA and autoimmune disorders is more pronounced in women. Its risk factors include arterial hypertension, dyslipidemia, cancer, allergic rhinitis, chronic obstructive pulmonary disease (COPD), urticarial, and chronic liver disease. Another important risk factor is patient’s age, with the highest risk observed in subjects aged 20–40. In the group of patients aged 20–40 years, approximately 65% are likely to develop an autoimmune disease within 5 years of being diagnosed with IDA [47]. In a study of 2581 pregnant women anti-TPO antibody level was higher in subjects with iron deficiency, with no differences in TT4 levels between study groups. Hypothyroidism and subclinical hypothyroidism were more prevalent in IDA group versus mild or no iron deficiency groups. The study has shown that the incidence of AITD was growing along with the severity of iron deficiency. In a meta-analysis by Luo et al. from 2021, ID in women of reproductive age resulted in a twofold increase in the risk of elevated titers of TPO- and/or Tg-antibodies [48].
3.6. Iron Function in the Synthesis and Tissue Actions of Thyroid Hormones
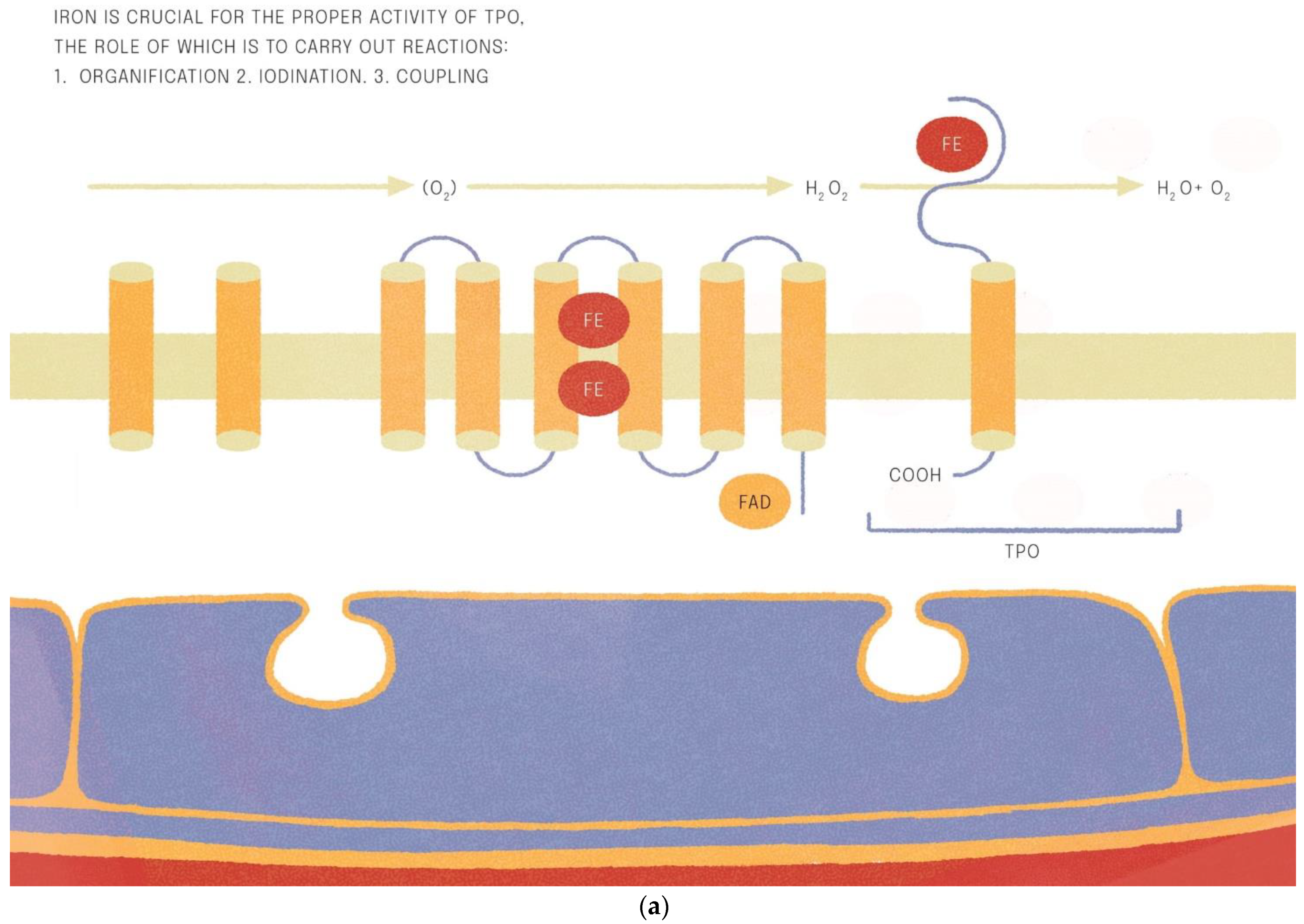
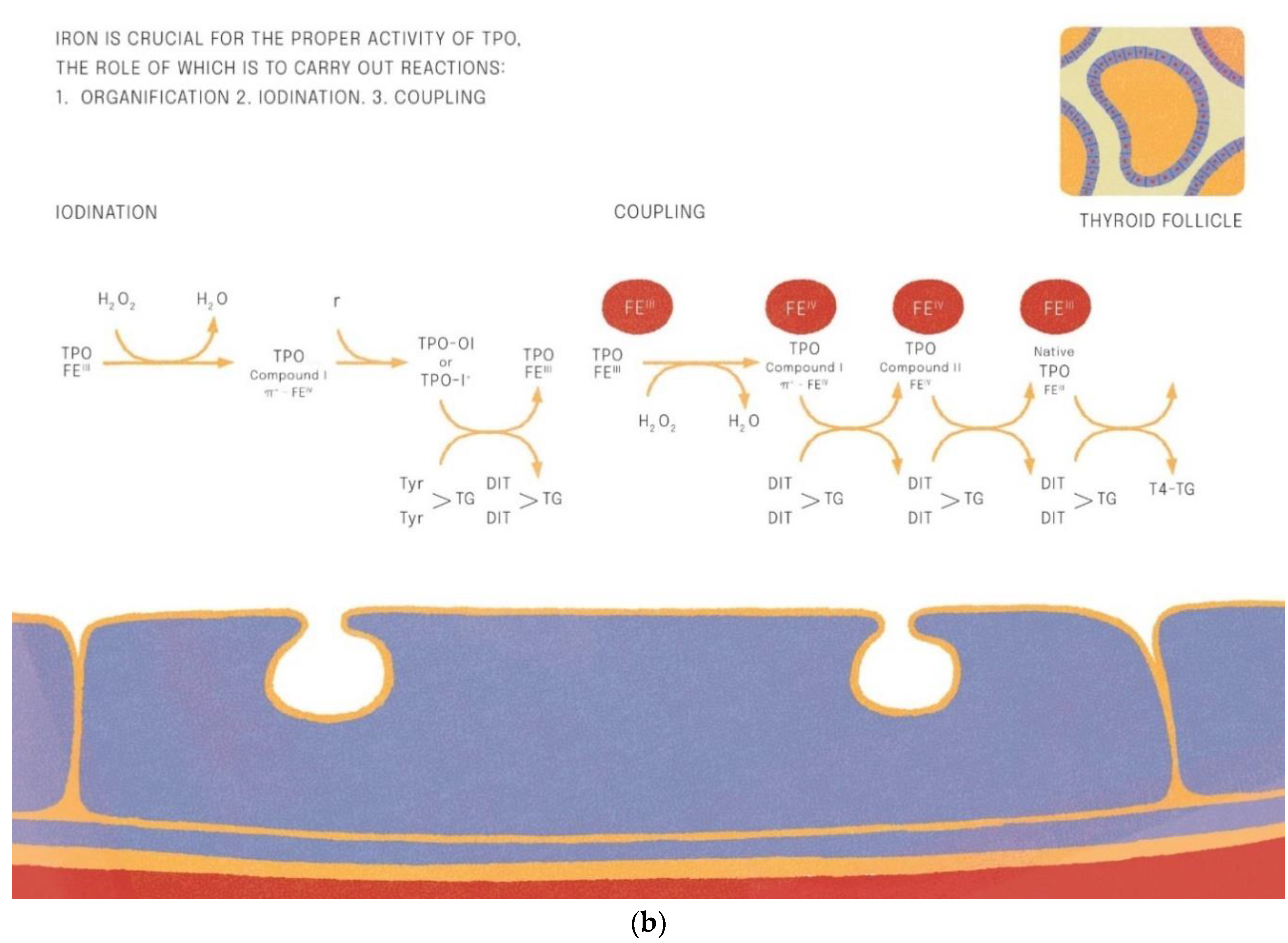
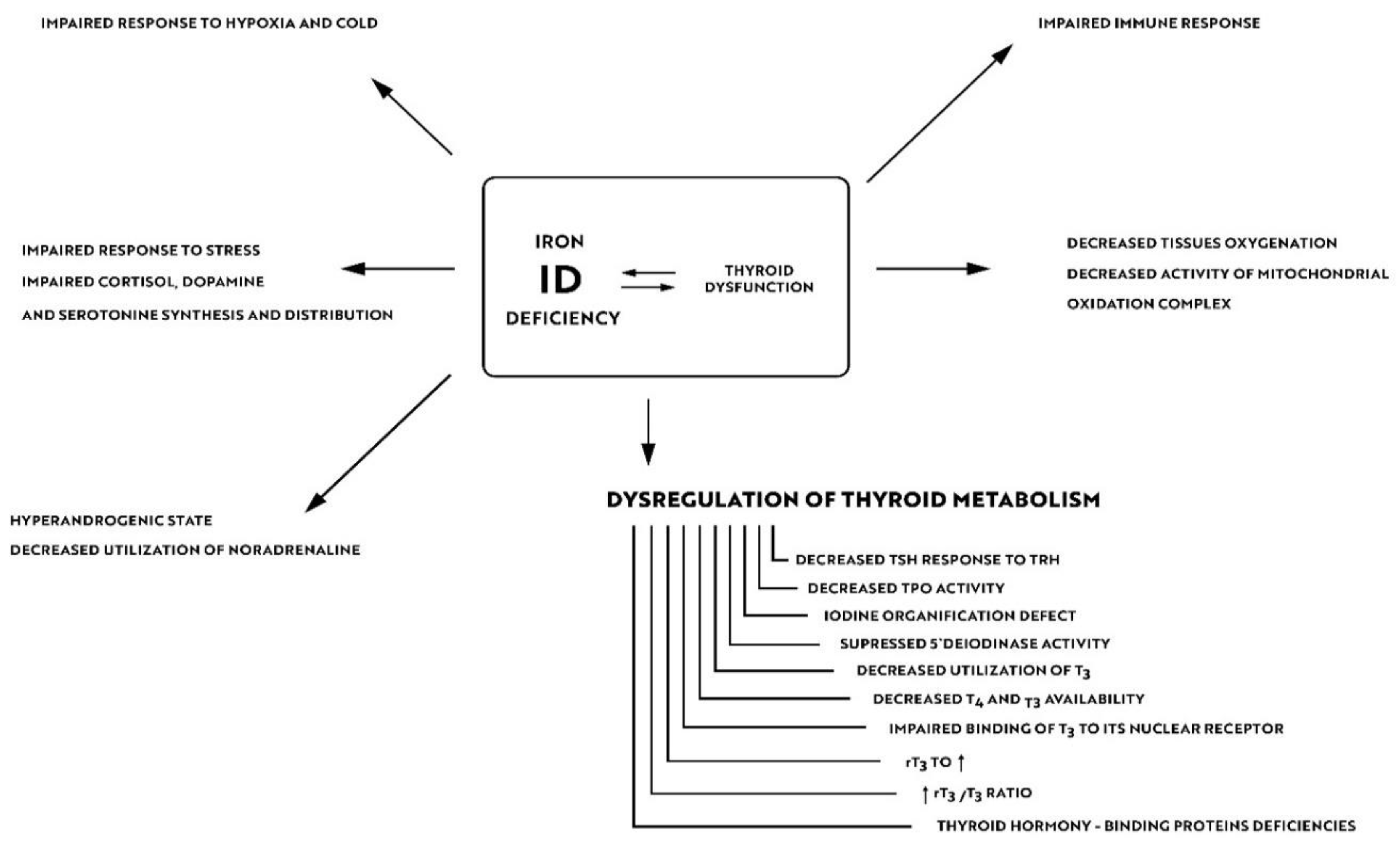
Iron is crucial for proper TPO activity, which is the key enzyme involved in biosynthesis of thyroid hormones (Figure 1a,b). Iron deficiency can reduce the activity of thyroid peroxidase (TPO). Studies have shown that the administration of hemin can increase the level and activity of TPO from 20% to 120% [49], while the administration of succinylacetone can decrease its activity by 25–37% [49]. A study with seven groups of rats showed that iron deficiency can reduce the activity of TPO. Three groups (ID-3, ID-7, ID-11) were administered food low in iron (3, 7 and 11 ppm iron, respectively). The other four groups were provided adequate iron intake (35 ppm). After 4 weeks, Hb, T3, and T4 levels were considerably lower in groups with ID, and TPO activity was reduced (by 56%, 45% and 33%, respectively) [50]. A study with human subjects confirmed a reduced activity of TPO in groups with ID (by 33–56% depending on the severity of iron deficiency) [51]. ID resulted in reduced activity of 5′-deiodinase [52] and slower utilization of T3 from the serum pool [53]. T3 binding to its nuclear receptor is also decreased [54] and iodine prophylaxis is less effective in ID. In Figure 2 we have presented the summary of the impact of iron deficiency on thyrometabolism (Figure 2).
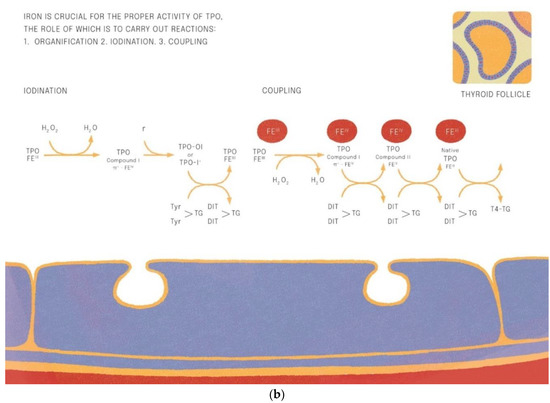
Figure 1.
(a,b). Iron is crucial for the proper activitity of TPO (thyroid peroxidase).
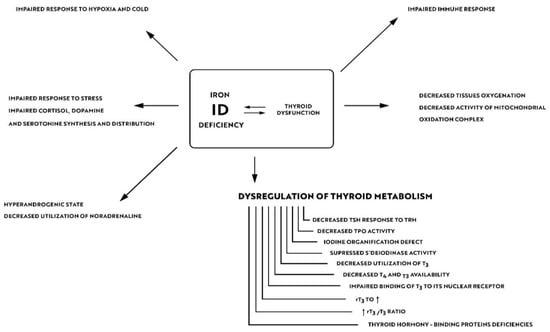
Figure 2.
Impact of iron deficiency on thyroid metabolism.
4. Iron Deficiency Impairs the Body’s Immune Response and Alters the Human Microbiome
Iron is necessary for immune response reactions. TRF1 (a protein responsible for iron transport into the lymphocytes) gene mutation can lead to immune deficiency states in humans, with low IgG levels and reduced B-cell and T-cell proliferation [55]. Iron is of key importance for appropriate activities of peroxidases and synthases involved in nitric oxide generation, which is crucial for maintaining proper functions of immune cells. Moreover, iron is involved in the regulation of cytokine production and signaling pathways [56]. ID can affect cytokine expression on lymphocytes, leading to an increase in cells with IFN-gamma expression and a decrease in cells with IL-4 expression [57]. Recent studies have shown the role of cytokines and chekokines in the pathogenesis of AITD. In thyroid tissue Th1 may be responsible for enhanced INF gamma level which stimulates CXCL10 production from the thyroid. Patients with a high degree of lymphocytic infiltration and a hypoechoic thyroid pattern are observed to have high levels of IFN-gamma [43]. INF gamma and TNF alpha, produced by Th1 cells, stimulate thyrocytes to release CXCL10 [43,44]. Iron deficiency impairs the TLR4 pathway signaling [58] and affects the activity of iron-dependent ubiquitin ligase [59] and regulation of SHP1 tyrosine phosphatase [60]. Nevertheless, iron deficiency can lead to the activation of the signaling pathways dependent on NF-kappaB, transcription factors necessary for the expression of genes crucial for innate and adaptive immunity, which may exacerbate inflammatory processes in the thyroid [61]. Dendritic cell activation involves the Nf-kappaB signaling pathway, which might be the cause of altered differentiation of CD4+ and subsequent thyroid inflammation [42].
Adequate cell-mediated immune response is delayed in ID [62]. A number of studies in humans have shown an impairment of innate immunity in subjects with ID. Bactericidal activity of macrophages is reduced, while neutrophils contain lower amounts of myeloperoxidase (MPO), involved in the generation of reactive oxygen species and responsible for intracellular pathogen death [63]. Children with ID had lower levels of IgG and IL-6, and decreased phagocytic activity with reduced oxidative burst in neutrophils [64]. ID affects blastogenesis and mitogenesis of T cells and protein kinase C activity [65]. In mice it can lead to a decrease in T-cell mediated antigen specific response, production of antibodies, and B-cell proliferation [66]. Moreover, a reduction in the induction of cyclin S, and delayed entry of B cells in phase S of the cell cycle during B-cell proliferation is observed [66]. Histone demethylation is of key importance for T-cell differentiation. Demethylation of cyclin E1 of histone H3K9 is iron dependent, which in case of iron deficiency may impair T-lymphocyte differentiation [67]. The most severely affected in ID was TLR-dependent B-lymphocyte proliferation, followed by B-lymphocyte response to BCR stimulation and relatively least affected T-lymphocyte response to TCR stimulation [66]. An effective immune response including self-tolerance, cell-mediated immunity, and humoral immunity plays a critical role in the development of AITD. The dysfunctional adaptive immunity response in iron deficiency states may favor the production of antithyroid antibodies by intrathyroidal B lymphocytes. Moreover, the Th1- and Tfh-prevalent autoimmune response is characteristic for AITD, which is also affected to a considerable extent by ID in the mechanisms of impaired response to the TCR transduced stimulation and disturbed T-lymphocyte differentiation.
AITD can lead to small intestinal bacterial overgrowth (SIBO) with altered composition of intestinal microbiota [68]. A hypothesis suggests that microbiota dysbiosis can lead to AITD. Microbiome can affect iron absorption, which in turn can alter composition and metabolic activity of microbiome and modulate intestinal effects on the immune system [69]. A study has shown that, in the absence of viable intestinal microbiota, iron absorption decreased by as much as 25% [70]. Moreover, ID can result in higher sensitivity of body cells to bacterial endotoxins [71]. Of course, we must keep in mind that patients with AITD are more likely to suffer from celiac disease and gastritis—for this reason we cannot exclude that the higher prevalence of iron deficiency in AITD patients partially results from malabsorption disorders [72]. Moreover, celiac disease is finally confirmed in 1 out of 31 patients undergoing diagnostic procedures because of iron deficiency. Therefore, in case of concomitant ID and AITD, we recommend diagnostic procedures for celiac disease and gastritis.
To summarize, the effect of iron deficiency on the body’s immune reactions, including the breakdown of immune tolerance, dysfunction of T lymphocytes, and distorted humoral response, can underly the possible mechanisms leading to the development of AITD.
5. Iron Deficiency and Thyroid Sensitivity to Environmental Factors
Iron homeostasis disruption can lead to higher sensitivity of the body to environmental pollutants. Functional groups of chemical compounds remove iron atoms from inside the cell, leading to its functional deficiency. Functional ID activates kinases and transcription factors able to trigger inflammatory reactions. Increased iron availability can diminish its cellular deficiency and counteract biological effects of chemical particles [73]. Iron is kinetically preferred by chemical particles due to its electropositivity, high affinity for oxygen-containing groups, and availability. Due to endocytosis of inorganic particles, their surface functional groups come into reaction with iron to produce chemical complexes [74]. Chemical particles have the continuous ability to export iron from cells, while the body responds to it with iron sequestration to such an extent to make it unavailable to chemicals. Therefore, toxic ferruginous bodies are formed [75].
Polychlorinated biphenyls (PCBs) including bisphenol A, phthalates, brominated flame retardants, and perfluorinated compounds can disrupt thyroid function [76]. Thyroid function can also be disturbed by cadmium, which accumulates in thyrocyte mitochondria [77], or by manganese, which may suppress TSH release by inhibiting dopamine secretion [78]. A study of 236 women, conducted by Benven S et al. [79] has shown higher titers of anti-TPO and anti-Tg antibodies in women from Group A who ingested swordfish versus Group B who received omega 3 dietary supplements. In Group A, mercury intake was 1000 mcg/month versus 25 mcg/month in Group B. An in vitro study by Fallahi et al. [80] investigated the effect of vanadium oxygen on thyroid growth and proliferation and secretion of CXCL8 and CXCL11 in thyrocytes. Animal studies have shown that iron deficiency may increase tissue sensitivity to the toxic effects of heavy metals including cadmium and manganese [81]. Vanadium oxygen (V205) was able to induce secretion of CXCL8 and CXCL11 by thyrocytes. This can trigger and maintain inflammatory processes in thyroid. In a study by Sun X et al. which involved 675 pregnant women, an exposure to heavy metals (vanadium, arsenic) was associated with lower levels of thyroid hormones and decreased birth weight of children [82]. Populations exposed to vanadium were observed to have higher incidence of thyroid cancer, for example in the Etna region. Vanadium oxygen can increase chemokine secretion by Th1 cells, synergically potentiating the effects of cytokines such as INF gamma and TNF alpha, which results in induction of inflammatory state in the thyroid gland [80]. Exposure to vanadyl sulphate results in a reduction of iron in the cellular nucleus and mitochondria. In response to the vanadyl sulphate induced iron loss, the iron import into the cell is increased in order to compensate for these sudden deficiencies. Intracellular iron deficiency leads to increased DMT1 expression. Vanadium has the ability to bind with iron in transferrin, lactoferrin and ferritin. Following vanadium exposure, the amount of non-heme iron in the nucleus and mitochondria decreased rapidly with its concomitant increase in the cellular supernatant [83]. Vanadium is able to interact with iron incorporated in iron body stores [84,85,86,87]. Similar to the biological effects of vanadium exposure, iron dependent mitochondrial proteins can be targeted by other heavy metals, which may exacerbate their toxicity and this effect is potentiated by iron deficiency.
A twofold increase in the incidence of thyroid lesions suspected for malignancy was observed in FNAB in polluted areas. Arena S et al. [88] studied a group of 391 patients undergoing thyroid FNAB in areas of petrochemical industry (Group A) and compared them to a control group of 622 patients living at a distance from exposure areas (Group B). Lymphocytic thyroiditis and suspected malignant lesions were observed considerably more frequently in Group A. In a Brazilian study by de Freitas CU et al. [89], higher anti-TPO titers were seen in patients exposed to petrochemical industry. Freire C et al. [90] in their study of inhabitants of industrial areas polluted with organochlorine (OC) pesticides found higher anti-TPO titers following methoxychlor exposure. Cells administered iron prior to their exposure to pollutants demonstrated lower oxidant levels. Exposure to silica resulted in increased levels of IL-6 and IL-8, although these cytokines were not released by cells rich in iron [86]. Studies have shown that silica exposure increased NF-kappaB activation at least fivefold. Mitochondrial sources of iron were complexed by silica which was then centrifuged into the nuclear fraction. This illustrates how chemical particles can disrupt the intracellular iron homeostasis.
Tissue damage, including the damage to the thyroid gland, is the final outcome of iron deficiency during their exposure to chemical compounds. Iron deficiency states result in an increased activity of MAP-kinases and transcription factors, which potentiates inflammatory processes in tissues. In response to the exposure to chemical particles iron import into cells is upregulated. Tissue damage is particularly likely to occur in cases of exposure to nanomolecules because of their small sizes and high interaction surfaces, which potentiates biological effects of iron deficiency. Nanomolecules may contain alcohol, diol, epoxide, ether, dibenzofurans, and benzodioxines [73]. In a Slovakian study, increased thyroid volume and higher incidence of thyroid dysfunction were seen in employees exposed to PCBs and polychlorinated dibenzodioxins and dibenzofurans [91], which confirmed positive correlation between AITD and the severity of OC exposure. Similarly, employees exposed to polybrominated diphenyl ethers and polybrominated biphenyls (PBBs) had elevated titers of thyroid antibodies and higher TSH levels [92]. Schell LM et al. [93] conducted a study of 115 adolescents from the Akwesasne Mohawk Nation living on the territory near the St. Lawrence River in the State of New York. For many years, waters of the St. Lawrence River and its three tributaries had been polluted by aluminum foundries. As a result, the PCBs dichlorodiphenyldichloroethylene (p,p’-DDE), hexachlorobenzene (HCB), and Mirex (an organochlorine insecticide) were included into the local food chain. Adolescents who had been breastfed in their infancy were found to have elevated anti-TPO levels. Vietnam veterans exposed to Agent Orange (2,4—dichlorophenoxyacetic acid and tetrachlorodibenzodioxin (TCDD)) were more likely to suffer from diabetes mellitus and a number of thyroid and pituitary disorders, with threefold increase in the incidence of Graves’ disease [94]. AITD was considerably more prevalent in regions around Chernobyl, affecting 6.4% of children and adolescents versus 2.4% of those living in regions not exposed to radioactive fallout [95]. In a study by Tajtakova M et al. [96] in which 324 children from regions of high exposure to nitrogen were compared to 596 children from control groups, anti-TPO and TSH levels were higher and thyroid hypoechogenicity was more frequent in nitrogen-exposed subjects. A study of 70 vitiligo patients conducted by Colucci R et al. [97] has shown that exposure to PCBS was associated with increased levels of IgG antibodies to T4, while exposure to nitrates, nitriles, and soy isoflavones was associated with increased levels of IgM and IgG antibodies to T3. Studies in mice have shown that animals with ID exposed to cigarette smoke had higher levels of alveolar macrophages, and were subject to more rapid changes in the lungs [98,99]. Animals with ID presented more severe intracellular inflammation in response to smog exposure [73].
6. Impact of Iron Deficiency on Residual Symptoms in Euthyroid Patients with Aitd
About 10–15% of hypothyroid patients complain of residual symptoms such as fatigue, lower quality of life, impaired cognitive functions and memory problems in spite of their thyrometabolic status being well-controlled [100]. In their study of 5000 subjects Ettleson et al. found that 79% of hypothyroid patients may present symptoms of “brain fog” [101]. Hypotheses that explain the development of „brain fog” in hypothyroidism focus on autoimmune processes, oxidative stress, and alterations in neurotransmitter levels [102]. Wet et al., in the their study of 199 subjects, confirmed a correlation between the presence of anti-TPO antibodies and an increased risk of depression and anxiety [103]. A study of AITD euthyroid mice has shown memory deficits, more prominent loss of synapses and astrocytes in the hippocampus and considerable reduction of neuroplasticity [104]. In a controversial study by Guldvog et al., a marked improvement in quality of life was achieved in patients with AITD following thyroidectomy [105]. Due to these residual symptoms, numerous patients with AITD seek alternative treatments, such as the use of triiodothyronine alone or desiccated porcine thyroid extract (DTE). As already mentioned, ID can result in impaired synthesis of neurotransmitters in the brain and reduced neuroplasticity. In a Japanese study of 11,876 participants, depression and experience of stress were more prevalent in the group of IDA subjects [106]. A Finnish study showed that iron can influence effectiveness of the hypothyroidism treatment. An experiment included 25 female patients with persistent symptoms of hypothyroidism in spite of being euthyroid. After 6–12 months of treatment with oral iron products the symptoms were relieved. At the onset of the treatment none of the women suffered from anemia, but all of them had ferritin levels < 60 mcg/L. Achievement of ferritin levels > 100 mcg/L resulted in reduction of residual symptoms in two-thirds of the studied patients [107]. Findings of these studies suggest that the role or iron is underestimated in AITD patients in everyday practice. As shown in Figure 2, ID can impair the body’s resistance to stress, increase sympathetic stimulation by reducing the utilization of norepinephrine and decrease tissue oxygenation. The beneficial effects of treatment with iron products may also result from the improvement of iodine utilization by the thyroid gland, and from better utilization and availability of thyroid hormones at the tissue level (including in the brain) due to the increased deiodinase and TPO activity and increased T3 binding to its nuclear receptor as well as due to the immunomodulatory effects of iron.
7. Epigenetics in AITD Development
Epigenetics can be a link that integrates the effects of environmental and genetic factors on the development of AITD [108]. The most recent research shows that environmental factors can influence genes by epigenetic modifications [109]. The most common type of genetic modifications is DNA methylation. Global hypomethylation of DNA in AITD can be responsible for overexpression of some genes associated with immune system modification, and for immune cell activation that results in autoimmune attack on the thyroid tissue [110,111]. Polymorphisms in DNA methylation-regulating genes (DNMT1, MTRR) were correlated with DNA hypomethylation observed in AITD [112]. Histones play an important role in the modulation of immune response and tolerance [113]. Fragments of DNA released from injured thyroid cells can be recognized by histone H2B, causing an activation of immune response genes with subsequent potentiation of autoimmunity to thyrocytes [48]. IFN-alpha could induce changes in Tg gene expression and trigger AITD by enhancement of Lys-4 residue methylation in histone E3 at the promoter area of Tg gene [114]. IFN-alpha plays an important role in immune response to viral infection as it stimulates an increase in H3K4me3 and H3K4me1 levels in thyrocytes [115]. Saramaru et al. reported that rs3758391 and rs746720 alleles in the gene of sirtuin 1—an enzyme involved in histone deacetylation—were associated with higher titers of autoantibodies in AITD [116].
miRNA is a form of small, non-coding RNA, composed of 18–25 nucleotides, with the ability to regulate gene expression, controlling up to 60% of RNA [117]. RNA miR223-3p and miR-155-5p regulate functions of the immune system [118,119]. Mi-R-155-5p and miR-146a-5p play a particular role in immune response modulation [120,121]. MiR-146a-5p potentiates expression of interleukin-1 receptor associated kinase 1 and TNF receptor, while its reduced expression potentiates dendritic cell activation and antigen presentation [121,122]. Altered expression of miR 155-5p and miRNA-146a-5p can promote development of autoimmune diseases via breakdown of immune tolerance. Bernecker et al. showed alterations in expressions of miRNA-146a-5p and miRNA-155-5p in thyroid cells in AITD patients [123]. Zhu et al. indicated higher expression of miRNA-142-5p in AITD and its positive correlation with anti-TPO titers [124]. Overexpression of miR-142 in thyroid cells resulted in reduced claudin 1 expression and increased permeability of the thyrocyte monolayer [124]. miR-125a-3p could target the IL-23 receptor, and decreased expression of miR-125a-3p could upregulate IL-23 receptor expression in AITD [125]. Long non-coding (Lnc) RNAs composed of more than 200 nucleotides are also involved in the development of AITD [126]. Lnc RNAs can regulate gene expression by epigenetic regulations, gene transcription, post-translational regulation, and alterations of miRNA [127,128]. They contribute to the regulation of production and differentiation of T cells, and every type of T cell contains different IncRNA [129,130]. Lnc RNA-IFNG-AS1 was upregulated in AITD, and it was associated with the frequency of circulating Th1 cells and INF-gamma expression [131]. lnc RNA-IFNG-AS1 can regulate INF-gamma expression in human CD4+ cells and promote Th1 lymphocyte-dependent responses [131]. In women, one X chromosome is randomly inactivated, which is called XCI (X chromosome inactivation) and can result in a mosaic pattern of cells expressing genes from either chromosome [132]. XCI may be influenced by histone modifications and DNA and microRNA methylation [133]. Skewed XCI occurs when the inactivation of one X chromosome is silenced more than the other one [133,134], and this may cause disruption in gene expression and lead to the development of autoimmune disease [135]. Brix et al. noticed an increased frequency of skewed CXI in female twins with AITD [136,137]
Iron is essential for DNA synthesis. Its deficiency may result in impaired DNA synthesis and subsequent alterations in programmed cell death [138]. ID potentiates DNA damage and may cause genomic instability, mimic radiation in damaging DNA by causing single- and double-strand breaks, oxidative lesions, or both [139]. In ID states, oxidative stress is more pronounced. An increase in oxidant levels and/or a decrease in antioxidant enzyme capacities is seen in IDA [140]. Iron deficiency can induce nuclear DNA base damage. An animal study has shown that iron deficiency increased oxidant levels in PMNs and potentiated liver mtDNA damage [141]. The activity of ribonucleotide reductase is decreased in iron-deficient cell cultures [142]; this could lead to decreased availability of deoxyribonucleotides for DNA repair. It is one of the major causes of imbalance between antioxidant enzymes and DNA breaking and repairing enzymes [143]. DNA polymerases, ribonucleotide reductases (RR), DNA glycolsylases, DNA primases, DNA helicasess, and DNA endonucleases are all enzymes that contain Fe-S clusters, which are involved in DNA replication, synthesis, and repair [142]. In ID states, increased chromosome fragility and reduced sister chromatid exchange are observed [144]. ID can disturb biogenesis and normal expression of microRNA [143]. MicroRNAs are involved in iron homeostasis through the post-translational regulation of genes related to the uptake, utilization and storage of iron. [145]. What is important, iron is crucial for the activity of co-factor of Drosha protein (Class 2 ribonuclease III enzyme) and Poly (rC)-binding protein 2 (Pcbp2). Drosha is responsible for the processing of pri-microRNAs into pre-microRNAs within the nucleus [146]. Therefore, abnormal heme biosynthesis and degradation may alter microRNA mediated DNA regulation. Furthermore, microRNA synthesis and expression depend on ROS and hypoxia, which are common in ID [147,148]. Hypoxia results in overexpression of Mir-373 and MiR210, with subsequent downregulation of genes involved in DNA repair: RAD 23B and RAD 52 [149]. DNA demethylation relies on the activity of iron-dependent methylcytosine dioxygenases (TET protein family), which need iron for methylcytosine-to-hydroxymethylcytosine conversion [150]. Iron deficiency can also impair histone modifications—removal of methyl groups from lysine residues is catalyzed by Jmj/AT-rich interactive domain (ARID)-containing histone demethylase (JARID) proteins, and iron is essential for their enzymatic activity [151].
Through the mechanisms of DNA repair and the effects of iron on the complex epigenetic interplay, iron deficiency can induce changes in the genome that potentially may promote the development of AITD.
8. Conclusions
The human lifestyle in the 21st century is inevitably associated with exposure to chemical compounds, pathogens, and stress, which implies an increased risk of autoimmune disorders and thyroid dysfunction. At least 30–50% of females with hypothyroidism and persistent residual symptoms in spite of adequate dose of levothyroxine can have underlying iron deficiency. Patients with AITD should undergo routine screening for ID and gastropathy. If ferritin level is lower than 70 mcg/L, diagnostic procedures for celiac disease or gastritis should be offered and iron supplementation should be started in order to restore adequate iron stores in the body and prevent tissue iron deficiency. Iron deficiency can lead to immune system dysfunction, cause epigenetic changes in human DNA, and potentiate tissue damage by chemical compounds acting as thyroid disruptors. The influence of iron deficiency on the development of AITD has been investigated in very few studies, and we believe that special attention should be paid to the issue of how ID can impair immune tolerance mechanisms. In conclusion, iron appears to be an active by-stander in the pathomechanism of AITD.
Author Contributions
Conceptualization: M.S. and E.B.-S.; validation: M.S. and K.G.-N.; investigation: M.S. and K.G.-N.; resources: M.S.; writing—original draft preparation: M.S.; writing—review and editing: M.S. and K.G.-N.; supervision: W.M. and E.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hiromatsu, Y.; Satoh, H.; Amino, N. Hashimoto’s thyroiditis: History and future outlook. Hormones 2013, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655, Erratum in N. Engl. J. Med. 2003, 349, 620. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef]
- Shero, N.; Fiset, S.; Plamondon, H.; Thabet, M.; Rioux, F.M. Increase serum cortisol in young guinea pig offspring in response to maternal iron deficiency. Nutr. Res. 2018, 54, 69–79. [Google Scholar] [CrossRef]
- Hu, X.; Wang, R.; Shan, Z.; Dong, Y.; Zheng, H.; Jesse, F.F.; Rao, E.; Takahashi, E.; Li, W.; Teng, W.; et al. Perinatal Iron Deficiency-Induced Hypothyroxinemia Impairs Early Brain Development Regardless of Normal Iron Levels in the Neonatal Brain. Thyroid 2016, 26, 891–900. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259, Erratum in Lancet 2017, 390, e38. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Bisbe, E.; Shander, A.; Spahn, D.R.; Muñoz, M. Management of Perioperative Iron Deficiency Anemia. Acta Haematol. 2019, 142, 21–29. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2011, 31, 1872–1880, Erratum in Eur. Heart J. 2011, 32, 1054. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R.; Jones, B.C. Iron in the brain. Nutr. Rev. 1993, 51, 157–170. [Google Scholar] [CrossRef]
- Pinero, D.J.; Jones, B.; Beard, J.L. Alterations in brain iron metabolism in response to dietary iron changes. J. Nutr. 2000, 130, 254–263. [Google Scholar] [CrossRef]
- Georgieff, M.K. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008, 36 Pt 6, 1267–1271. [Google Scholar] [CrossRef]
- Youdim, M.B. Nutrient deprivation and brain function: Iron. Nutrition 2000, 16, 504–508. [Google Scholar] [CrossRef]
- McEchron, M.D.; Cheng, A.Y.; Liu, H.; Connor, J.R.; Gilmartin, M.R. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr. Neurosci. 2005, 8, 195–206. [Google Scholar] [CrossRef]
- Insel, B.J.; Schaefer, C.A.; McKeague, I.W.; Susser, E.S.; Brown, A.S. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry 2008, 65, 1136–1144. [Google Scholar] [CrossRef]
- Min, Y.; Wang, X.; Chen, H.; Yin, G. The exploration of Hashimoto’s Thyroiditis related miscarriage for better treatment modalities. Int. J. Med. Sci. 2020, 17, 2402–2415. [Google Scholar] [CrossRef]
- Lee, H.J.; Li, C.W.; Hammerstad, S.S.; Stefan, M.; Tomer, Y. Immunogenetics of autoimmune thyroid diseases: A comprehensive review. J. Autoimmun. 2015, 64, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Tanigawa, K.; Akama, T.; Yoshihara, A.; Ishii, N.; Suzuki, K. Innate immune activation and thyroid autoimmunity. J. Clin. Endocrinol. Metab. 2011, 96, 3661–3671. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, M.; Kahaly, G.J. Polyglandular autoimmune syndromes: Immunogenetics and long-term follow-up. J. Clin. Endocrinol. Metab. 2003, 88, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Benvenga, S.; Antonelli, A. The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): Review of the literature and report of a large series. Autoimmun. Rev. 2019, 18, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Brix, T.H.; Hegedüs, L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin. Endocrinol. 2012, 76, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tomer, Y.; Davies, T.F. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr. Rev. 2003, 24, 694–717. [Google Scholar] [CrossRef]
- Simmonds, M.J. GWAS in autoimmune thyroid disease: Redefining our understanding of pathogenesis. Nat. Rev. Endocrinol. 2013, 9, 277–287. [Google Scholar] [CrossRef]
- Lo, M.S.; Towne, M.; VanNoy, G.E.; Brownstein, C.A.; Lane, A.A.; Chatila, T.A.; Agrawal, P.B. Monogenic Hashimoto thyroiditis associated with a variant in the thyroglobulin (TG) gene. J. Autoimmun. 2018, 86, 116–119. [Google Scholar] [CrossRef]
- Desailloud, R.; Hober, D. Viruses and thyroiditis: An update. Virol. J. 2009, 6, 5. [Google Scholar] [CrossRef]
- Morohoshi, K.; Takahashi, Y.; Mori, K. Viral infection and innate pattern recognition receptors in induction of Hashimoto’s thyroiditis. Discov. Med. 2011, 12, 505–511. [Google Scholar]
- Frost, J.N.; Tan, T.K.; Abbas, M.; Wideman, S.K.; Bonadonna, M.; Stoffel, N.U.; Wray, K.; Kronsteiner, B.; Smits, G.; Campagna, D.R.; et al. Hepcidin-Mediated Hypoferremia Disrupts Immune Responses to Vaccination and Infection. Med 2021, 2, 164–179.e12. [Google Scholar] [CrossRef]
- Bach, J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef]
- Aleksandrov, Y.; Semikov, V.; Shulutko, A.; Gogokhia, T.; Gorbacheva, A.; Mansurova, G. Subacute thyroiditis and COVID-19 (review). Georgian Med. News. 2021, 311, 98–103. (In Russian) [Google Scholar]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Wöll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Iddah, M.A.; Macharia, B.N. Autoimmune thyroid disorders. ISRN Endocrinol. 2013, 2013, 509764. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Chen, H.; Liu, X.; Zheng, X.; Shi, H.; Jiang, L.; Cui, D. Analysis of Regulatory T Cell Subsets and Their Expression of Helios and PD-1 in Patients with Hashimoto Thyroiditis. Int. J. Endocrinol. 2019, 2019, 5368473. [Google Scholar] [CrossRef]
- Álvarez-Sierra, D.; Marín-Sánchez, A.; Ruiz-Blázquez, P.; de Jesús Gil, C.; Iglesias-Felip, C.; González, Ó.; Casteras, A.; Costa, R.F.; Nuciforo, P.; Colobran, R.; et al. Analysis of the PD-1/PD-L1 axis in human autoimmune thyroid disease: Insights into pathogenesis and clues to immunotherapy associated thyroid autoimmunity. J. Autoimmun. 2019, 103, 102285. [Google Scholar] [CrossRef]
- Nanba, T.; Watanabe, M.; Inoue, N.; Iwatani, Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid 2009, 19, 495–501. [Google Scholar] [CrossRef]
- Xue, H.; Yu, X.; Ma, L.; Song, S.; Li, Y.; Zhang, L.; Yang, T.; Liu, H. The possible role of CD4+CD25highFoxp3+/CD4+IL-17A+ cell imbalance in the autoimmunity of patients with Hashimoto thyroiditis. Endocrine 2015, 50, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, Y.; Wang, S.; Zhao, N.; Qin, J.; Li, Y.; Fan, C.; Shan, Z.; Teng, W. Circulating Exosomes Activate Dendritic Cells and Induce Unbalanced CD4+ T Cell Differentiation in Hashimoto Thyroiditis. J. Clin. Endocrinol. Metab. 2019, 104, 4607–4618. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Frascerra, S.; Galetta, F.; Franzoni, F.; Corrado, A.; Miccoli, M.; Benvenga, S.; Paolicchi, A.; Ferrannini, E.; et al. Circulating chemokine (CXC motif) ligand (CXCL)9 is increased in aggressive chronic autoimmune thyroiditis, in association with CXCL10. Cytokine 2011, 55, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Jogdand, G.M.; Mohanty, S.; Devadas, S. Regulators of Tfh Cell Differentiation. Front. Immunol. 2016, 7, 520. [Google Scholar] [CrossRef] [PubMed]
- Dubska, M.; Banga, J.P.; Plochocka, D.; Hoser, G.; Kemp, E.H.; Sutton, B.J.; Gardas, A.; Gora, M. Structural insights into autoreactive determinants in thyroid peroxidase composed of discontinuous and multiple key contact amino acid residues contributing to epitopes recognized by patients’ autoantibodies. Endocrinology 2006, 147, 5995–6003. [Google Scholar] [CrossRef]
- Orgiazzi, J. Thyroid autoimmunity. Presse Med. 2012, 41, e611–e625. [Google Scholar] [CrossRef]
- Chang, R.; Chu, K.A.; Lin, M.C.; Chu, Y.H.; Hung, Y.M.; Wei, J.C. Newly diagnosed iron deficiency anemia and subsequent autoimmune disease: A matched cohort study in Taiwan. Curr. Med. Res. Opin. 2020, 36, 985–992. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- Fayadat, L.; Niccoli-Sire, P.; Lanet, J.; Franc, J.L. Role of heme in intracellular trafficking of thyroperoxidase and involvement of H2O2 generated at the apical surface of thyroid cells in autocatalytic covalent heme binding. J. Biol. Chem. 1999, 274, 10533–10538. [Google Scholar] [CrossRef]
- Hurrell, R.F. Bioavailability of iodine. Eur. J. Clin. Nutr. 1997, 51 (Suppl. 1), S9–S12. [Google Scholar]
- Hess, S.Y.; Zimmermann, M.B.; Arnold, M.; Langhans, W.; Hurrell, R.F. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J. Nutr. 2002, 132, 1951–1955. [Google Scholar] [CrossRef]
- Beard, J.; Tobin, B.; Green, W. Evidence for thyroid hormone deficiency in iron-deficient anemic rats. J. Nutr. 1989, 119, 772–778. [Google Scholar] [CrossRef]
- Dillman, E.; Gale, C.; Green, W.; Johnson, D.G.; Mackler, B.; Finch, C. Hypothermia in iron deficiency due to altered triiodothyronine metabolism. Am. J. Physiol. 1980, 239, R377–R381. [Google Scholar] [CrossRef]
- Smith, S.M.; Finley, J.; Johnson, L.K.; Lukaski, H.C. 1994. Indices of in vivo and in vitro thyroid hormone metabolism in irondeficient rats. Nutr. Res. 1994, 14, 729–739. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.; et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016, 48, 74–78. [Google Scholar] [CrossRef]
- Hershko, C.; Peto, T.E.; Weatherall, D.J. Iron and infection. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 660–664. [Google Scholar] [CrossRef]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Bell, M.; Jensen, R.J.; Gunter, E.; Buchanan, I.; Larned, J.; Kazembe, P.N.; Dobbie, H.; et al. The effects of iron deficiency on lymphocyte cytokine production and activation: Preservation of hepatic iron but not at all cost. Clin. Exp. Immunol. 2001, 126, 466–473. [Google Scholar] [CrossRef]
- Wang, L.; Harrington, L.; Trebicka, E.; Shi, H.N.; Kagan, J.C.; Hong, C.C.; Lin, H.Y.; Babitt, J.L.; Cherayil, B.J. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J. Clin. Investig. 2009, 119, 3322–3328. [Google Scholar] [CrossRef]
- Salahudeen, A.A.; Thompson, J.W.; Ruiz, J.C.; Ma, H.W.; Kinch, L.N.; Li, Q.; Grishin, N.V.; Bruick, R.K. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 2009, 326, 722–726. [Google Scholar] [CrossRef]
- Gomez, M.A.; Alisaraie, L.; Shio, M.T.; Berghuis, A.M.; Lebrun, C.; Gautier-Luneau, I.; Olivier, M. Protein tyrosine phosphatases are regulated by mononuclear iron dicitrate. J. Biol. Chem. 2010, 285, 24620–24628. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Kochanowski, B.A.; Sherman, A.R. Cellular growth in iron-deficient rats: Effect of pre- and postweaning iron repletion. J. Nutr. 1985, 115, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Spear, A.T.; Sherman, A.R. Iron deficiency alters DMBA-induced tumor burden and natural killer cell cytotoxicity in rats. J. Nutr. 1992, 122, 46–55. [Google Scholar] [CrossRef]
- Hassan, T.H.; Badr, M.A.; Karam, N.A.; Zkaria, M.; El Saadany, H.F.; Abdel Rahman, D.M.; Shahbah, D.A.; Al Morshedy, S.M.; Fathy, M.; Esh, A.M.H.; et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine 2016, 95, e5395. [Google Scholar] [CrossRef]
- Kuvibidila, S.R.; Kitchens, D.; Baliga, B.S. In vivo and in vitro iron deficiency reduces protein kinase C activity and translocation in murine splenic and purified T cells. J. Cell Biochem. 1999, 74, 468–478. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Wu, Q.; An, P.; Huang, L.; Wang, J.; Chen, C.; Chen, X.; Zhang, F.; Ma, L.; et al. Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat. Commun. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Chen, Z.; Genuth, S.; Paterson, A.; Zhang, L.; Wu, X.; Li, S.M.; Cleary, P.; Riggs, A.; Harlan, D.M.; et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes 2014, 63, 1748–1762. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Pleasants, J.R.; Wostmann, B.S. Effect of intestinal microflora on iron and zinc metabolism, and on activities of metalloenzymes in rats. J. Nutr. 1972, 102, 101–107. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953, Erratum in Nat. Rev. Microbiol. 2005, 3, 268. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A. Air pollution particles and iron homeostasis. Biochim. Biophys. Acta 2016, 1860, 2816–2825. [Google Scholar] [CrossRef]
- Dugger, D.L.; Irby, B.N.; McConnell, B.L.; Cummings, W.W.; Mattman, R.W. The exchange of twenty metal ions with the weakly acidic silanol group of silica gel. J. Phys. Chem. 1964, 68, 757–760. [Google Scholar] [CrossRef]
- Koerten, H.K.; Brederoo, P.; Ginsel, L.A.; Daems, W.T. The endocytosis of asbestos by mouse peritoneal macrophages and its long-term effect on iron accumulation and labyrinth formation. Eur. J. Cell. Biol. 1986, 40, 25–36. [Google Scholar]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Cadmium effects on the thyroid gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [CrossRef]
- Soldin, O.P.; Aschner, M. Effects of manganese on thyroid hormone homeostasis: Potential links. Neurotoxicology 2007, 28, 951–956. [Google Scholar] [CrossRef]
- Benvenga, S.; Vigo, M.T.; Metro, D.; Granese, R.; Vita, R.; Le Donne, M. Type of fish consumed and thyroid autoimmunity in pregnancy and postpartum. Endocrine 2016, 52, 120–129. [Google Scholar] [CrossRef]
- Fallahi, P.; Foddis, R.; Elia, G.; Ragusa, F.; Patrizio, A.; Frenzilli, G.; Benvenga, S.; Cristaudo, A.; Antonelli, A.; Ferrari, S.M. Differential modulation by vanadium pentoxide of the secretion of CXCL8 and CXCL11 chemokines in thyroid cells. Mol. Med. Rep. 2018, 17, 7415–7420. [Google Scholar] [CrossRef]
- Shukla, A.; Agarwal, K.N.; Shukla, G.S. Effect of latent iron deficiency on metal levels of rat brain regions. Biol. Trace Elem. Res. 1989, 22, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, W.; Zhang, B.; Shen, X.; Hu, C.; Chen, X.; Jin, S.; Jiang, Y.; Liu, H.; Cao, Z.; et al. Maternal Heavy Metal Exposure, Thyroid Hormones, and Birth Outcomes: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Stonehuerner, J.; Soukup, J.M.; Dailey, L.A.; Kesic, M.J.; Cohen, M.D. Iron diminishes the in vitro biological effect of vanadium. J. Inorg. Biochem. 2015, 147, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, E.; Rade, J. Relationships between iron and vanadium metabolism: The association of vanadium with bovine lactoferrin. Toxicol. Lett. 1980, 5, 381–387. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Winterbourn, C.C.; Stern, A. Tetravalent vanadium releases ferritin iron which stimulates vanadium-dependent lipid peroxidation. Free Radic. Res. Commun. 1991, 12–13 Pt 1, 125–129. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Grady, J.K.; Shao, J.; Arosio, P.; Santambrogio, P.; Chasteen, N.D. Vanadyl(IV) binding to mammalian ferritins. An EPR study aided by site-directed mutagenesis. J. Inorg. Biochem. 2000, 80, 107–113. [Google Scholar] [CrossRef]
- Arena, S.; Latina, A.; Baratta, R.; Burgio, G.; Gullo, D.; Benvenga, S. Chronic lymphocytic thyroiditis: Could it be influenced by a petrochemical complex? Data from a cytological study in South-Eastern Sicily. Eur. J. Endocrinol. 2015, 172, 383–389. [Google Scholar] [CrossRef]
- de Freitas, C.U.; Grimaldi Campos, R.A.; Rodrigues Silva, M.A.; Panachão, M.R.; de Moraes, J.C.; Waissmann, W.; Roberto Chacra, A.; Maeda, M.Y.; Minazzi Rodrigues, R.S.; Gonçalves Belchor, J.; et al. Can living in the surroundings of a petrochemical complex be a risk factor for autoimmune thyroid disease? Environ. Res. 2010, 110, 112–117. [Google Scholar] [CrossRef]
- Freire, C.; Koifman, R.J.; Sarcinelli, P.N.; Simões Rosa, A.C.; Clapauch, R.; Koifman, S. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ. Res. 2013, 127, 7–15. [Google Scholar] [CrossRef]
- Langer, P.; Tajtáková, M.; Fodor, G.; Kocan, A.; Bohov, P.; Michálek, J.; Kreze, A. Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated biphenyls. Eur. J. Endocrinol. 1998, 139, 402–409. [Google Scholar] [CrossRef]
- Bahn, A.K.; Mills, J.L.; Snyder, P.J.; Gann, P.H.; Houten, L.; Bialik, O.; Hollmann, L.; Utiger, R.D. Hypothyroidism in workers exposed to polybrominated biphenyls. N. Engl. J. Med. 1980, 302, 31–33. [Google Scholar] [CrossRef]
- Schell, L.M.; Gallo, M.V.; Ravenscroft, J.; DeCaprio, A.P. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ. Res. 2009, 109, 86–92. [Google Scholar] [CrossRef]
- Yi, S.W.; Hong, J.S.; Ohrr, H.; Yi, J.J. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: The Korean veterans health study. Environ. Res. 2014, 133, 56–65. [Google Scholar] [CrossRef]
- Agate, L.; Mariotti, S.; Elisei, R.; Mossa, P.; Pacini, F.; Molinaro, E.; Grasso, L.; Masserini, L.; Mokhort, T.; Vorontsova, T.; et al. Thyroid autoantibodies and thyroid function in subjects exposed to Chernobyl fallout during childhood: Evidence for a transient radiation-induced elevation of serum thyroid antibodies without an increase in thyroid autoimmune disease. J. Clin. Endocrinol. Metab. 2008, 93, 2729–2736. [Google Scholar] [CrossRef]
- Tajtáková, M.; Semanová, Z.; Tomková, Z.; Szökeová, E.; Majoros, J.; Rádiková, Z.; Seböková, E.; Klimes, I.; Langer, P. Increased thyroid volume and frequency of thyroid disorders signs in schoolchildren from nitrate polluted area. Chemosphere 2006, 62, 559–564. [Google Scholar] [CrossRef]
- Colucci, R.; Lotti, F.; Arunachalam, M.; Lotti, T.; Dragoni, F.; Benvenga, S.; Moretti, S. Correlation of Serum Thyroid Hormones Autoantibodies with Self-Reported Exposure to Thyroid Disruptors in a Group of Nonsegmental Vitiligo Patients. Arch. Environ. Contam. Toxicol. 2015, 69, 181–190. [Google Scholar] [CrossRef]
- Sato, K.; Inoue, S.; Igarashi, A.; Tokairin, Y.; Yamauchi, K.; Kimura, T.; Nishiwaki, M.; Nemoto, T.; Nakano, H.; Sato, M.; et al. Effect of Iron Deficiency on a Murine Model of Smoke-induced Emphysema. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 588–597. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Diez Roux, A.V.; Gassett, A.J.; Jacobs, D.R.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704, Erratum in Lancet 2016, 388, 660. [Google Scholar] [CrossRef]
- Samuels, M.H.; Bernstein, L.J. Brain Fog in Hypothyroidism: What Is It, How Is It Measured, and What Can Be Done About It. Thyroid 2022, 32, 752–763. [Google Scholar] [CrossRef]
- Ettleson, M.D.; Raine, A.; Batistuzzo, A.; Batista, S.P.; McAninch, E.; Teixeira, M.C.T.V.; Jonklaas, J.; Laiteerapong, N.; Ribeiro, M.O.; Bianco, A.C. Brain Fog in Hypothyroidism: Understanding the Patient’s Perspective. Endocr. Pract. 2022, 28, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Flores, M.; Warda, F.; Mooradian, A. Pathophysiology and Clinical Features of Neuropsychiatric Manifestations of Thyroid Disease. J. Endocr. Soc. 2022, 6, bvab194. [Google Scholar] [CrossRef] [PubMed]
- Watt, T.; Hegedüs, L.; Bjorner, J.B.; Groenvold, M.; Bonnema, S.J.; Rasmussen, A.K.; Feldt-Rasmussen, U. Is Thyroid Autoimmunity per se a Determinant of Quality of Life in Patients with Autoimmune Hypothyroidism? Eur. Thyroid J. 2012, 1, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Y.; Yang, H.; Xu, Y.; Cai, Y.; Liu, T.; Xia, Q.; Zhu, D.; Wang, F. Hashimoto’s Thyroiditis Induces Hippocampus-Dependent Cognitive Alterations by Impairing Astrocytes in Euthyroid Mice. Thyroid 2021, 31, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Guldvog, I.; Reitsma, L.C.; Johnsen, L.; Lauzike, A.; Gibbs, C.; Carlsen, E.; Lende, T.H.; Narvestad, J.K.; Omdal, R.; Kvaløy, J.T.; et al. Thyroidectomy Versus Medical Management for Euthyroid Patients With Hashimoto Disease and Persisting Symptoms: A Randomized Trial. Ann. Intern. Med. 2019, 170, 453–464. [Google Scholar] [CrossRef]
- Hidese, S.; Saito, K.; Asano, S.; Kunugi, H. Association between iron-deficiency anemia and depression: A web-based Japanese investigation. Psychiatry Clin. Neurosci. 2018, 72, 513–521. [Google Scholar] [CrossRef]
- Soppi, E. Iron deficiency is the main cause of symp-tom persistence in patients treated for hypothyroidism. Thyroid 2015, 25, A74. [Google Scholar]
- Cañas, C.A.; Cañas, F.; Bonilla-Abadía, F.; Ospina, F.E.; Tobón, G.J. Epigenetics changes associated to environmental triggers in autoimmunity. Autoimmunity 2016, 49, 1–11. [Google Scholar] [CrossRef]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218 Pt 1, 71–79. [Google Scholar] [CrossRef]
- Cai, T.T.; Muhali, F.S.; Song, R.H.; Qin, Q.; Wang, X.; Shi, L.F.; Jiang, W.J.; Xiao, L.; Li, D.F.; Zhang, J.A. Genome-wide DNA methylation analysis in Graves’ disease. Genomics 2015, 105, 204–210. [Google Scholar] [CrossRef]
- Limbach, M.; Saare, M.; Tserel, L.; Kisand, K.; Eglit, T.; Sauer, S.; Axelsson, T.; Syvänen, A.C.; Metspalu, A.; Milani, L.; et al. Epigenetic profiling in CD4+ and CD8+ T cells from Graves’ disease patients reveals changes in genes associated with T cell receptor signaling. J. Autoimmun. 2016, 67, 46–56. [Google Scholar] [CrossRef]
- Arakawa, Y.; Watanabe, M.; Inoue, N.; Sarumaru, M.; Hidaka, Y.; Iwatani, Y. Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clin. Exp. Immunol. 2012, 170, 194–201. [Google Scholar] [CrossRef]
- Yan, N.; Zhou, J.Z.; Zhang, J.A.; Cai, T.; Zhang, W.; Wang, Y.; Muhali, F.S.; Guan, L.; Song, R.H. Histone hypoacetylation and increased histone deacetylases in peripheral blood mononuclear cells from patients with Graves’ disease. Mol. Cell. Endocrinol. 2015, 414, 143–147. [Google Scholar] [CrossRef]
- Stefan, M.; Jacobson, E.M.; Huber, A.K.; Greenberg, D.A.; Li, C.W.; Skrabanek, L.; Conception, E.; Fadlalla, M.; Ho, K.; Tomer, Y. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J. Biol. Chem. 2011, 286, 31168–31179. [Google Scholar] [CrossRef]
- Stefan, M.; Wei, C.; Lombardi, A.; Li, C.W.; Concepcion, E.S.; Inabnet, W.B., 3rd; Owen, R.; Zhang, W.; Tomer, Y. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc. Natl. Acad. Sci. USA 2014, 111, 12562–12567. [Google Scholar] [CrossRef]
- Sarumaru, M.; Watanabe, M.; Inoue, N.; Hisamoto, Y.; Morita, E.; Arakawa, Y.; Hidaka, Y.; Iwatani, Y. Association between functional SIRT1 polymorphisms and the clinical characteristics of patients with autoimmune thyroid disease. Autoimmunity 2016, 49, 329–337. [Google Scholar] [CrossRef]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef]
- Vicente, R.; Noël, D.; Pers, Y.M.; Apparailly, F.; Jorgensen, C. Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat. Rev. Rheumatol. 2016, 12, 496, Erratum in Nat. Rev. Rheumatol. 2016, 12, 211–220. [Google Scholar] [CrossRef]
- Rothchild, A.C.; Sissons, J.R.; Shafiani, S.; Plaisier, C.; Min, D.; Mai, D.; Gilchrist, M.; Peschon, J.; Larson, R.P.; Bergthaler, A.; et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, E6172–E6181. [Google Scholar] [CrossRef]
- Seddiki, N.; Brezar, V.; Ruffin, N.; Lévy, Y.; Swaminathan, S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 2014, 142, 32–38. [Google Scholar] [CrossRef]
- Chatzikyriakidou, A.; Voulgari, P.V.; Georgiou, I.; Drosos, A.A. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand. J. Immunol. 2010, 71, 382–385. [Google Scholar] [CrossRef]
- Park, H.; Huang, X.; Lu, C.; Cairo, M.S.; Zhou, X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J. Biol. Chem. 2015, 290, 2831–2841. [Google Scholar] [CrossRef]
- Bernecker, C.; Lenz, L.; Ostapczuk, M.S.; Schinner, S.; Willenberg, H.; Ehlers, M.; Vordenbäumen, S.; Feldkamp, J.; Schott, M. MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid 2012, 22, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Zhang, W.; Zhang, W.; Fan, L.; Wang, L.; Liu, Y.; Liu, S.; Guo, Y.; Wang, Y.; et al. MicroRNA-142-5p contributes to Hashimoto’s thyroiditis by targeting CLDN1. J. Transl. Med. 2016, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liu, Y.; Tian, J.; Ma, J.; Tang, X.; Yang, J.; Rui, K.; Zhang, Y.; Mao, C.; Lu, L.; et al. Decreased expression of microRNA-125a-3p upregulates interleukin-23 receptor in patients with Hashimoto’s thyroiditis. Immunol. Res. 2015, 62, 129–136. [Google Scholar] [CrossRef]
- Stachurska, A.; Zorro, M.M.; van der Sijde, M.R.; Withoff, S. Small and Long Regulatory RNAs in the Immune System and Immune Diseases. Front. Immunol. 2014, 5, 513. [Google Scholar] [CrossRef] [PubMed]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Ollikainen, N.; Guttman, M. Long non-coding RNAs: Spatial amplifiers that control nuclear structure and gene expression. Nat. Rev. Mol. Cell. Biol. 2016, 17, 756–770. [Google Scholar] [CrossRef]
- Huang, W.; Thomas, B.; Flynn, R.A.; Gavzy, S.J.; Wu, L.; Kim, S.V.; Hall, J.A.; Miraldi, E.R.; Ng, C.P.; Rigo, F.; et al. Retraction Note: DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature 2018, 562, 150. [Google Scholar] [CrossRef]
- Aune, T.M.; Crooke, P.S., 3rd; Spurlock, C.F., 3rd. Long noncoding RNAs in T lymphocytes. J. Leukoc. Biol. 2016, 99, 31–44. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Tian, J.; Ma, J.; Tang, X.; Rui, K.; Tian, X.; Mao, C.; Lu, L.; Xu, H.; et al. The Long Noncoding RNA IFNG-AS1 Promotes T Helper Type 1 Cells Response in Patients with Hashimoto’s Thyroiditis. Sci. Rep. 2015, 5, 17702. [Google Scholar] [CrossRef]
- Briggs, S.F.; Reijo Pera, R.A. X chromosome inactivation: Recent advances and a look forward. Curr. Opin. Genet. Dev. 2014, 28, 78–82. [Google Scholar] [CrossRef]
- Gendrel, A.V.; Heard, E. Noncoding RNAs and epigenetic mechanisms during X-chromosome inactivation. Annu. Rev. Cell. Dev. Biol. 2014, 30, 561–580. [Google Scholar] [CrossRef]
- Minks, J.; Robinson, W.P.; Brown, C.J. A skewed view of X chromosome inactivation. J. Clin. Investig. 2008, 118, 20–23. [Google Scholar] [CrossRef]
- Seidel, M.G.; Rami, B.; Item, C.; Schober, E.; Zeitlhofer, P.; Huber, W.D.; Heitger, A.; Bodamer, O.A.; Haas, O.A. Concurrent FOXP3- and CTLA4-associated genetic predisposition and skewed X chromosome inactivation in an autoimmune disease-prone family. Eur. J. Endocrinol. 2012, 167, 131–134. [Google Scholar] [CrossRef][Green Version]
- Brix, T.H.; Knudsen, G.P.; Kristiansen, M.; Kyvik, K.O.; Orstavik, K.H.; Hegedüs, L. High frequency of skewed X-chromosome inactivation in females with autoimmune thyroid disease: A possible explanation for the female predisposition to thyroid autoimmunity. J. Clin. Endocrinol. Metab. 2005, 90, 5949–5953. [Google Scholar] [CrossRef]
- Yin, X.; Latif, R.; Tomer, Y.; Davies, T.F. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann. N. Y. Acad. Sci. 2007, 1110, 193–200. [Google Scholar] [CrossRef]
- Aksu, B.Y.; Hasbal, C.; Himmetoglu, S.; Dincer, Y.; Koc, E.E.; Hatipoglu, S.; Akcay, T. Leukocyte DNA damage in children with iron deficiency anemia: Effect of iron supplementation. Eur. J. Pediatr. 2010, 169, 951–956. [Google Scholar] [CrossRef]
- Ames, B.N. Micronutrient deficiencies. A major cause of DNA damage. Ann. N. Y. Acad. Sci. 1999, 889, 87–106. [Google Scholar] [CrossRef]
- Aslan, M.; Horoz, M.; Kocyigit, A.; Ozgonül, S.; Celik, H.; Celik, M.; Erel, O. Lymphocyte DNA damage and oxidative stress in patients with iron deficiency anemia. Mutat. Res. 2006, 601, 144–149. [Google Scholar] [CrossRef]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Naitoh, Y.; Kohno, H.; Tokunaga, R.; Taketani, S. Iron deprivation decreases ribonucleotide reductase activity and DNA synthesis. Life Sci. 1992, 50, 2059–2065. [Google Scholar] [CrossRef]
- Zohora, F.; Bidad, K.; Pourpak, Z.; Moin, M. Biological and Immunological Aspects of Iron Deficiency Anemia in Cancer Development: A Narrative Review. Nutr. Cancer 2018, 70, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kobayashi, K.; Ndong, M.; Yamamoto, Y.; Katsumata, S.; Suzuki, K.; Uehara, M. Activation of Nrf2/Keap1 signaling and autophagy induction against oxidative stress in heart in iron deficiency. Biosci. Biotechnol. Biochem. 2015, 79, 1366–1368. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Clarke, S. Influence of microRNA on the maintenance of human iron metabolism. Nutrients 2013, 5, 2611–2628. [Google Scholar] [CrossRef]
- Weitz, S.H.; Gong, M.; Barr, I.; Weiss, S.; Guo, F. Processing of microRNA primary transcripts requires heme in mammalian cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1861–1866. [Google Scholar] [CrossRef]
- Nallamshetty, S.; Chan, S.Y.; Loscalzo, J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radic Biol. Med. 2013, 64, 20–30. [Google Scholar] [CrossRef]
- Bao, B.; Azmi, A.S.; Li, Y.; Ahmad, A.; Ali, S.; Banerjee, S.; Kong, D.; Sarkar, F.H. Targeting CSCs in tumor microenvironment: The potential role of ROS-associated miRNAs in tumor aggressiveness. Curr. Stem. Cell. Res. Ther. 2014, 9, 22–35. [Google Scholar] [CrossRef]
- Crosby, M.E.; Kulshreshtha, R.; Ivan, M.; Glazer, P.M. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009, 69, 1221–1229, Erratum in Cancer Res. 2009, 69, 3240. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- Barks, A.K.; Liu, S.X.; Georgieff, M.K.; Hallstrom, T.C.; Tran, P.V. Early-Life Iron Deficiency Anemia Programs the Hippocampal Epigenomic Landscape. Nutrients 2021, 13, 3857. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).